Abstract
IκB kinase γ (IKKγ) (also known as NEMO, Fip-3, and IKKAP-1) is the essential regulatory component of the IKK complex; it is required for NF-κB activation by various stimuli, including tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), phorbol esters, lipopolysaccharides, and double-stranded RNA. IKKγ is encoded by an X-linked gene, deficiencies in which may result in two human genetic disorders, incontinentia pigmenti (IP) and hypohidrotic ectodermal dysplasia with severe immunodeficiency. Subsequent to the linkage of IKKγ deficiency to IP, we biochemically characterized the effects of a mutation occurring in an IP-affected family on IKK activity and NF-κB signaling. This particular mutation results in premature termination, such that the variant IKKγ protein lacks its putative C-terminal Zn finger and, due to decreased mRNA stability, is underexpressed. Correspondingly, IKK and NF-κB activation by TNF-α and, to a lesser extent, IL-1 are reduced. Mutagenesis of the C-terminal region of IKKγ was performed in an attempt to define the role of the putative Zn finger and other potential functional motifs in this region. The mutants were expressed in IKKγ-deficient murine embryonic fibroblasts (MEFs) at levels comparable to those of endogenous IKKγ in wild-type MEFs and were able to associate with IKKα and IKKβ. Substitution of two leucines within a C-terminal leucine zipper motif markedly reduced IKK activation by TNF-α and IL-1. Another point mutation resulting in a cysteine-to-serine substitution within the putative Zn finger motif affected IKK activation by TNF-α but not by IL-1. These results may explain why cells that express these or similar mutant alleles are sensitive to TNF-α-induced apoptosis despite being able to activate NF-κB in response to other stimuli.
The IκB kinase (IKK) complex, composed of the IKKα and IKKβ catalytic subunits (5, 20, 24, 38) and the IKKγ (NEMO) regulatory subunit (27, 37), is the key to activation of the NF-κB/Rel family of transcription factors (12, 26). NF-κB dimers are found mainly in the cytoplasm of resting cells in a complex with specific inhibitors, the IκB proteins (26). Upon activation, NF-κB dimers enter the nucleus in response to stimuli, such as viral and bacterial infections, phorbol esters, antigens, and the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 1 (IL-1) (26). NF-κB regulates important target genes encoding chemokines, cytokines, adhesion molecules, and even its own inhibitors, IκBα and IκBβ. Furthermore, NF-κB activation is required for preventing TNF-α-induced cell death (1, 16, 35, 36). Extracellular stimuli initiate signaling cascades, which lead to the phosphorylation of both IKKα and IKKβ catalytic subunits (4). Once activated, IKK phosphorylates the IκB proteins at specific N-terminal residues (serines 32 and 36 for human IκBα) and thereby targets them for ubiquitination-dependent proteolysis (12).
Gene targeting in mice revealed that IKKβ is essential for IKK and NF-κB activation by proinflammatory cytokines and for preventing TNF-α-induced cell death. Like RelA−/− mice, which lack the p65 subunit of NF-κB (2), Ikkβ−/− mice die at midgestation due to TNF-α-induced liver apoptosis (14, 15, 33). IKKα, however, is neither required nor sufficient for NF-κB activation in response to TNF-α or other proinflammatory stimuli. Instead, it is required for proper development and differentiation of the epidermis and its appendices (8, 13, 32). This function of IKKα, which cannot be provided by IKKβ, is not dependent on NF-κB activation or the kinase activity of IKKα (9). Recently, however, two new functions of IKKα which do depend on its kinase activity were identified. First, IKKα is required for IκBα degradation and NF-κB activation in mammary epithelial cells in response to a member of the TNF cytokine family called RANK ligand (3). Second, IKKα is required for activation of p52-containing NF-κB dimers through a mechanism that is independent of IκB degradation but is dependent on the processing of the NF-κB2 p100 precursor polypeptide to the mature p52 subunit (30).
IKKγ was identified by two independent approaches. Genetic complementation of cells that were unable to activate NF-κB and therefore were extremely sensitive to apoptosis resulted in the isolation of the IKKγ (NEMO) cDNA (37). Simultaneously, extensive purification of the IKK complex resulted in the isolation of several polypeptides that were clearly distinct from the previously identified IKKα and IKKβ subunits (27). IKKγ has several distinct structural motifs, including two coiled-coil regions that are separated by α helices, a leucine zipper (LZ) motif, and a putative Zn finger at the extreme C terminus (26). The region responsible for the interaction with IKKα and IKKβ lies between residues 44 and 86 in the N-terminal domain of IKKγ (18). It is still not clear how the single Ikkγ locus gives rise to multiple gene products. Although IKKγ lacks catalytic functions, it is essential for NF-κB activation (17, 28, 29, 37). Cells that lack IKKγ contain only low-molecular-weight IKK complexes (37), which most likely correspond to IKKα and IKKβ homo- and heterodimers (38). In addition to the assembly of high-molecular-weight IKK complexes, which seem to contain four catalytic subunits (21), IKKγ is also required for IKK activation by a variety of stimuli (27). This regulatory function was revealed through the generation of a C-terminal truncation mutant of IKKγ that, despite being able to assemble high-molecular-weight IKK complexes, failed to support IKK activation (27).
Genetic studies were used to further investigate the physiological role of IKKγ in regulating NF-κB activation in vivo (17, 28, 29). Both the human and the mouse IKKγ/Ikkγ loci are located on the X chromosome. Consistent with this chromosomal location, disruption of the Ikkγ gene in the mouse results in the death of male hemizygous embryos due to severe liver apoptosis similar to that exhibited by Ikkβ−/− or RelA−/− mice (17, 28). Interestingly, Ikkγ+/− females, which are born alive, display a severe but self-limiting inflammatory skin disorder which was shown to be identical to a human X-linked genetic disorder known as incontinentia pigmenti (IP) (17, 29). Indeed, mutations in the human IKKγ locus were shown to be responsible for IP (31). Recently, other mutations in the IKKγ locus which, unlike the IP mutations, do not severely reduce NF-κB activation, were shown to underlie an X-linked immunodeficiency syndrome called hypohidrotic ectodermal dysplasia with severe immunodeficiency (EDA-ID) (6, 10, 39). Unlike IP, which mostly affects female carriers (the males that inherit the mutant allele die during gestation), EDA-ID mostly affects males, probably because heterozygous females do not experience a considerable NF-κB deficiency. It was previously proposed, based on an analysis of Ikkγ+/− mice, that a severe NF-κB deficiency, which sensitizes cells that express the mutant allele to TNF-α-induced apoptosis, is essential for the genesis of IP (17).
Although IKKγ is the only subunit absolutely essential for activation of the IKK complex by diverse stimuli, very little is known about its mechanism of action. It was shown that an amino-terminal α-helical region of IKKγ associates with the carboxyl-terminal segment of either IKKα or IKKβ and thereby promotes the assembly of the high-molecular-weight IKK complex (18, 23). However, it is not clear which features within the C-terminal region of IKKγ, which is required for full activation by proinflammatory cytokines but not for complex assembly, mediate its function (27). Interestingly, most of the mutations in IP, as well as EDA-ID, which do not prevent the expression of the IKKγ polypeptide are located within its C-terminal region (6, 10, 31, 39). Here we describe an IP-linked frameshift mutation that removes completely the putative Zn finger from IKKγ. The resulting variant IKKγ polypeptide supports reduced IKK and NF-κB activation by either TNF-α or IL-1. To further characterize the function of the IKKγ C-terminal region, we generated several point mutations within the LZ, a putative SH3 binding motif, and the Zn finger. We found that amino acid substitutions within the LZ result in a substantial reduction of IKK activation by both TNF-α and IL-1, whereas an amino acid substitution within the Zn finger severely affects IKK activation by TNF-α but not IL-1. Cells that express IKKγ mutants that no longer respond to TNF-α are hypersensitive to TNF-α-induced apoptosis. These results suggest that the C-terminal region of IKKγ may contain distinct functional motifs required for connecting IKK to different upstream activators.
MATERIALS AND METHODS
Plasmid construction and site-directed mutagenesis.
The PCM-hygro-IKKγ (murine) expression vector was provided by A. Israël (Institut Pasteur, Paris, France). The full-length coding sequence of murine IKKγ was amplified by PCR. BamHI and XhoI sites were introduced into the 5′ and 3′ ends, respectively. The cDNA fragment was then subcloned into the pBabePuro retroviral vector carrying the puromycin resistance gene. All subcloning and mutagenesis steps were done by using a QuickChange kit (Stratagene, La Jolla, Calif.) as recommended by the manufacturer. A full description of the PCR primers used to generate the different mutants will be provided upon request.
Cell culturing, transfection, and retroviral infection.
Wild-type (WT) and IP primary human embryonic fibroblasts (17) were maintained in Chang medium D (Irvine Scientific, Santa Ana, Calif.). WT and IKKγ-deficient mouse embryonic fibroblasts (MEFs) were described previously (17). MEFs and 293T cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, l-glutamine (2 mM; Life Technologies), penicillin (1,000 U/ml), and streptomycin (1,000 μg/ml). Viral stocks were produced by transfecting ∼2 × 106 293T cells per 60-mm dish with 2 μg of pBabePuro IKKγ constructs and 2 μg of the pCLECO vector (22). Supernatants containing fully packaged retrovirus were recovered 48 h after transfection and were immediately used for infection or stored at −80°C. Infection was achieved by incubating IKKγ-deficient MEFs with 500 μl of viral stock in the presence of Polybrene (8 μg/ml). At 6 h after infection, supernatants were removed and the cells were cultured for an additional 32 h in complete medium. Infected cells were selected with puromycin (2 μg/ml), and individual populations expressing each construct were isolated after about 10 days. The sensitivities of parental and reconstituted Ikkγ fibroblasts expressing different IKKγ constructs as well as WT and IP human fibroblasts to TNF-α-induced apoptosis were examined as described previously (17). Apoptotic nuclei were scored by counting at least 100 cells in five random fields for each experimental condition. Each experiment was done in duplicate.
Immunoprecipitation, kinase, and immunoblot assays and EMSAs.
Mouse fibroblasts expressing different IKKγ constructs as well as WT and IP human fibroblasts were treated or not treated with TNF-α (10 ng/ml) or IL-1 (10 ng/ml). At various times, the IKK complex was immunoprecipitated from cell lysates with an anti-IKKγ monoclonal antibody (MAb) (c73-764; Pharmingen), and activity was measured by an immunocomplex kinase assay with glutathione S-transferase-IκBα(1-54) as a substrate (27). Gel loading was normalized by immunoblotting with an anti-IKKα MAb (IMG-136; 1:500; Imgenex). Immunoblot analysis was done as previously described (8). Electrophoretic mobility shift assays (EMSAs) were performed as described previously (27). Briefly, 10 μg of whole-cell extracts was incubated with 2 μg of poly(dI-dC) and 5,000 cpm of labeled oligonucleotide probes. After 30 min of incubation, samples were resolved on native 5% polyacrylamide gels.
Genomic DNA isolation and CSGE.
Peripheral blood samples from an IP-affected patient and her unaffected son were obtained; together with male fibroblasts from the same family that expressed either WT or IP IKKγ alleles, these samples were used for the extraction of genomic DNA with a Qiagen Genomic-tip system as instructed by the manufacturer. PCR amplification was performed with 100 ng of genomic DNA and primers for individual IKKγ exons as previously reported (31). Conformation-sensitive gel electrophoresis (CSGE) was performed as described previously (7), with the exception that samples were run on 15% acrylamide gels.
Gel filtration analysis.
Lysates from unstimulated WT and IP human embryonic fibroblasts were chromatographed on a Superose 6 gel filtration column as described previously (5). The level of expression and the position of elution of the different IKK subunits were determined after immunoblotting.
RESULTS
Identification of an IKKγ exon 10 mutation in an IP-affected family.
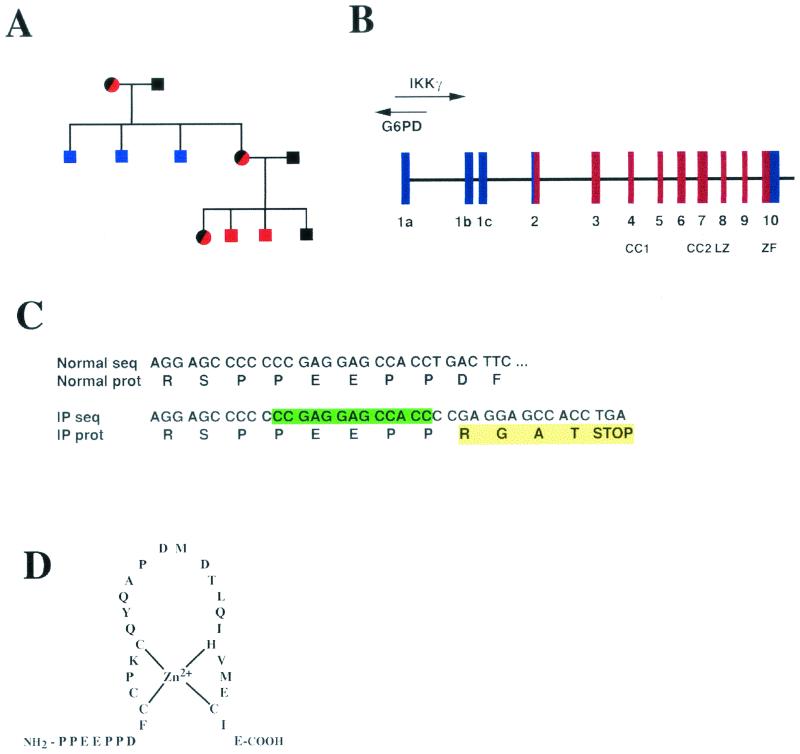
IKKγ is an X-linked gene in humans and mice (11, 17, 28, 29). Several IKKγ mutations that result in IP in heterozygous females have been described (31). Based on similarities to the phenotype of Ikkγ+/− female mice, it was postulated that the IP mutations result in a severe loss of function in cells that express the mutant allele after X chromosome inactivation (17). However, hypomorphic mutations in IKKγ that result in reduced NF-κB activation in surviving males with EAD-ID have been described (6, 10, 39). We and Roberts et al. previously studied human fibroblasts derived from either unaffected (WT) or stillborn male progeny of an IP-affected mother within an IP-affected family that has been monitored for three generations (Fig. 1A) (17, 25). To molecularly define the mutation in this family, we amplified all coding and noncoding IKKγ exons (Fig. 1B) and performed CSGE to detect mismatches in DNA heteroduplexes that contain one WT strand and one strand of mutant DNA. We detected two bands for the exon 10 PCR product derived from the IP-affected mother (data not shown). Sequencing of this PCR product revealed a 13-bp duplication following a cytosine tract (Fig. 1C). This duplication results in the addition of four novel amino acids and a premature stop codon after proline 393 (Fig. 1C). These changes result in the complete removal of the putative Zn finger at the extreme C terminus of IKKγ (Fig. 1D).
FIG. 1.
Molecular identification of an IP-linked mutation. (A) Pedigree of the IP-affected family being studied. Red and black circles denote carrier females; black squares denote nonaffected males; blue squares denote undiagnosed and deceased males; and red squares denote carrier and deceased males. (B) Genomic structure and organization of the human IKKγ gene (31). Coding and noncoding exons are shown in red and blue, respectively. G6PD, glucose-6-phosphate dehydrogenase. (C) Nucleotide (seq) and protein (prot) sequences of normal and mutant (IP) IKKγ alleles at the region of the mutation. The nucleotide sequence shows a 13-base duplication following a cytosine tract found in the IP-affected patient. The protein sequence shows the addition of four novel amino acids after proline 393 of human IKKγ, which results in a truncated protein that lacks all of the putative Zn finger. (D) Sequence of the putative Zn finger.
The IKKγ Zn finger is not required for assembly of the IKK complex.
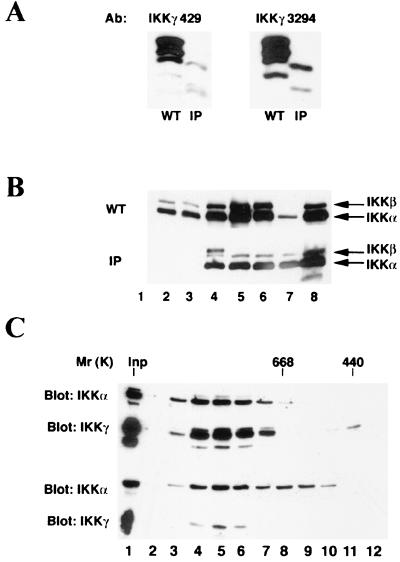
In a previous study, we were unable to detect IKKγ expression by immunoblot analysis of two primary fibroblast cultures that carry the IP mutation described above. These cultures were established from an aborted male fetus as well as a newborn male who died at birth, both of which were delivered by the same IP-affected mother (17). To find the reason for the inability to detect expression of the variant IP IKKγ polypeptides, we mapped the epitope recognized by the anti-IKKγ MAb (c73-1794) used in the previous study. We found that this particular MAb recognizes an epitope located within the putative Zn finger (data not shown). When we repeated the analysis with two other, more recently generated anti-IKKγ antibodies, we found reduced amounts of variant IKKγ polypeptides with sizes smaller than those of WT polypeptides (Fig. 2A). Using Northern blot analysis, we were unable to detect IKKγ mRNA expression in these IP fibroblasts (17), but the use of more sensitive reverse transcription-PCR analysis allowed the detection of reduced amounts of IKKγ transcripts in these IP fibroblasts (data not shown). We assume that the mutant mRNA is destabilized as a result of nonsense-mediated decay.
FIG. 2.
Characterization of the variant IKKγ polypeptides encoded by the IP allele. (A) Reduced expression of truncated IKKγ polypeptides detected in IP fibroblasts. Whole-cell extracts from WT and IP fibroblasts were subject to immunoblot analysis with MAb c73-429 and rabbit polyclonal antibody 3294. (B) Interaction of WT- and IP-encoded IKKγ polypeptides with IKKα and IKKβ. Extracts of WT and IP male fibroblasts were immunoprecipitated with control immunoglobulin G (lane 1) or the following antibodies: MAb IMG-324 (lane 2), MAb c73-1794 (lane 3), MAb c73-429 (lane 4), MAb c73-764 (lane 5), and polyclonal antibody 3294 (lane 6), all against IKKγ; anti-IKKα antibody M-280 (lane 7); and MAb B78-1 against IKKα (lane 8). The samples were then probed with antibodies against IKKα (IMG-136) and IKKβ (MAb 10AG2; Upstate Biotechnology Inc.). As shown by the signals in lanes 7 and 8, the two extracts contained similar amounts of IKKα and IKKβ. (C) WT (top panel) and IP (bottom panel) cell lysates were chromatographed on a Superose 6 gel filtration column. The levels of the different IKK subunits present in each fraction were determined by immunoblotting with anti-IKKα (MAb IMG-136) and anti-IKKγ (MAb c73-1794). Positions at which molecular weight markers (in thousands) eluted from this column are indicated. Inp, input.
We next examined whether IKKα and IKKβ can still physically interact with the mutant IKKγ polypeptides. Immunoblot analysis indicated that precipitation of the mutant IKKγ polypeptides with different antibodies brought down both IKKα and IKKβ (Fig. 2B). However, immunoprecipitation with anti-IKKγ MAb c73-1794 and MAb IMG-324, both of which are directed against the putative Zn finger, did not result in the isolation of either IKKα or IKKβ from the IP fibroblasts (Fig. 2B, lanes 2 and 3). To compare the sizes of the WT and mutant IKK complexes, cell lysates from WT and IP fibroblasts were prepared and separated by gel filtration on a Superose 6 column. IKK usually elutes from this column as two major species, a large one of approximately 900 kDa and a smaller one of approximately 300 kDa (5, 38). Although IKKα and IKKβ, as well as IKK activity, are found in both species, TNF-α stimulation elevates the activity of only the larger complex (38). Immunoblot analysis indicated that IKKα, IKKβ (data not shown), and IKKγ were present mostly in the larger complex in WT fibroblasts (Fig. 2C, top panels). However, only a portion of the total IKKα and IKKβ pools (approximately 50%) coeluted with the larger IKK complex in IP cell extracts (Fig. 2C, bottom panels). A considerable fraction of the IKKα and IKKβ catalytic subunits eluted at between 400 and 600 kDa in IP cell lysates (Fig. 2C and data not shown). This midsize complex, found only in IP cell extracts, did not contain any IKKγ polypeptides, all of which eluted with the larger IKK complex (Fig. 2C). Therefore, the putative Zn finger is not required for binding to IKKα or IKKβ or for generation of the high-molecular-weight cytokine-regulated IKK complex. However, in the absence of sufficient amounts of IKKγ polypeptides, IKKγ-free complexes containing IKKα and IKKβ did appear. Despite the large reduction in IKKγ expression, there was only a twofold reduction in the amounts of IKKα and IKKβ catalytic subunits eluting within the high-molecular-weight complex of IP cell lysates relative to WT cell lysates. These results suggest that there is an excess of IKKγ over IKKα and IKKβ in WT fibroblasts.
Effect of a C-terminal truncation on IKK activation.
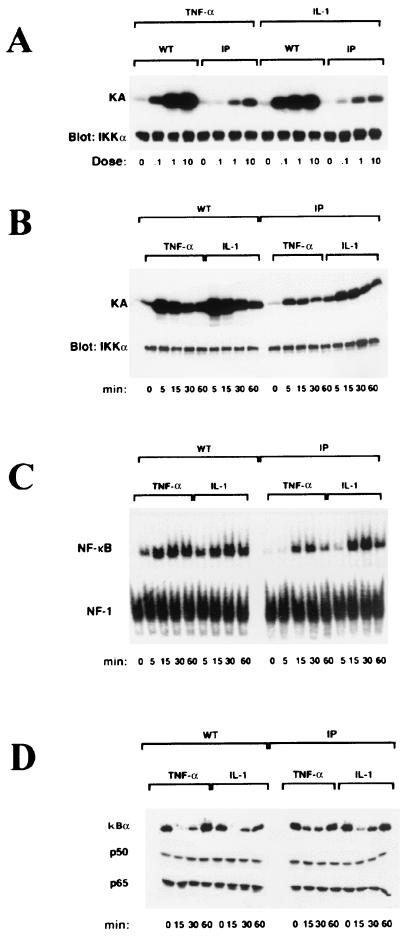
We next examined whether deletion of the IKKγ Zn finger affected IKK activation by the proinflammatory cytokines TNF-α and IL-1. WT and IP fibroblasts were stimulated for 5 min with TNF-α or IL-1. Increasing amounts of either TNF-α or IL-1 resulted in a dose-dependent increase in IKK activity (Fig. 3A). The level of TNF-α- or IL-1-stimulated IKK activity was higher in WT fibroblasts at the different concentrations tested (Fig. 3A). Next, we stimulated WT and IP fibroblasts with saturating doses (10 ng/ml) of TNF-α or IL-1 and measured IKK activity at different times. In both cell lines, IKK activity peaked at 5 min following TNF-α or IL-1 stimulation (Fig. 3B). Furthermore, NF-κB DNA binding activity (Fig. 3C) and IκBα degradation (Fig. 3D) were induced in WT and IP fibroblasts by TNF-α and IL-1. Surprisingly, despite the marked reductions in IKKγ expression and IKK activity, the differences in the levels of induced NF-κB DNA binding activity between WT and IP fibroblasts were not very dramatic. Nevertheless, TNF-α-induced NF-κB activity in IP fibroblasts was considerably lower than that in WT fibroblasts, whereas IL-1-induced NF-κB activity was not as different between the two cell lines (Fig. 3C). Correspondingly, IκBα degradation in IP fibroblasts was slower after TNF-α stimulation than after IL-1 stimulation (Fig. 3D). The expression of the p50 and p65 subunits of NF-κB, however, was normal in both cell lines (Fig. 3D). These results suggest that the putative Zn finger of IKKγ is required for maximal IKK activation by either TNF-α or IL-1. Based on the more sensitive mobility shift assay, the activation of NF-κB by TNF-α is more dependent on full IKK activity, which requires the IKKγ Zn finger, than is the response to IL-1.
FIG. 3.
Analysis of IKK and NF-κB activation in WT and IP fibroblasts. (A) WT and IP human male fibroblasts were treated or not treated with different doses (0.1, 1, and 10 ng/ml) of TNF-α or IL-1 for 5 min. Cells were lysed, and IKK activity (KA) was measured by an immunocomplex kinase assay with an anti-IKKα (M-280) and glutathione S-transferase-IκBα(1-54) as a substrate. The samples were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and autoradiographed. The membrane was reprobed (Blot: IKKα) with MAb IMG-136 against IKKα as a loading control. (B) Same as in panel A, except that cells were treated with saturating doses of TNF-α (10 ng/ml) or IL-1 (10 ng/ml) for different times as indicated prior to the determination of kinase activity. (C) Induction of NF-κB DNA binding activity in WT and IP fibroblasts treated as described above. Whole-cell extracts (10 μg per sample) were incubated with an NF-κB probe. The same extracts were also incubated with an NF-1 probe used as a control. DNA binding activity was determined by EMSAs. (D) Western blot analysis of IκBα, p50, and p65 in whole-cell extracts prepared from WT and IP fibroblasts treated as described for panel B.
Characterization of IKKγ LZ and Zn finger amino acid substitutions.
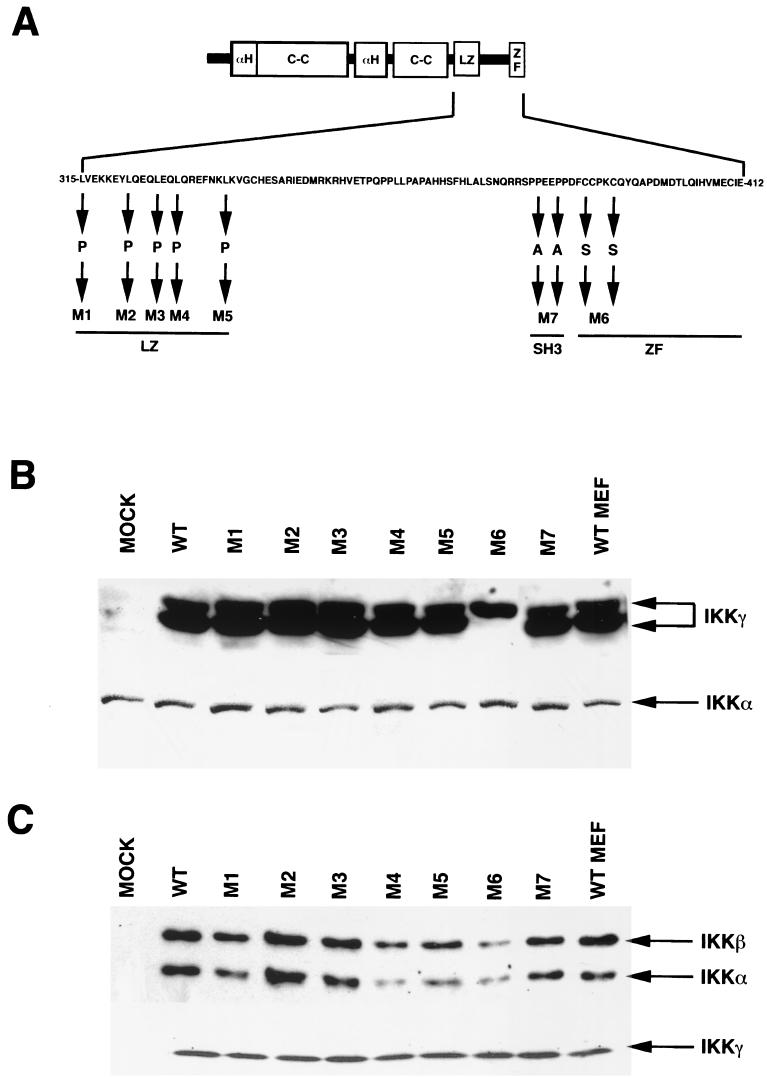
LZ and Zn finger motifs are known to mediate protein-protein interactions. To investigate the role of these motifs in IKKγ function, we substituted L315, L322, L326, L329, and L336 within the C-terminal LZ with proline residues and C389 and C393 within the base of the Zn finger with serine residues (Fig. 4A). We also replaced P360 and P363, within a proline-rich region that may be recognized by SH3 domains, with alanine residues (Fig. 4A). With the pBabePuro retroviral expression system, the various mutants were introduced into IKKγ-deficient 3T3 cells (17). None of the mutants and none of the WT IKKγ constructs contained N- or C-terminal epitope tags to avoid potential interference with IKK complex assembly or activation. The levels of expression of WT IKKγ and mutant IKKγ in the reconstituted cells were similar to those of endogenous IKKγ in WT MEFs, with the exception of mutant M6, which was expressed at slightly reduced levels (Fig. 4B). The levels of expression of IKKα were similar in all the reconstituted cells (Fig. 4B, bottom panel). It was previously shown that IKKγ migrates as a doublet, with several other minor polypeptides, on denaturing polyacrylamide gels (17, 27). Disruption of the mouse Ikkγ locus eliminates all IKKγ isoforms (17). Interestingly, the M6 mutation (C389S and C393S) in the IKKγ Zn finger resulted in the expression of only the slower-migrating isoform (Fig. 4B). The cause for this difference is currently unknown. Consistent with an analysis of C-terminal truncation mutants (27) (see above), all of the different substitution mutants of IKKγ were able to bind both IKKα and IKKβ (Fig. 4C).
FIG. 4.
Mutational analysis of the IKKγ C-terminal region. (A) Scheme showing the structural features of full-length IKKγ and the amino acid substitutions introduced into different functional motifs (C-C, coiled coil; αH, α-helical region; ZF, Zn finger; SH3, SH3 binding site). The mutantsgenerated were as follows: M1 (L315P), M2 (L322P), M3 (L326P), M4 (L329P), M5 (L336P), M6 (C389S and C393S), and M7 (P360A and P363A). (B) IKKγ-deficient MEFs were reconstituted with WT IKKγ or the different amino acid substitution mutants described in panel A. Levels of expression of IKKγ in the different reconstituted cells were similar to those found in WT MEFs (control) that were not infected by any retrovirus. The membrane was reprobed with MAb IMG-136 against IKKα as a loading control (bottom panel). (C) Amino acid substitutions within the C-terminal region of IKKγ do not affect binding to IKKα or IKKβ. IKKγ was immunoprecipitated from reconstituted cells or WT fibroblasts with a polyclonal antibody directed against its N terminus followed by immunoblotting with antibodies against IKKα and IKKβ. The membrane was reprobed with a rabbit polyclonal antibody against IKKγ as a loading control (bottom panel).
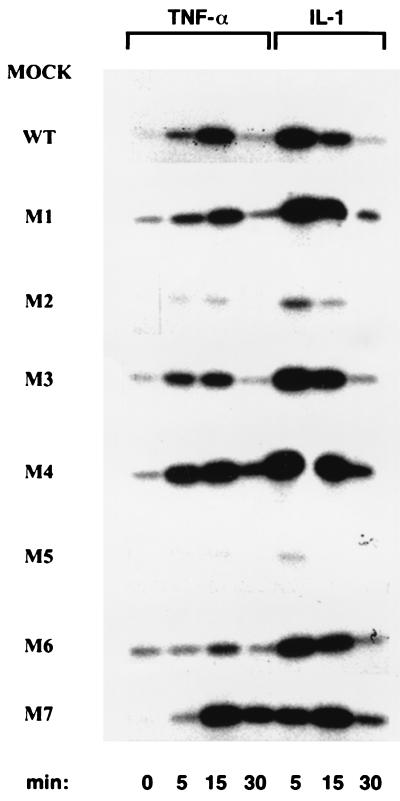
We next assayed the IKK activity associated with the different mutants and its regulation by TNF-α or IL-1. Whereas WT IKKγ and LZ mutants M1, M3, and M4 were able to reconstitute cytokine-responsive IKK activity, very little IKK activity was generated by LZ mutants M2 and M5 following TNF-α or IL-1 stimulation (Fig. 5). This lower level of IKK activity was not due to a problem with the expression or folding of mutants M2 and M5, since they were both expressed at levels similar to that of WT IKKγ and were able to associate with both IKKα and IKKβ. These results suggest that the M2 and M5 mutations may affect the ability of IKKγ to interact with upstream activators not yet identified. Interestingly, a higher level of basal IKK activity was generated by Zn finger mutant M6 (Fig. 5). Whereas IL-1 further increased the IKK activity associated with mutant M6 to a level similar to that associated with WT IKKγ, TNF-α elicited only a minor increase in the level of IKK activity (Fig. 5). The levels of NF-κB DNA binding activity generated by the various mutants were proportional to their levels of IKK activity (data not shown).
FIG. 5.
Effects of amino acid substitutions within the C-terminal region of IKKγ on IKK activation by TNF-α or IL-1. IKKγ-deficient cells that were either mock infected or reconstituted with either WT or amino acid substitution mutants of IKKγ were left untreated or treated with either TNF-α (10 ng/ml) or IL-1 (10 ng/ml). Immunocomplex kinase assays were performed on cells lysed at the indicated times as described in the legend to Fig. 3B. The membrane was reprobed with MAb IMG-136 against IKKα as a loading control (data not shown).
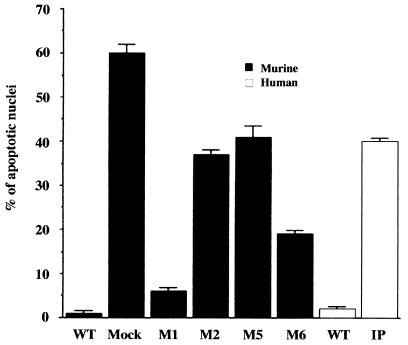
NF-κB activation protects cells from TNF-α-induced apoptosis (16). We previously suggested that increased sensitivity to TNF-α-induced apoptosis may underlie much of the pathology seen in IP-affected patients and Ikkγ+/− mice (17). We therefore examined the sensitivities of the various reconstituted mouse fibroblast cell lines as well as WT and IP human fibroblasts to TNF-α-induced apoptosis. Both WT mouse and human fibroblasts were resistant to TNF-α-induced apoptosis, whereas Ikkγ− mouse fibroblasts and human fibroblasts expressing the IP allele were highly sensitive (Fig. 6). The expression of an IKKγ mutant that restores close to a normal level of IKK activity, M1, conferred TNF-α resistance to Ikkγ− fibroblasts, whereas the expression of mutants that exhibit a defective response to TNF-α failed to restore full TNF-α resistance. Importantly, the expression of mutant M6, which restores a nearly normal level of IL-1-induced IKK activity but exhibits only a weak response to TNF-α (Fig. 5), caused only a partial reduction in TNF-α sensitivity (Fig. 6).
FIG. 6.
Effects of amino acid substitutions within the C-terminal region of IKKγ on susceptibility to TNF-α-induced apoptosis. WT mouse fibroblasts or Ikkγ− fibroblasts that were either mock reconstituted or reconstituted with WT or C-terminal substitution mutants of IKKγ as well as WT and IP human fibroblasts were treated with TNF-α (50 ng/ml). After a 24-h incubation period, cells were fixed, stained with 4′,6′-diamidino-2-phenylindole (DAPI), and mounted with a coverslip. Values shown are percentages of apoptotic nuclei per field scored by using a fluorescence microscope; error bars indicate standard deviations.
DISCUSSION
Several lines of evidence indicate that the IKK complex is the key to NF-κB activation by TNF-α, IL-1, and most other stimuli (12). Mouse knockout studies have confirmed that IKKβ is the major protein kinase required for IκB phosphorylation and eventual degradation in response to proinflammatory cytokines (14, 15, 33). Although lacking catalytic activity, the regulatory subunit of the IKK complex, IKKγ, plays an even more critical role in IKK and NF-κB activation. The N-terminal domain of IKKγ is required both for the binding of IKKα and IKKβ and their assembly into a high-molecular-weight complex essential for activation (18, 19, 27). C-terminal deletion mutants of IKKγ were found to be unable to support IKK activation, even though they can still promote IKK holoenzyme assembly (19, 27).
In this study, we biochemically characterized the effect of a mutation in the human IKKγ locus, which cosegregates over three generations with the IP disease phenotype, on IKK and NF-κB activation by proinflammatory stimuli. This mutation generates truncated IKKγ polypeptides which lack the putative Zn finger located at the extreme C terminus. Although IKK activation by both TNF-α and IL-1 is decreased by removal of the Zn finger, part of the reduction is simply due to the approximate 50% reduction in the level of the high-molecular-weight IKK complex caused by reduced IKKγ expression (Fig. 2). However, the reduction in TNF-α- or IL-1-induced IKK activation exceeds 50%, suggesting that the Zn finger of IKKγ makes an important, although not essential, contribution to signaling. It also appears that despite the considerable decrease in IKK activation in IP fibroblasts (Fig. 3A), the reduction in NF-κB activation is not as large and is mostly limited to the response to TNF-α and not to IL-1 (Fig. 3C). These findings suggest that only a small amount of IKK activation is required for NF-κB activation. In support of this notion, it was recently shown that caspase 3 mediates the proteolysis of IKKβ, which eliminates its kinase activity. However, effective reduction of IKK activation and diminished NF-κB activation, which sensitizes cells to TNF-α-induced apoptosis, require multiple treatments with TNF-α (34). These results suggest the existence of excess or surplus IKK complexes in levels that exceed those that are required for NF-κB activation. This excess IKK could be important for the protection of cells from TNF-α-induced apoptosis.
Although the analysis of the particular IP mutation that we have studied suggests that the Zn finger may not be essential for IKK activation, the identification of point mutations in EDA-ID-affected patients (6, 10, 39), one of which results in a substitution of a cysteine at the base of the Zn finger, suggests that this motif is of functional importance. Indeed, we found that substitutions of other cysteines at the base of the Zn finger of mouse IKKγ result in a mutant, M6, that has almost normal responsiveness to IL-1 but is defective in the response to TNF-α (Fig. 5).
Several other findings of the present study highlight the importance of the C-terminal region of IKKγ. Using site-directed mutagenesis, we found that the LZ located within this region is not required for IKK complex assembly but is important for responsiveness to both TNF-α and IL-1. These results are consistent with those of previous reports demonstrating the importance of the C-terminal region of IKKγ through the generation and analysis of large deletion mutants. As discussed above, mutations within the Zn finger also have an adverse effect on IKK activation. The EDA-ID-linked substitution affecting C417 in human IKKγ prevents NF-κB activation and B-cell immunoglobulin class switching following CD40L stimulation (10). We found that substitutions of two other cysteines, C389 and C393, which oppose the equivalent of C417 (C410 in the mouse) at the base of the Zn finger (Fig. 1D), reduced the response to TNF-α but had hardly any effect on the response to IL-1. These results suggest that the C terminus of IKKγ may contain distinct but overlapping interaction surfaces that are required for connecting the IKK complex to different upstream activators. Despite the nearly normal response of mutant M6 (C389S and C393S) of IKKγ to IL-1, these mutations fail to restore resistance to TNF-α-induced apoptosis when expressed in IKKγ-deficient fibroblasts. Notably, all the mutations in IKKγ which reduce NF-κB activation by TNF-α also result in increased sensitivity to TNF-α-induced apoptosis.
In a separate study, we found that the inhibition of TNF-α signaling alleviates most, if not all, of the IP-like symptoms in Ikkγ+/− mice (C. Makris, unpublished data). Thus, it is likely that the signaling pathway most critical to the genesis of IP is the TNF-α signaling pathway and that the most important outcome of the disease-linked mutations affecting the C terminus of IKKγ is increased sensitivity to TNF-α-induced apoptosis.
Acknowledgments
We thank the IP-affected patient and her son for providing blood samples.
C.M. was supported by a postdoctoral fellowship from the Cancer Research Institute. This work was supported by grants from the National Institutes of Health (ES04151 and AI43477) and the California Cancer Research Program.
REFERENCES
- 1.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-α induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 2.Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-169. [DOI] [PubMed] [Google Scholar]
- 3.Cao, Y., G. Bonizzi, T. Seagroves, Greten, F., R. Johnson, E. Schmidt, and M. Karin. 2001. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107:763-775. [DOI] [PubMed] [Google Scholar]
- 4.Delhase, M., and M. Karin. 1999. The IκB kinase: a master regulator of NF-κB, innate immunity and epidermal differentiation. Cold Spring Harbor Symp. Quant. Biol. 64:491-503. [DOI] [PubMed] [Google Scholar]
- 5.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 6.Doffinger, R., A. Smahi, C. Bessia, F. Geissmann, J. Feinberg, A. Durandy, C. Bodemer, S. Kenwrick, S. Dupuis-Girod, S. Blanche, P. Wood, S. H. Rabia, D. J. Headon, P. A. Overbeek, F. Le Deist, S. M. Holland, K. Belani, D. S. Kumararatne, A. Fischer, R. Shapiro, M. E. Conley, E. Reimund, H. Kalhoff, M. Abinun, A. Munnich, A. Israel, G. Courtois, and J. L. Casanova. 2001. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-κB signaling. Nat. Genet. 27:277-285. [DOI] [PubMed] [Google Scholar]
- 7.Ganguly, A., M. J. Rock, and D. J. Prockop. 1993. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc. Natl. Acad. Sci. USA 90:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu, Y., V. Baud, M. Delhase, P. Zhang, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of the IκB kinase. Science 284:316-320. [DOI] [PubMed] [Google Scholar]
- 9.Hu, Y., V. Baud, T. Oga, K. Kim, Y. Kazuhiko, and M. Karin. 2001. IKKα controls formation of the epidermis independently of NF-κB via a differentiation inducing factor. Nature 410:710-714. [DOI] [PubMed] [Google Scholar]
- 10.Jain, A., C. A. Ma, S. Liu, M. Brown, J. Cohen, and W. Strober. 2001. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohidrotic ectodermal dysplasia. Nat. Immunol. 2:223-228. [DOI] [PubMed] [Google Scholar]
- 11.Jin, D. Y., and K. T. Jeang. 1999. Isolation of full-length cDNA and chromosomal localization of human NF-κB modulator NEMO to Xq28. J. Biomed Sci. 6:115-120. [DOI] [PubMed] [Google Scholar]
- 12.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 13.Li, Q., Q. Lu, J. Y. Hwang, D. Büscher, K.-F. Lee, J. C. Izpsua-Belmonte, and I. M. Verma. 1999. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 13:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, Q., D. Van Antwerp, F. Mercurio, K.-F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 15.Li, Z.-W., W. Chu, Y. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKβ subunit of IκB kinase (IKK) is essential for NF-κB activation and prevention of apoptosis. J. Exp. Med. 189:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, Z.-G., H. Hu, D. V. Goeddel, and M. Karin. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis, while NF-κB activation prevents cell death. Cell 87:565-576. [DOI] [PubMed] [Google Scholar]
- 17.Makris, C., V. L. Godfrey, G. Krähn-Senftleben, T. Takahashi, J. L. Roberts, T. Schwarz, L. Feng, R. S. Johnson, and M. Karin. 2000. Female mice heterozygote for IKKγ/NEMO deficiencies develop a genodermatosis similar to the human X-linked disorder incontinentia pigmenti. Mol. Cell 15:969-979. [DOI] [PubMed] [Google Scholar]
- 18.May, M. J., F. D'Acquisto, L. A. Madge, J. Glockner, J. S. Pober, and S. Ghosh. 2000. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289:1550-1554. [DOI] [PubMed] [Google Scholar]
- 19.Mercurio, F., B. W. Murray, A. Shevchenko, B. L. Bennett, D. B. Young, J. W. Li, G. Pascual, A. Motiwala, H. Zhu, M. Mann, and A. M. Manning. 1999. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol. Cell. Biol. 19:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 21.Miller, B. S., and E. Zandi. 2001. Complete reconstitution of human IKK complex in yeast. Assessment of its stoichiometry and the role of IKKγ on the complex activity in the absence of stimulation. J. Biol. Chem. 276:36320-36326. [DOI] [PubMed] [Google Scholar]
- 22.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed]
- 23.Poyet, J. L., S. M. Srinivasula, J. H. Lin, T. Fernandes-Alnemri, S. Yamaoka, P. N. Tsichlis, and E. S. Alnemri. 2000. Activation of the IκB kinases by RIP via IKKγ/NEMO-mediated oligomerization. J. Biol. Chem. 275:37966-37977. [DOI] [PubMed] [Google Scholar]
- 24.Regnier, C. H., H. Y. Song, X. Gao, D. V. Goeddel, Z. Cao, and M. Rothe. 1997. Identification and characterization of an IκB kinase. Cell 90:373-383. [DOI] [PubMed] [Google Scholar]
- 25.Roberts, J. L., B. Morrow, C. Vega-Rich, C. M. Salafia, and H. M. Nitowsky. 1998. Incontinentia pigmenti in a newborn male infant with DNA confirmation. Am. J. Med. Genet. 75:159-163. [DOI] [PubMed] [Google Scholar]
- 26.Rothwarf, D. M., and M. Karin. 1999. The NF-κB activation pathway: a paradigm in information transfer from membrane to nucleus. SciSTKE 1999:RE1. [Online.]www.stke.org/cgi/content/fullOC_sigtrans;1999/5/re1. [DOI] [PubMed]
- 27.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKKγ is an essential regulatory subunit of the IκB kinase complex. Nature 395:297-300. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph, D., W. C. Yeh, A. Wakeham, B. Rudolph, D. Nallainathan, J. Potter, A. J. Elia, and T. W. Mak. 2000. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 14:854-862. [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt-Supprian, M., W. Bloch, G. Courtois, K. Addicks, A. Israel, K. Rajewsky, and M. Pasparakis. 2000. NEMO/IKKg-deficient mice model incontinentia pigmenti. Mol. Cell 5:981-992. [DOI] [PubMed] [Google Scholar]
- 30.Senftleben, U., Y. Cao, G. Xiao, F. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S.-C. Sun, and M. Karin. 2001. Activation by IKKα of a second, evolutionarily conserved, NF-κB signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 31.Smahi, A., G. Courtois, P. Vabres, S. Yamaoka, S. Heuertz, A. Munnich, A. Israël, N. S. Heiss, S. M. Klauck, P. Kioschis, S. Wiemann, A. Poustka, T. Esposito, T. Bardaro, F. Gianfrancesco, A. Ciccodicola, M. D'Urso, H. Woffendin, T. Jakins, D. Donnai, H. Stewart, S. J. Kenwrick, S. Aradhya, T. Yamagata, M. Levy, R. A. Lewis, et al. 2000. Genomic rearrangement in NEMO impairs NF-κB activation and is a cause of incontinentia pigmenti. Nature 405:466-472. [DOI] [PubMed] [Google Scholar]
- 32.Takeda, K., O. Takeuchi, T. Tsujimura, S. Itami, O. Adachi, T. Kawai, H. Sanjo, K. Yoshikawa, N. Terada, and S. Akira. 1999. Limb and skin abnormalities in mice lacking IKKα. Science 284:313-316. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka, M., M. E. Fuentes, K. Yamaguchi, M. H. Durnin, S. A. Dalrymple, K. L. Hardy, and D. V. Goeddel. 1999. Embryonic lethality, liver degeneration, and impaired NF-κB activation in IKKβ-deficient mice. Immunity 10:421-429. [DOI] [PubMed] [Google Scholar]
- 34.Tang, G., J. Yang, Y. Minemoto, and A. Lin. 2001. Blocking caspase-3-mediated proteolysis of IKKβ suppresses TNFα-induced apoptosis. Mol. Cell 8:1005-1016. [DOI] [PubMed] [Google Scholar]
- 35.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNFα-induced apoptosis by NF-κB. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 36.Wang, C.-Y., M. W. Mayo, and A. S. Baldwin, Jr. 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274:784-787. [DOI] [PubMed] [Google Scholar]
- 37.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israël. 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 38.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 39.Zonana, J., M. E. Elder, L. C. Schneider, S. J. Orlow, C. Moss, M. Golabi, S. K. Shapira, P. A. Farndon, D. W. Wara, S. A. Emmal, and B. M. Ferguson. 2000. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKKγ (NEMO). Am. J. Hum. Genet. 67:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]