Abstract
Objective:
Endovascular abdominal aortic aneurysm repair (EAR) requires long-term surveillance for endoleak or increase in aneurysm diameter. We analyzed the natural history of and risk factors for endoleak development.
Summary Background Data:
Endoleak is a common complication of EAR that can lead to aneurysm enlargement and even rupture. Following EAR, imaging studies are used to identify leaks since patients with endoleak may require additional endovascular interventions or conversion to open repair. No criteria currently exist for cessation or reduction in frequency of screening imaging studies.
Methods:
Data on 220 patients undergoing EAR were retrospectively reviewed. Kaplan-Meier survival analysis and Cox proportional hazards regression were used with the end point being new endoleak development. Potential risk factors included preoperative aneurysm diameter, number of negative surveillance studies, and postoperative increase in diameter.
Results:
A total of 52 patients (24%) who underwent EAR had endoleak detected during postoperative follow-up, which averaged 19 months (range, 0.4–101 months). One, 6-, 12-, and 24- month endoleak-free survival was 90%, 80%, 77%, and 73%, respectively. Three leaks occurred after year 2, at postoperative months 24, 48, and 85. Increasing number of negative screening studies was negatively associated with risk for endoleak development (B = −3.122, P < 0.001), while increase in aneurysm diameter was positively associated with risk for endoleak (B = 0.072, P = 0.04).
Conclusion:
Risk for endoleak declines as the number of negative postoperative scans increases, but new endoleaks are identified as late as 7 years following EAR. Reduction in screening frequency cannot be uniformly recommended at this time. Patients with documented aneurysm expansion should be monitored carefully and endoleak should be suspected.
Serial computed tomography (CT) scans and/or ultrasounds are required following endovascular abdominal aortic aneurysm repair (EAR) to identify endoleak or aneurysm enlargement. Postoperative CT scan data on 220 patients undergoing EAR are examined to determine the rate, temporal course, and predictors for late endoleak development.
Endovascular abdominal aortic aneurysm repair (EAR) has become an increasingly popular therapeutic option, especially for patients with comorbidities that make open repair high risk. The technique has less than certain success, and multiple cases of aneurysm rupture following EAR with a variety of devices have been reported.1–7 Successful EAR requires exclusion of the aneurysm sac from the systemic circulation;8 incomplete exclusion results in endoleak with aneurysm sac pressurization, and ongoing risk for rupture.9
Endoleak is a complication unique to EAR, and is defined as blood flow within the aneurysm sac but outside the endoluminal graft. Endoleaks occur in 10% to 40% of patients following EAR4,6,10–13 and are classified (types I–IV) by the source of communication between the systemic circulation and the aneurysm sac.14 Most endoleaks are discovered during the first 30 postoperative days (primary endoleaks), but late endoleaks (secondary endoleaks) are also well recognized.15
Screening for endoleak, aneurysm enlargement, graft migration, or other postoperative complications can be accomplished with a combination of computed tomography (CT) scanning and duplex ultrasound. Published follow-up recommendations for image screening after EAR without endoleak suggest screening at 1, 6, and 12 months postoperative and then every year thereafter.16 There are no established criteria for reduction in frequency of screening studies for patients who remain endoleak-free years after EAR. The objective of this study was to determine the temporal course of secondary endoleak occurrence, to identify risk factors for late endoleak development, and to determine if imaging is needed in late endoleak-free follow-up.
METHODS
We reviewed a database of 220 patients that underwent EAR from 1993 to 2002. Longitudinal data regarding aneurysm size and endoleak status gathered from ultrasound, CT scan, and arteriogram studies were prospectively acquired and retrospectively reviewed.
Postoperative CT scans were routinely performed with arterial-phase IV contrast with 3-mm cuts within the first 30 postoperative days, at 6 months, and yearly thereafter (provided no indication was discovered for increased frequency). CT scans were reviewed by both radiology staff and the senior author, with the consensus interpretations regarding aneurysm diameter and endoleak status recorded into the database. Duplex ultrasounds were performed in an accredited vascular laboratory by a certified technician and interpreted by vascular surgery staff.
Statistical methods included Kaplan-Meier survival analysis and Cox proportional hazards regression, with the end point defined as first endoleak development. Patients who never experienced endoleak were censored from analysis at the time of their final negative follow-up study. Endoleak risk factors included number of negative screening studies prior to endoleak detection or final endoleak-free follow-up, maximum preoperative aneurysm diameter, and postoperative change in aneurysm diameter. Summary data shown are mean ± standard error. All statistical analyses were performed using the Statistical Package for the Social Sciences software (SPSS, Inc, Chicago, IL).
RESULTS
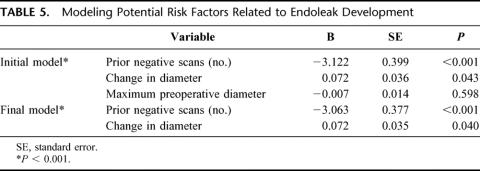
Of 220 patients analyzed, 88% were male and 12% were female (Table 1). The mean patient age was 72.2 ± 0.56 years (range, 43–92 years). Mean aneurysm diameter at the time of repair was 56 ± 0.75 mm (36–110 mm), with a mean change in diameter from operation to end point of −5.5 ± 0.56 mm. The majority of patients (73%) underwent repair with the Ancure prosthesis (Guidant Corporation, Menlo Park, CA), followed by the Excluder (23%) (W.L. Gore & Associates, Inc. Flagstaff, AZ) and AneuRx (5%) (Medtronic AVE, Santa Rosa, CA) devices, respectively (Table 2).
TABLE 1. Patient and Device Data at Time of Endovascular Aneurysm Repair (n = 220)
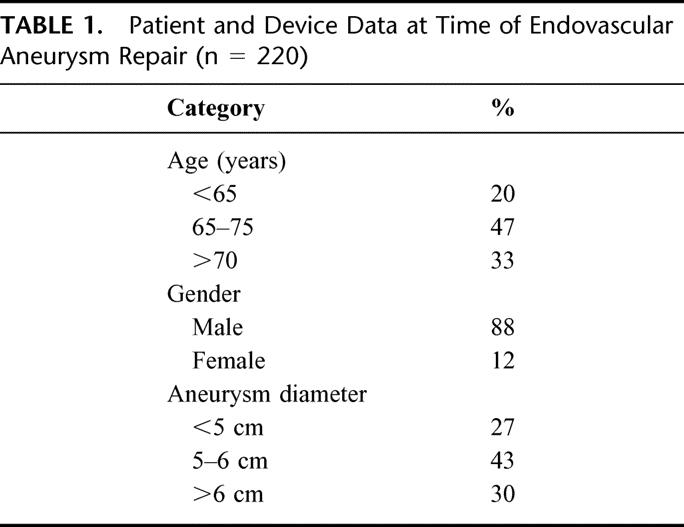
TABLE 2. Devices by Configuration
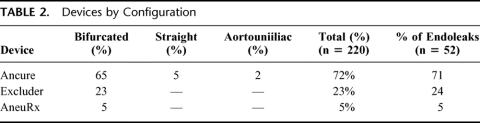
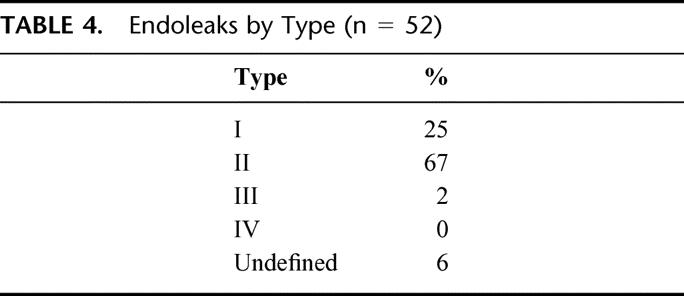
All patients who underwent EAR had CT scans performed as part of their postoperative imaging, while 52 (24%) of patients had ultrasound follow-up in addition to CT. Fifty-two patients (24%) had endoleak detected during postoperative follow-up. There were no significant differences in endoleak related to device (Table 2). One-, 6-, 12-, and 24-month survival without endoleak was 90%, 80%, 77%, and 73%, respectively (Fig. 1 and Table 3). The majority of endoleaks were type II (67.3%); 25% were type I, 1.9% were type III, and 5.8% were type indeterminate (Table 4). Three endoleaks occurred after year 2, at post-EAR months 25 (type II), 48 (type II), and 85 (type I). Mean time from operation to endoleak detection was 10 ± 6.4 months (range 0.1–85 months) for type I endoleaks and 4.6 ± 1.6 months (range 0.1–48.1 months) for type II endoleaks. There were no post-EAR aneurysm ruptures.
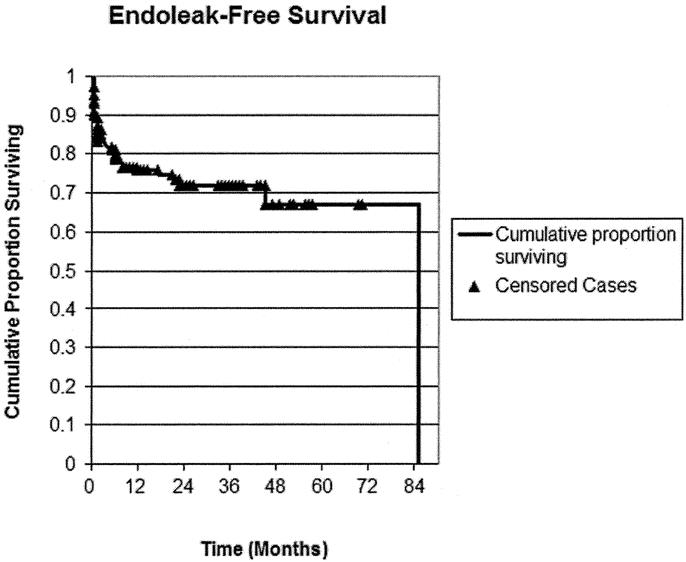
FIGURE 1. Kaplan-Meier curve: freedom from endoleak. ▴, censored cases (final, event-free follow-up).
TABLE 3. Kaplan-Meier Survival Analysis: Endoleak-Free Survival Through 48 Months
TABLE 4. Endoleaks by Type (n = 52)
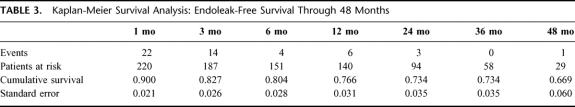
Cox proportional hazards regression modeling of potential risk factors associated with endoleak development is presented in Table 5. The number of negative screening studies prior to endoleak (or to final endoleak-free follow-up) was negatively associated with risk for endoleak (B = −3.122, P < 0.001), and change in aneurysm diameter was positively associated with risk for endoleak (B = 0.072, P = 0.04). Preoperative aneurysm diameter was not related to endoleak development (B = −0.007, P = 0.60).
TABLE 5. Modeling Potential Risk Factors Related to Endoleak Development
DISCUSSION
Endoleak classification is based upon the source of communication between the aneurysm sac and the systemic circulation. Type I endoleaks have blood flow into the aneurysm sac around the proximal or distal graft attachment sites, or around an inadequately sealed iliac occluder plug. Type II endoleaks involve blood flow into the aneurysm sac from the visceral aortic branches (such as the inferior mesenteric, lumbar, or accessory renal arteries) without communication around the attachment sites. Type III endoleak denotes flow between separated graft modules or through a graft defect, and type IV endoleak refers to flow through porosities in the graft material during the early postoperative period. In some cases, the source of endoleak cannot be determined with imaging studies; this is referred to as endoleak of undetermined origin. As many as 12% of patients require additional procedures following EAR to manage endoleak17; extender cuff insertion and conversion to open repair are treatment options for type I or type III endoleaks, while type II endoleaks are often amenable to catheter-based embolization or laparoscopic vessel ligation. The risk of endoleak as well as aneurysm enlargement requires late image screening following EAR.
Prevention of death from aneurysm rupture is the primary goal of EAR. Following EAR, adequate aneurysm exclusion is generally thought to be associated with aneurysm shrinkage,18,19 whereas presence of endoleak allows continued aneurysm sac pressurization and risk for rupture. While the clinical importance of endoleak in general (and type II endoleak in particular) has been questioned in the past,20 several authors have demonstrated a significant relationship between endoleak and aneurysm expansion or even rupture.1,5,21 The recent description of a series of post-EAR aneurysm ruptures associated with endoleak in the absence of detectable expansion, including type II endoleaks, has further emphasized the importance of endoleak detection, surveillance, and potential need for intervention.7
Correlation between preoperative aneurysm diameter greater than 6 cm and endoleak development has been demonstrated by others,22 although we did not observe such a relationship in our patient cohort. The association between large preoperative aneurysm diameter and increased endoleak occurrence could be related to either a propensity for larger aneurysm sacs to harbor type II endoleaks without spontaneous thrombosis or the more severe proximal neck angulation associated with larger aneurysms inviting type I leaks. While not evaluated in this study, other reports suggest aortic neck angulation impacts type I endoleak risk and requires special consideration when planning late imaging follow-up.23 We used the Ancure prosthesis in a majority of our patients and certainly preferred it for patients with substantial proximal neck angulation. The ability of this unsupported graft to accommodate proximal angulation may have provided us a more favorable leak rate proximally in patients with angulated necks. However, our sample size is too small to provide proof of such a hypothesis.
Proximal neck dilation is recognized as a complication following EAR, with risk increasing over time.22,23 While substantial dilation of the proximal neck may be related to patient-anatomy selection, it is also possible that the proximal neck dilates over time as extrinsic force from self-expanded prostheses impart radial force in addition to that from aortic pulsation. Our latest endoleak occurred in the presence of aortic neck dilation 85 months after operation. Two of the 3 secondary endoleaks occurring after year 2 were type II leaks. It is possible that instead of new events, these may represent endoleaks present since EAR but undetected because of either the sensitivity of the screening techniques or the intermittent nature of the leak. Although the true duration of any late endoleak is difficult to accurately determine, 1 of these patients ultimately required coil embolization, illustrating the potential clinical importance secondary type II endoleak detection.
The significant increase in risk for endoleak development in the setting of postoperative aneurysm expansion described in this study underscores the interrelation between these 2 imaging findings. Aneurysm expansion should prompt suspicion of an associated endoleak, the treatment of which may result in subsequent reduction of aneurysm diameter.23 Aneurysm expansion can also occur, however, without associated endoleak development; this phenomenon is referred to as endotension.15 Endotension may indicate unsuccessful exclusion and ongoing risk for rupture; however, controversy persists regarding the clinical significance of endotension. We have thus far a single patient with endotension and therefore little experience with its management.
While there is generalized agreement that patients with endoleak should either be closely followed or treated, the management of patient-years status-post EAR with an increasing number of negative surveillance imaging studies remains unclear.
The difficulty in providing long-term follow-up for patients after EAR limits the overall value of EAR treatment. Patient travel, costs associated with multiple imaging studies, patients lost to follow-up, as well as clinician time and effort all collide to make follow-up in large numbers of EAR patients problematic. The ability to reduce the frequency of imaging studies in a cohort of patients without endovascular leak 2 or more years after operation would be desirable from cost and convenience perspectives for both patient and vascular surgeon. This study demonstrates a decreased risk for new endoleak development as the number of negative screening studies increases as well as an increased risk for endoleak in the presence of an expanding native aneurysm. Considered together, these findings suggest that it might be desirable to adopt more liberal screening regimens for patients with multiple negative surveillance imaging studies and stable aneurysm size. Our enthusiasm for such modification in follow-up is tempered by the occurrence of new endoleaks as late as 85 months following EAR, especially since the consequences of missed endoleak are potentially catastrophic.
Limitations
Our study has several limitations. Delayed contrast CT image acquisition has been recently suggested to enhance the sensitivity for endoleak detection24; our failure to use this technique routinely may have resulted in missed endoleaks. CT scanning was the only postoperative screening modality used in the majority (76.3%) of our patients, and we did not identify additional leaks with ultrasound as an adjuvant test. Although not included in our initial model, aortic neck angulation, the presence of mural thrombus, and excessive neck calcification have been proposed as other risk factors for endoleak.25 Patients with excessive neck calcification (which can create difficulty with obtaining an adequate proximal seal) or mural thrombus (which can predispose to graft slippage and migration) are not considered good candidates for EAR at our institution; these risk factors were therefore not included in our analysis but might be important predictors of endoleak at centers where EAR is undertaken in patients with these aneurysm characteristics. Neck angulation was excluded from our analysis due to difficulty in reproducible measurement of this characteristic both preoperatively and postoperatively. Finally, the small number of patients at risk for endoleak beyond 4 years in this study limits our ability to draw definitive conclusions about the late incidence of secondary endoleak, although our observation of endoleak events past this 4-year time point is particularly concerning.
Contrasted CT scanning has been recommended as the primary means of post-EAR imaging16 and continues to be the preferred method at our institution. Although our early screening regimen used both CT and ultrasound, cost and ability to maintain patient follow-up can be adversely impacted by the use of multiple tests. We primarily use ultrasound to further characterize endoleaks identified by prior CT scans or to gain additional information in patients with indeterminate CT scans; data provided by ultrasound such as intraluminal flow velocity and direction can be helpful in predicting endoleak resolution and/or planning endoleak management.26,27 The clinical utility of MRA and other noninvasive means of screening for endoleak remain to be defined.
CONCLUSION
This study identifies a significant reduction in risk for late endoleak with an increasing number of negative screening imaging studies. However, late endoleak occurrence past 2 postoperative years was still observed. Interval increase in aortic diameter was predictive of endoleak presence. Although we cannot make specific recommendations for reduction in frequency or cessation of late follow-up screening at this time, long-term follow-up in the future may allow definition of a post-EAR endoleak-free subgroup that can be safely followed by aneurysm size alone. In the future, larger studies may be able to recommend individualized late screening regimens based on screening history and aneurysm size.
Discussions
Dr. Eric D. Endean (Lexington, Kentucky): I would like to thank the authors for providing me with a copy of their manuscript in advance for my review.
Endoluminal repair of abdominal aortic aneurysm is in many respects attractive. However, enthusiasm is tempered by the inability to apply the technique to all patients and the risk of late rupture despite an initial successful repair. It is believed that endoleak may be responsible for pressurizing the aneurysm sac and as a consequence results in risk for aneurysm expansion and rupture. The fact that endoleak may develop at some time after endoluminal repair has led to the recommendation for postoperative surveillance protocols to detect endoleak. Currently, it is thought that life-long follow-up is needed. These studies increase the cost of endoluminal repair and patients find the repeated studies inconvenient.
Dr. Naslund and his colleagues have reported on the incidence of endoleaks as well as the time course of its development following endoluminal repair of abdominal aortic aneurysms with the hope that criteria can be established to eventually limit or eliminate the need for indefinite follow-up studies. Unfortunately, their findings parallel those of others, and, because of the finding of late endoleaks, they continue to recommend long-term follow-up. I have a few questions to pose to the authors.
First, you mention that both CT scans and duplex ultrasounds were used to detect endoleaks. Could you elaborate on which modality you found most useful, whether the studies were complementary, or how often each was used?
Second, the authors evaluated as risk factors for the development of endoleak negative screening studies prior to detection, maximum preoperative aneurysm diameter, and postoperative change in aneurysm diameter. Did you look at other potential factors that may have contributed to endoleak such as the degree of angulation of the neck, presence of any endoleak seen at the time of endograph deployment, the presence or absence of iliac (common or internal iliac) artery aneurysm, patency of the inferior mesenteric artery, associated occlusive disease, or some measure pertaining to the difficulty of successfully completing the endograph?
Third, while it is perhaps beyond the scope of this paper, what approach do you take in regards to treatment when a new type II endoleak is identified?
Fourth, your data evaluate the development of any type of endoleak over time. Have you broken down the data to look at the time course for the development of each type of endoleak?
Finally, in the manuscript you suggest that at least some endoleaks found on follow-up studies may represent leaks that had been present since repair but were undetected. While this is possible, it would seem that endoleaks do develop at some time after repair. Would you comment on the mechanisms that lead to these late developing endoleaks, and in particular the type II endoleaks?
I enjoyed reading this manuscript. It is well written and addresses a timely and important problem in vascular surgery. The authors have detailed their experience from a large and impressive series of patients.
Dr. William D. Jordan, Jr. (Birmingham, Alabama): I want to congratulate Dr. Naslund and his colleagues for reporting on the need for postoperative surveillance after endovascular aneurysm repair. This report of more than 220 patients over a 9-year period represents both pioneering work done prior to FOA approval of endovascular aneurysm repair and also continued the high level standard of care that these Vanderbilt surgeons have continued to practice.
More specifically, Dr. Naslund has addressed the issue of endoleak or the identification of contrast outside the lumen of the stent but within the aneurysm sac. Despite 9 years of experience with this problem, we do not fully understand the implications of an endoleak. Regardless, Dr. Naslund may have offered a practical tool using the absence of an of an endoleak as an opportunity to limit the careful surveillance that has been so often prescribed after this new advance in aneurysm treatment. I realize his final recommendations said not, but I think perhaps there is a gem in here that we can use. Their series identified shrinking aneurysms in most patients after repair and no ruptures in this cohort of 220 consecutive patients.
I have only 3 questions for Dr. Naslund. First, at UAB we are also using the surrogate of endoleak in evaluating the success of this type of treatment. Does the absence of endoleak really assure us of success? Only 5 short days ago, early in the wee hours of Thanksgiving morn, I had to convert a patient who was 85 years old and suffered a rupture even in the absence of endoleak. This patient was visiting from another state when he presented with his problem to our institution. What was missed with this patient? Are we simply following an endoleak because we can see it? Do we follow this entity because it is available on CT or ultrasound? There have been recent developments in assessing the pressure within the aneurysm sac. Might this be a better surrogate if we can follow the actual pressure rather than the presence of contrast?
Secondly, you describe a 24% endoleak rate and a secondary intervention rate of 12%. What was your secondary intervention rate required in these 220 patients? Additionally, are these secondary interventions always required or simply these radiographic findings that we are treating in hopes of improving on our results?
Finally, at our own institution in Birmingham, we have used maximum aneurysm diameter as an endpoint to follow these patients. You also used diameter in your report. However, some authors have suggested that we use total volume as a better measurement and thus a better surrogate to assess the successful exclusion of the aneurysm. Do you have any experience with these volumetric measurements using the 3-dimensional software that is commonly being promoted today? Do you think this will be a valuable tool for us in the future?
I certainly want to thank Dr. Naslund for the opportunity to review the manuscript and for forwarding it to me well in advance of the meeting. I am impressed with the work at your institution and I look for more scientific information to assist us in treating our aneurysm patients.
Dr. L. D. Britt (Norfolk, Virginia): Would you please expand on the technical difficulties, if any, if you screen and you find an irreversible problem necessitating an open approach?
Dr. Ali F. Aburahma (Charleston, West Virginia): I also want to congratulate you for this presentation, but I think there is something still lacking in terms of the risk factor for endoleak. Specifically, 2 of them extensive calcification and the presence of extensive thrombus. I also want to echo Dr. Jordan's comment. I think most of the trend in the last few years has been to look to the endotension or sac pressure measurement as a better predictor of future ruptures and so forth. And I wonder if you have done this in the last few years?
Dr. Thomas C. Naslund (Nashville, Tennessee): Dr. Endean asked several questions regarding follow-up with CT versus ultrasound. My personal opinion is that CT is the best modality for follow-up. The reason I feel this way is because resolution available with modern scanners and the computer software availability of modern scanners provide images far superior to those available from ultrasound. Furthermore, surface ultrasound is more technician-dependent than is CT scanning. I feel that some centers do a good job utilizing ultrasound for endograft follow-up, but they are selective, very experienced, and generally have only 1 or 2 technicians performing the studies.
I think that ultrasound and CT scan can be complementary follow-up studies. Endoleaks can be missed with both modalities. Ultrasound provides information important about limb blood flow that cannot be obtained with CT scanning and may be able to find endoleaks that are missed by CT scanning. Some centers select ultrasound in favor of CT scan; others select a combination of the 2 studies. It is perfectly reasonable to have both modalities utilized, but I will generally utilize CT scanning alone for routine follow-up.
The next question involved other risk factors for endoleak such as neck angulation. Neck angulation is a difficult entity to define in that we have no hard and fast rule for angle measurement. But, in response to the question, we did not assess angle in this study. We certainly appreciate the fact that angulation invites the opportunity for proximal leak.
Similarly, we did not study operative endoleaks and how they might predict secondary endoleaks over time. Part of this is due to the limited scope of our study and part of this is due to difficulty in both detection and documentation of operative endoleak in the operative note.
Sometimes I find myself unsure based on operative notes as to whether an extension was done because a type I endoleak was present or simply suspected. Since I resolve type I endoleaks intraoperatively, there are none at the end of the procedure. Dr. Endean also commented regarding the iliac artery aneurysms as an independent risk factor for late endoleak. While this is certainly true based on possibility of distal type I leaks, we did not study this as an independent risk factor.
In addition, we did not assess the inferior mesenteric artery patency preoperatively as a risk factor. While many patients have patent inferior mesenteric arteries, it is not a particularly difficult type II endoleak to manage postoperatively if it is a source of ongoing leak. It is without doubt a risk factor for type II leak since it is impossible to have a type II leak involving the inferior mesenteric artery unless the inferior mesenteric artery is patent.
Regarding my approach to type II leaks, I generally leave the type II leak with observation alone for the first 6 months. I counsel the patient in advance that they will have upwards of 30% chance of having one at 1 month. At 6 months, I make a plan, in part depending upon the size of the aneurysm, to resolve the leak. Thus far, all type II leaks have been able to be managed with endoluminal techniques. Undetected endoleaks are obviously difficult to assess. I can't determine whether or not I am missing endoleaks and identifying them later as secondary leaks or if indeed the patient progresses from an endoleak-free state to a late endoleak development. The technique of CT scanning has evolved to include not only CTA images but also delayed scans. Early in my experience, I did not routinely have such scans done and certainly could have missed leaks without appreciating it. Two of the late leaks were type II leaks, and it is not possible for me to comment as to whether or not they were secondary leak or simply missed primary leaks. However, the latest leak was at 85 months, which was a type I endoleak. It was a minor leak but nonetheless was undoubtedly new. It was secondary to proximal neck dilation.
Dr. Jordan asked if the absence of endoleak assures that we have successfully managed the patient's aneurysm. The answer is unequivocally no. Unfortunately, this technique has a lot of pitfalls, and that is why the follow-up becomes so important. Dr. Jordan's experience of having a ruptured aneurysm with no previously identified endoleaks has been reported by others as well. I worry about late endoleak and I also worry about device migration as a cause for endoleak formation and ultimate aneurysm rupture. For this reason I tend to select the device that I think has the lowest risk for migration, assuming the anatomy is compatible. I find the complication of migration most disturbing of all risk factors.
There are a couple of questions related to sensing of pressure in the aneurysm sac. There is new microchip technology that allows implantation of a device in the aneurysm sac and allows remote pressure evaluations. Such pressure evaluations could possibly even be done in the office or from a remote location, such as the patient's home, and provide some information regarding physiology within the aneurysm sac.
Unfortunately, all I can say right now is that it is a wonderful research tool. I have no personal experience with it, but I think it will teach us a lot about what is going on in the aneurysm sac after endovascular grafting. I am sure it will lead us in new directions, but I don't know where. I am reluctant to suggest that it will offer a meaningful method of follow-up because all it will provide is sac pressure measurements. Issues about migration and endoleak may or may not be defined by pressure measurement alone. I feel that the follow-up issues regarding aneurysm repair are too complicated to be solved by aneurysm sac pressure alone.
Finally, Dr. Jordan asked a question about measurement of aneurysm volume versus aneurysm diameter. I think that aneurysm volume assessments are probably better. I have not been utilizing them mostly because the programs that allow volume measurements are fairly new and I have follow-up that goes back as long as 9 years. I have recorded data in a consistent fashion over time, which started out with simple diameters, and thus far I continue with simple diameters.
I continue to watch the data of others, and I am personally more concerned about lost to follow-up patients or lack of follow-up than I am subtle differences between aneurysm diameter measure or volume measurements that might be present. The biggest problem of all is lost follow-up. It is the problem that I struggle with the most and which I think is most important in patients undergoing endovascular aneurysm repair.
Regarding the question of technical difficulty of converting a patient from complication of endovascular aneurysm repair, in general it is safe to say that conversions are more difficult than primary aneurysm operations. The reason for this is that there is a lot of fibrosis that occurs around areas of interest such as the neck of the aneurysm. This is especially true with grafts that have fixation systems that pierce the artery wall. Once such a graft is placed and ballooning occurs in the attachment system, undoubtedly there is some hemorrhage around the aorta inducing a fibrotic reaction, making subsequent exposure difficult. What is also important to note is that some grafts, most notably the Ancure graft, have hooks that pierce the artery wall, causing injury to fingers probing around the aortic neck.
As far as technical tips on conversions, I am very reluctant to convert patients and generally go to extreme efforts to avoid conversion utilizing advanced endovascular techniques. To date, all of my late conversions have been done for infection. I have not yet converted a patient for endovascular leak and therefore can provide little in terms of specific expertise for this type of operation. I have resorted to laparoscopic help to manage type II endoleaks on a couple of occasions with success. The best tip I could provide for conversion of an endovascular graft is understanding completely the original operation, the device involved, the techniques involved, and whether or not there is any type of positive fixation between the prothesis and artery. It is fair to say that the Ancure graft is going to be the hardest to explant. The newer Cook Zenith graft with its suprarenal fixation would probably also be difficult to explant. It is my understanding that it is best to leave the suprarenal stent in place and simply sew a conventional graft underneath it. The other 2 grafts available on the market today seem to come out fairly easily.
Another question was related to calcium in the proximal neck and thrombus in the proximal neck. Thrombus in the proximal neck impairs any type of positive fixation between the device and graft and therefore invites migration. The Ancure graft resisted migration if the hooks were able to be placed in a segment of a normal vessel. This may also be true of the Zenith graft but, in general, I would avoid the neck that harbors significant thrombus.
Calcium in the proximal neck can provide a rigid constraint that prevents the graft from expanding and molding to the architecture of the neck and therefore increases the risk of type I leak. Similarly, thrombus in the neck can provide slippage, mechanical failure, or leak. During the process of patient selection, the proximal neck is without doubt the most important aspect of anatomy to consider when offering a patient an endovascular graft.
Footnotes
Reprints: Thomas C. Naslund, MD, Division of Vascular Surgery, 1161 22nd Avenue South, D5237 MCN, Nashville, TN 37232. E-mail: thomas.naslund@vanderbilt.edu.
Research grant support from Guidant Endovascular Technologies, Inc. (EVT, Menlo Park, CA); research grant support and speaker for WL Gore & Associates, Inc. (Flagstaff, AZ); scientific advisor for LeMaitre Vascular, Inc. (Burlington, MA).
REFERENCES
- 1.Harris PL, Vallabhaneni SR, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32:739–749. [DOI] [PubMed] [Google Scholar]
- 2.Politz JK, Newman VS, Stewart MT. Late abdominal aortic aneurysm rupture after AneuRx repair: a report of three cases. J Vasc Surg. 2000;31:599–606. [PubMed] [Google Scholar]
- 3.Zarins CK, White RA, Fogarty TJ. Aneurysm rupture after endovascular repair using the AneuRx stent graft. J Vasc Surg. 2000;31:960–970. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg RK, Lawrence-Brown M, Bhandari G, et al. An update of the Zenith endovascular graft for abdominal aortic aneurysms: initial implantation and mid-term follow-up data. J Vasc Surg. 2001;33(suppl 2):157–164. [DOI] [PubMed] [Google Scholar]
- 5.Hinchliffe RJ, Singh-Ranger R, Davidson IR, et al. Rupture of an abdominal aortic aneurysm secondary to type II endoleak. Eur J Vasc Endovasc Surg. 2001;22:563–565. [DOI] [PubMed] [Google Scholar]
- 6.Faries PL, Brener BJ, Connelly TL, et al. A multicenter experience with the Talent endovascular graft for the treatment of abdominal aortic aneurysms. J Vasc Surg. 2002;35:1123–1128. [DOI] [PubMed] [Google Scholar]
- 7.Fransen Ga G, Vallabhaneni SSr, Van Marrewijk Cj C, et al. Rupture of infra-renal aortic aneurysm after endovascular repair: a series from EUROSTAR Registry. Eur J Vasc Endovasc Surg. 2003;26:487–493. [DOI] [PubMed] [Google Scholar]
- 8.Sonesson B, Dias N, Malina M, et al. Intra-aneurysm pressure measurements in successfully excluded abdominal aortic aneurysm after endovascular repair. J Vasc Surg. 2003;37:733–738. [DOI] [PubMed] [Google Scholar]
- 9.Baum RA, Carpenter JP, Cope C, et al. Aneurysm sac pressure measurements after endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2001;33:32–41. [DOI] [PubMed] [Google Scholar]
- 10.Moore WS. The Guidant Ancure bifurcation endograft: five-year follow-up. Semin Vasc Surg. 2003;16:139–143. [DOI] [PubMed] [Google Scholar]
- 11.Zarins CK. The US AneuRx Clinical Trial: 6-year clinical update 2002. J Vasc Surg. 2003;37:904–908. [DOI] [PubMed] [Google Scholar]
- 12.Pfammatter T, Lachat ML, Kunzli A, et al. Short-term results of endovascular AAA repair with the Excluder bifurcated stent-graft. J Endovasc Ther. 2002;9:474–480. [DOI] [PubMed] [Google Scholar]
- 13.Makaroun M, Zajko A, Sugimoto H, et al. Fate of endoleaks after endoluminal repair of abdominal aortic aneurysms with the EVT device. Eur J Vasc Endovasc Surg. 1999;18:185–190. [DOI] [PubMed] [Google Scholar]
- 14.White GH, Yu W, May J, et al. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg. 1997;4:152–168. [DOI] [PubMed] [Google Scholar]
- 15.Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–1060. [DOI] [PubMed] [Google Scholar]
- 16.Eskandari MK, Yao JS, Pearce WH, et al. Surveillance after endoluminal repair of abdominal aortic aneurysms. Cardiovasc Surg. 2001;9:469–471. [DOI] [PubMed] [Google Scholar]
- 17.Faries PL, Cadot H, Agarwal G, et al. Management of endoleak after endovascular aneurysm repair: cuffs, coils, and conversion. J Vasc Surg. 2003;37:1155–1161. [DOI] [PubMed] [Google Scholar]
- 18.Bertges DJ, Chow K, Wyers MC, et al. Abdominal aortic aneurysm size regression after endovascular repair is endograft dependent. J Vasc Surg. 2003;37:716–723. [DOI] [PubMed] [Google Scholar]
- 19.Farner MC, Carpenter JP, Baum RA, et al. Early changes in abdominal aortic aneurysm diameter after endovascular repair. J Vasc Interv Radiol. 2003;14(2 Pt 1):205–210. [DOI] [PubMed] [Google Scholar]
- 20.Resch T, Ivancev K, Lindh M, et al. Persistent collateral perfusion of abdominal aortic aneurysm after endovascular repair does not lead to progressive change in aneurysm diameter. J Vasc Surg. 1998;28:242–249. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura JS, Moore WS. Clinical consequences of periprosthetic leak after endovascular repair of abdominal aortic aneurysm: Endovascular Technologies Investigators. J Vasc Surg. 1998;27:606–613. [DOI] [PubMed] [Google Scholar]
- 22.Mohan IV, Laheij RJ, Harris PL. Risk factors for endoleak and the evidence for stent-graft oversizing in patients undergoing endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2001;21:344–349. [DOI] [PubMed] [Google Scholar]
- 23.Liewald F, Ermis C, Gorich J, et al. Influence of treatment of type II leaks on the aneurysm surface area. Eur J Vasc Endovasc Surg. 2001;21:339–343. [DOI] [PubMed] [Google Scholar]
- 24.Rozenblit AM, Patlas M, Rosenbaum AT, et al. Detection of endoleaks after endovascular repair of abdominal aortic aneurysm: value of unenhanced and delayed helical CT acquisitions. Radiology. 2003;227:426–433. [DOI] [PubMed] [Google Scholar]
- 25.Cao P, Verzini F, Parlani G, et al. Predictive factors and clinical consequences of proximal aortic neck dilatation in 230 patients undergoing abdominal aorta aneurysm repair with self-expandable stent-grafts. J Vasc Surg. 2003;37:1200–1205. [DOI] [PubMed] [Google Scholar]
- 26.Arko FR, Filis KA, Siedel SA, et al. Intrasac flow velocities predict sealing of type II endoleaks after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2003;37:8–15. [DOI] [PubMed] [Google Scholar]
- 27.Solis MM, Ayerdi J, Babcock GA, et al. Mechanism of failure in the treatment of type II endoleak with percutaneous coil embolization. J Vasc Surg. 2002;36:485–491. [DOI] [PubMed] [Google Scholar]