Abstract
Objective:
To examine recurrence and survival rates for patients treated with hepatic resection only, radiofrequency ablation (RFA) plus resection or RFA only for colorectal liver metastases.
Summary Background Data:
Thermal destruction techniques, particularly RFA, have been rapidly accepted into surgical practice in the last 5 years. Long-term survival data following treatment of colorectal liver metastasis using RFA with or without hepatic resection are lacking.
Methods:
Data from 358 consecutive patients with colorectal liver metastases treated for cure with hepatic resection ± RFA and 70 patients found at laparotomy to have liver-only disease but not to be candidates for potentially curative treatment were compared (1992–2002).
Results:
Of 418 patients treated, 190 (45%) underwent resection only, 101 RFA + resection (24%), 57 RFA only (14%), and 70 laparotomy with biopsy only or arterial infusion pump placement (“chemotherapy only,” 17%). RFA was used in operative candidates who could not undergo complete resection of disease. Overall recurrence was most common after RFA (84% vs. 64% RFA + resection vs. 52% resection only, P < 0.001). Liver-only recurrence after RFA was fourfold the rate after resection (44% vs. 11% of patients, P < 0.001), and true local recurrence was most common after RFA (9% of patients vs. 5% RFA + resection vs. 2% resection only, P = 0.02). Overall survival rate was highest after resection (58% at 5 years); 4-year survival after resection, RFA + resection and RFA only were 65%, 36%, and 22%, respectively (P < 0.0001). Survival for “unresectable” patients treated with RFA + resection or RFA only was greater than chemotherapy only (P = 0.0017).
Conclusions:
Hepatic resection is the treatment of choice for colorectal liver metastases. RFA alone or in combination with resection for unresectable patients does not provide survival comparable to resection, and provides survival only slightly superior to nonsurgical treatment.
Complete resection provides the best survival for colorectal liver metastases. Radiofrequency ablation alone or in combination with resection provides a small but significant advantage over nonsurgical management but does not provide durable survival comparable to complete resection.
Liver metastases from colorectal carcinoma are the leading cause of cancer-related morbidity and mortality in the West. The great majority of patients with colorectal liver metastases present with unresectable disease, while complete surgical resection remains the sole curative treatment of patients with disease confined to the liver. Although newer systemic chemotherapy can provide improvement in median survival time and quality of life for some patients, survival for unresected patients beyond 5 years is uncommon.1 Traditional limits to hepatic resection have been exceeded as advancements in hepatic surgery and postoperative patient management enable safe resection of up to 80% of the functional liver parenchyma with a mortality of 5% or less in major centers.2–4 The primary obstacles to complete resection in the majority of patients that present with colorectal liver metastases are the need to treat bilobar or bulky disease and the need to leave sufficient residual functional hepatic parenchyma after resection to support posthepatectomy hepatic function.
Strategies designed to increase the proportion of patients who are candidates for complete surgical treatment of liver metastases are emerging. Neoadjuvant chemotherapy,5,6 preoperative portal vein embolization,7 and 2-stage resection approaches8,9 contribute to an increase in the number of patients who can undergo potentially curative treatment. Despite these innovative strategies, the great majority of patients with liver-only metastases from colorectal carcinoma are not candidates for complete surgical resection.
To compliment resectional strategies when complete resection of all metastases is not possible, a number of tumor ablative techniques have been explored. Currently, the most widely used tumor ablative technique for treatment of colorectal liver metastasis is radiofrequency ablation (RFA), which has been shown to be safe and feasible in patients with unresectable hepatic tumors.10–13 Outcome following RFA is difficult to interpret, since most studies report recurrence per lesion rather than per patient and report outcome for populations with mixed tumor types using different techniques of ablation with different equipment. Further, follow-up data are immature, with 1-year survival reported to be 78%12,14–16 and 3-year survival for percutaneous RFA to be 46%.16 Unfortunately, up to 40% of lesions recur and 12% of patients are found to have recurrence at a treatment site at only 1 year following RFA.16,17 RFA combined with resection has recently been proposed as an option for unresectable patients.18,19 Thus, RFA has been reserved as an adjunctive tool to resection, when complete resection is not possible, either alone or in combination with resection.15,18,19
We performed a retrospective analysis of patients operated for colorectal metastases confined to the liver. Surgical and survival outcome of those who underwent resection only, RFA only, or resection of dominant lesions combined with RFA of additional tumors were compared. Patients operated during the same period by the same surgeons who were determined to be ineligible for complete resection and/or ablation, but were found at laparotomy to have no evidence of extrahepatic disease were used as a reference group for survival comparison. Patterns of recurrence, recurrence-free, and overall survival were analyzed.
PATIENTS AND METHODS
A consecutive series of 418 patients were identified from our prospective hepatobiliary database that underwent their first laparotomy for treatment of colorectal liver metastases and were found to have disease confined to the liver at exploration. All patients were operated between 1992 and 2002 at University of Texas M.D. Anderson Cancer Center in Houston, TX. Two major groups were identified: 1 group, including 348 patients, was treated for cure with hepatic resection only, RFA + resection, or RFA only (Fig. 1). Use of RFA alone or in combination with resection was confined to patients in whom no resection could be designed which would permit complete resection of disease leaving sufficient vascularized hepatic parenchyma to support postresection hepatic function. Therefore, RFA was used as a component of therapy when patients were considered to be “unresectable” based on preoperative imaging or intraoperative findings. The second group (70 patients, “chemotherapy only” group) was found to have disease too extensive for curative therapy based on disease distribution or extent (but no extrahepatic disease) based on preoperative or intraoperative findings. These patients underwent chemotherapy (systemic, intraarterial via hepatic artery infusion pump placed at the index laparotomy, or intraarterial plus systemic chemotherapy).
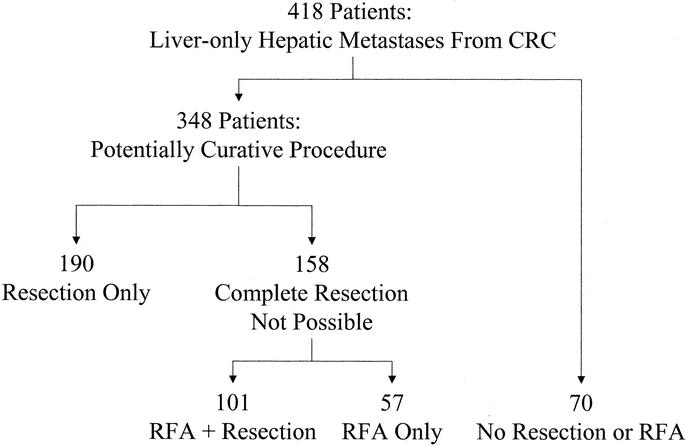
FIGURE 1. Distribution of patient groups studied. CRC, colorectal cancer.
All 418 patients had pathologic confirmation of colorectal liver metastasis. Preoperative imaging included chest radiograph or chest computed tomography when indicated, as well as abdominopelvic imaging with computed tomography or magnetic resonance imaging in all patients. No patient studied underwent prior hepatic resection or RFA. Complete tumor resection or RFA with preservation of sufficient hepatic parenchyma was the key selection criterion for surgical treatment. Patients were considered for RFA even if they had tumor abutting a major hepatic vein branch or the inferior vena cava. RFA was not performed adjacent to major biliary structures, particularly at the hepatic duct confluence. Intraoperative ultrasound was used in all patients.
All patients underwent resection and/or RFA during open laparotomy by a hepatobiliary surgeon. No patients were treated percutaneously. Intraoperative ultrasonography was used to guide placement of the RF needle into the lesions to be treated by RFA. All patients who underwent RFA were treated using the RadioTherapeutics RF 2000 or RF 3000 generator system (Boston Scientific, Natick, MA) using a previously described 2-phase algorithm.12 Briefly, following satisfactory deployment of the multiple array needle electrode, initial power was applied at 50 W (RF 2000 with 3.5-cm array) or 80 W (RF 3000 with 4.0-cm array) and then increased in 10 W increments at 1-minute intervals. Power and tissue impedance were monitored continuously from the RF generator until power “roll-off” occurred as a result of coagulative necrosis of the tissue in the treatment field. After a 20-second pause, power was reapplied at 75% of the maximum power achieved until power roll-off again occurred. The needle was then retracted and removed for tumors ≤ 2.5 cm. For tumors > 2.5 cm in diameter, the multiple array was repositioned for multiple treatments to obtain complete destruction of tumor and at least a 1-cm zone of surrounding liver parenchyma when possible.
The Brisbane 2000 terminology of liver anatomy and resections was used.20 Briefly, extended right hepatectomy (resection of Couinaud segments IV + V + VI + VII + VIII ± I), extended left hepatectomy (resection of Couinaud segments II + III + IV + V + VIII ± I), right hepatectomy (resection of Couinaud segments V + VI + VII + VIII ± I), left hepatectomy (resection of Couinaud segments II + III + IV ± I), bisegmentectomy (resection of 2 adjacent Couinaud segments), or segmentectomy (resection of a single Couinaud segment) were performed.
Patient demographics, primary tumor and liver tumor factors, operative factors, pathologic findings, recurrence patterns, and survival were analyzed. The Cox proportional hazard model was used to analyze differences in risk factors for survival. Survival was estimated using Kaplan-Meier analysis; differences in survival were analyzed using the log-rank test. Differences in tumor recurrence rates between treatment groups were analyzed using the Fisher exact test and 2-sided χ2 tests. Differences were considered to be statistically significant when the P value was < 0.05.
RESULTS
Descriptive Characteristics
All patients were treated and followed at the University of Texas M.D. Anderson Cancer Center between March 1992 and June 2002. Of the 348 patients treated for cure, 190 (55%) underwent resection only, 101 (29%) RFA + resection, and 57 (16%) RFA only. Sixty-one percent of the patients were male. The median patient age for the whole group was 60 years (range, 23–88 years).
Of the 190 patients who underwent resection only, 122 (64%) underwent resection of 4 or more Couinaud segments (91 hepatectomies, 31 extended hepatectomies) and 46 patients (24%) underwent additional procedures, including contralateral hepatic resection (22 patients), intraarterial pump placement (11 patients), or some combination of other procedures (14 patients).
Of the 101 patients who underwent RFA + resection, 56 (55%) underwent resection of 4 or more Couinaud segments (37 hepatectomies, 19 extended hepatectomies) and 27 patients (27%) underwent a procedure in addition to RFA + resection (additional hepatic resection in 8 patients, intraarterial pump placement in 19 patients).
A total of 110 tumors were ablated in 57 patients who underwent RFA only. The median number of tumors treated per patient was 1 (range, 1–8). A single tumor was ablated in 31 patients, 2 tumors in 13 patients, 3 tumors in 9 patients, 4 tumors in 3 patients, 5 tumors in 2 patients, and 8 tumors in 1 patient. Median tumor size treated was 2.5 cm. Tumors were ablated in all 8 Couinaud segments. Eight patients (14%) underwent intraarterial pump placement at the time of the ablational procedure.
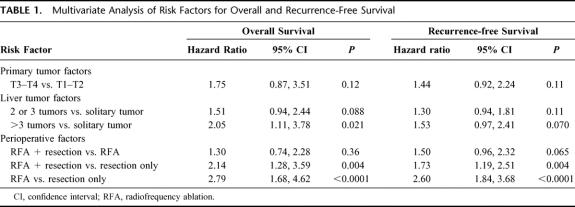
Risk factors for survival less than 5 years have been described in detail.21 These risk factors are described to predict outcome in surgically treated patients. We analyzed the entire cohort of patients treated for cure (348) using these criteria (patient factors: gender, race, age > 65 years at surgery; primary tumor factors: T classification, node status, colon vs. rectal location, recurrence-free interval > 12 months; liver tumor factors: prehepatic resection CEA > 200, number of tumors [solitary tumor vs. 2 to 3 tumors vs. > 3 tumors], size of largest tumor > 5 cm; perioperative factors: less than hepatectomy vs. hepatectomy or more, resection margin positivity, operative blood loss > 1000 mL, and treatment [resection vs. RFA + resection vs. RFA only]). In univariate analysis, only tumor number > 3 and treatment by RFA + resection or RFA only predicted decreased overall survival. These factors remained significant in multivariate analysis of overall survival as well (Table 1). RFA only (P < 0.0001) or RFA + resection (P = 0.004) were risk factors for decreased survival compared with resection only. There was no survival difference for patients treated with RFA + resection versus RFA only. In the analysis of recurrence-free survival, only treatment including RFA predicted lower survival probability (Table 1).
TABLE 1. Multivariate Analysis of Risk Factors for Overall and Recurrence-Free Survival
To determine whether the survival advantage for patients who underwent resection only was due to intergroup differences in known risk factors for survival, comparisons between the resection only, RFA + resection, and RFA only groups were made for all the risk factors listed above. No differences between groups in any of the patient, primary tumor, liver tumor, or perioperative factors were found (data not shown).
Patterns of Tumor Recurrence
Differences in recurrence patterns were observed among the treatment groups. Recurrence of any kind occurred most often after RFA only (84%) versus RFA + resection (63%) versus resection only (52%, P < 0.001; Fig. 2A). The dominant pattern of recurrence was intrahepatic; recurrence anywhere in the liver (including true local recurrence) without extrahepatic recurrence was fourfold more frequent after RFA than after resection only (44% vs. 11%, P < 0.001), and nearly double the rate after RFA + resection (28%, P < 0.001; Fig. 2B). Strictly defined local recurrence (recurrence in the Couinaud segment treated or at the margin of resection) was most common following RFA only (9% of patients) compared with 5% after RFA + resection and only 2% after resection only (P = 0.02). The proportion of patients in each group found to have distant metastasis as a component of failure was similar among the 3 surgical groups (41% for resection, 37% for RFA + resection, 40% for RFA only, P = not significant).

FIGURE 2. Recurrence following resection only, RFA + resection and RFA only. Recurrence at any local or distant site (A, *P < 0.001 compared with resection only), recurrence at any site within the liver, without evidence of distant metastasis (B, *P < 0.001 compared with resection only), and true local recurrence (C, *P = 0.02 compared with resection only) (patients in C are a subgroup of those in B).
Overall Survival
Overall survival for the group treated for cure (348 patients) is shown in Figure 3A. Median follow-up for the entire group was 21 months (range, 4–112 months). Overall survival for the subgroups treated for cure is presented in Figure 3B. Resection provided the best survival (3-year survival 73%, 4-year 65%, 5-year 58%, P < 0.0001). There was no significant difference in survival rate between the RFA + resection and the RFA only groups at 3 years (43% vs. 37%) or 4-years (36% vs. 22%, P = not significant).
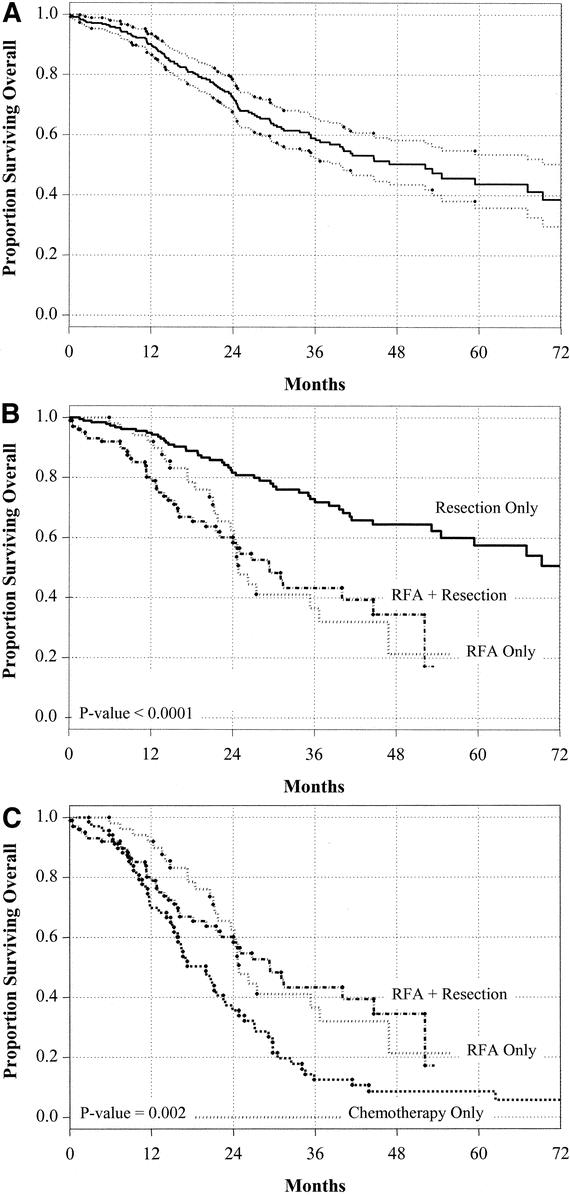
FIGURE 3. Overall survival stratified by treatment. Overall survival for the entire group treated with curative intent (A), stratified by surgical treatment of cure (B), and stratified by treatment of “unresectable” patients (C).
Patients underwent RFA as a component of therapy because they were considered “unresectable” as defined above. For this reason, a similar control group was identified with unresectable disease confined to the liver proven by laparotomy, the “chemotherapy only” group. Overall survival for these 3 “unresectable” groups is shown in Figure 3C. There is a statistically significant difference in survival for patients treated with RFA as a component of therapy versus chemotherapy only whether compared as a group (158 treated with RFA vs. 70 with chemotherapy only, P = 0.002) or when each subgroup is compared (RFA + resection vs. chemotherapy, P = 0.003; RFA only vs. chemotherapy, P = 0.005). Tumor number was predictive of poor survival in multivariate analysis. Overall survival was best for patients treated with solitary tumors, intermediate for those with 2 to 3 tumors, and worst for those with > 3 tumors (P < 0.0001, Fig. 4A). Similarly, overall survival with treatment of 2 to 3 tumors was best for resection and worst with RFA only (P = 0.0078, Fig. 4B). Importantly, survival for treatment of a solitary tumor by RFA did not provide survival comparable to resection of a solitary lesion (P = 0.025, Fig. 4C). Finally, the number of tumors, although predictive of survival for the entire group of patients treated for cure, was not a predictor of survival in the resection only group (overall survival following resection of solitary tumor vs. 2 to 3 tumors vs. > 3 tumors, P = 0.26).

FIGURE 4. Overall survival stratified by tumor number. Overall survival for all surgically treated patients stratified by number of tumors (A), for the subset of patients with 2 or 3 tumors stratified by surgical treatment (B), and for the subset of patients with solitary tumors stratified by surgical treatment (C).
Recurrence-Free Survival
Recurrence-free survival for curatively treated patients is found in Figure 5; the group overall in Figure 5A, and stratified by treatment in Figure 5B. Although there was no significant difference in recurrence-free survival for patients treated with RFA + resection versus RFA only, the survival for patients who were treated with resection only was best (P < 0.0001). Recurrence-free survival for the entire cohort was best for patients treated with solitary tumors (P = 0.0023).

FIGURE 5. Recurrence-free survival. Recurrence-free survival for the entire group treated for cure (A) and stratified by treatment type (B).
DISCUSSION
The benefit of complete resection of colorectal liver metastases is well recognized. The 5-year overall survival rate following resection found in the present study (58%) matches the findings of Choti et al22 for patients treated over a similar period (58%, 1993–1999). This higher survival rate than the survival rate reported in large series (33%–36% 5-year survival) examining patients treated from the 1960s through the mid-1990s4,21,22 likely reflects improvement in patient selection, perioperative and postoperative care, multidisciplinary treatment, and an appropriately aggressive approach to safe hepatic resection.22
The surgical approach to liver metastases in this study is aggressive; 4 or more Couinaud segments were resected in 64% of resection only patients and 24% underwent an associated procedure. When patients were not candidates for complete resection, patients were considered for combined resection + RFA as described by other authors.15,18,19 Dominant lesions were resected in patients with bilobar disease, and the contralateral lesions that could not be resected were ablated. This group was treated aggressively as well: 55% of patients treated with RFA + resection underwent a hepatectomy or more, 27% with an associated procedure. Resection of larger lesions was used when possible to avoid the unacceptably high recurrence rate after treatment of large tumors with RFA.23 Further, RFA cannot be used within 1 to 2 cm of the hepatic hilum due to the risk for bile duct stricture and fistula.12,13,24 RFA only was reserved for patients with tumors in proximity to vascular structures, which prevented resection that would leave sufficient functional parenchyma to support posthepatectomy function. Other limitations of RFA, such as risk for damage to diaphragm, adjacent stomach, or colon, were avoided in our patients because all patients were treated during open procedure in which such structures could be protected from RF produced heat.
The “unresectable” patients in the present series, that is, those treated with RFA as a component of therapy, were analyzed in 2 ways. First, RFA + resection and RFA only were compared with the gold standard of resection. The only detectable difference between groups was in the anatomic distribution of tumors, which made complete resection impossible in the RFA groups. No differences in tumor sizes and numbers, patient or primary tumor characteristics were evident to explain overall survival differences between groups. Despite this similarity in traditional predictors of survival, treatment with resection provided significantly better outcome. This appeared to be associated with a significantly higher intrahepatic failure rate and a higher true local recurrence rate associated with RFA, which was further reflected in differences in recurrence-free survival. Finally, despite the selection bias for RFA of solitary tumors in difficult locations (where a margin-negative resection was not possible), the highly significant difference in survival for patients with solitary tumors treated with resection only versus RFA only further strengthens the hypothesis that RFA cannot be considered an equivalent to complete hepatic resection. The finding that RFA + resection is associated with an intermediate intrahepatic and true local recurrence rate further supports this contention, although the survival curves for all patients treated with RFA as a component of therapy overlap.
Next, the “unresectable” patients were compared with the best available control group of unresectable patients; patients who were treated during the same time period, by the same surgeons, who underwent laparotomy and were found to have disease confined to the liver but were not candidates for resection and/or RFA. A statistical survival advantage for complete surgical treatment including RFA was found, although the survival curves are converging. Whether this survival advantage will be sustained with longer follow-up is unknown, especially with improving outcomes using newer systemic chemotherapy regimens. Three-year survival rate for RFA + resection (42%) or RFA only (37%) is similar to that reported in the literature.16,17 The present study is the first to our knowledge to compare outcome after RFA or RFA combined with hepatic resection to a studied control group and demonstrate an advantage over nonsurgical therapy.
CONCLUSION
These data provide evidence that when complete resection cannot be achieved, selective use of RFA can provide a modest survival benefit over chemotherapy alone for patients with colorectal metastases confined to the liver. The higher local recurrence rate, higher intrahepatic failure rate, and associated lower recurrence-free and overall survival rate for patients treated with RFA as a component of therapy compared with resection alone could not be explained by differences in the known patient, primary tumor, liver tumor, or perioperative factors, which predict recurrence and survival for patients treated surgically for colorectal metastases. Although patient selection for RFA may bias the analysis, selection was based on anatomy, not biology, as known risk factors for survival were not different among studied groups, but survival even in the subgroup of patients with solitary tumors treated with RFA was lower than the group treated with resection.
The proliferation of ablation techniques such as RFA for treatment of metastatic liver tumors has unfortunately preceded the analysis of outcome for the treatment modality. Proposal for a randomized trial comparing RFA versus resection for potentially resectable colorectal liver metastases must take into consideration existing data, such as these and may be inappropriate at this time.
ACKNOWLEDGMENTS
The authors thank Storm Weaver for secretarial assistance with manuscript preparation.
Discussions
Dr. Paul S. Dale (Macon, Georgia): I would like to congratulate Dr. Curley and his co-authors for their significant contributions supporting the use of radiofrequency ablation to treat nonresectable colorectal metastases.
In the early 1990s, we utilized cryosurgical ablation in many institutions to treat these patients. Then in late 1990, radiofrequency ablation appeared to be safer and offered very effective treatment of these patients and, therefore, many of us switched from the cold to the heat ablation.
Your report confirms that there is a low morbidity associated with utilizing radiofrequency ablation, but, more importantly, it does identify a benefit or an increase in survival for patients who are treated with radiofrequency ablation in those that underwent radiofrequency with or without resection.
Coming from a smaller institution with a cryo unit attracting dust in the corner, I am very excited about these results that showed that there was an improved survival in your patients who underwent radiofrequency ablation. Your results appear to support what we have long since thought, that we are doing some good by trying to ablate these patients with nonresectable tumors.
I would like to ask 2 basic questions regarding these results—really just 1 question at this point—and that involves adjuvant therapy.
Most recently, reports from Kemeny and from the John Wayne Cancer Institute have shown that adding regional hepatic artery infusion pump therapy along with systemic topoisomerase-1 inhibitor or CPT-11 appears to improve survival in these patients who underwent radiofrequency ablation. And I was wondering if you utilized this drug combination in your patients, either early in the study period or later on, and do you plan on doing this in future studies?
Dr. Richard J. Howard (Gainesville, Florida): The authors presented a very large series of patients with colorectal metastases presented to a variety of modalities, including resection, resection with RFA alone, or chemotherapy. All patients were operated on by the authors. And the modality seemed to be determined at the time of operation, depending on whether they were resectable or not. I would like to ask the authors a couple of questions.
One, it seems that the main determinant of survival was not necessarily the mode of treatment but, rather, the patient's disease, because the disease extent seemed to determine the modality of treatment. Is that a fair statement? Or am I wrong?
Why the patients who were treated with chemotherapy alone were unresectable or untreated with RFA, what were their characteristics? In the paper I was given last night, the median number of tumors in the patients treated with RFA was only 1. Why weren't those patients resected?
Do you have any indication of what is the success rate of treating the patients? You said your rate was only 6% in the liver, local recurrence rate. Most series have reported a larger local recurrence rate. Why is yours so low?
We used to treat patients with hepatocellular carcinomas with RFA as a way of controlling the tumor prior to liver transplantation. We did that with a small number and then gave it up because we were really concerned with studying—after we studied the explant of the liver—the number of patients whose tumor treatment was really ineffective and had viable tumor that had been treated with RFA.
Furthermore, some of them we are concerned that maybe the RFA made it worse in that they had vascular invasion it seemed at a higher rate than hepatocellular carcinomas. Have you had a chance to resect tumors that were treated with RFA either by yourself or others and found anything similar?
Dr. J. Michael Henderson (Cleveland, Ohio): This is the other end of the spectrum from the 2 liver papers you had earlier this afternoon. These are the real world. These are your patients with metastatic disease. And you have got all your treatment options here. I think that is what is important about this paper.
I do have a similar question with regard to unresectability in that it is clear that over 50% of your RFA-only patients have more tumors. The median size was 2.5-cm for ablated lesions. And I really had some problems, I read the manuscript, wondering what made those unresectable tumors? You know, in the operating room I understand often that that is a decision that is made at that time.
The other thing that struck me as I had the opportunity to look at the manuscript is that a lot of this is really about tumor biology. I think the survival numbers are impressive. And the value of this manuscript really spells out what can and should be expected with those truly resectable lesions at the current time,
For your resectable lesions you are looking at 5-year survivals of 58%, 2-year survivals of 73%, and you are looking at local recurrence in the liver in the 11% range in that group of patients.
Clearly, the biology is different in the ones that you deemed unresectable at that time who had either resection and RFA, so you are looking at, again, relatively small lesions, 3-year survivals of 43%, 4-year survivals of 36%. But looking at local recurrences, overall local recurrence is 63% with local recurrence in the liver at 23% and local recurrence at the site in the 5% range. There is very different biology. And I think whatever you are doing at M.D. Anderson, you are clearly stratifying them into their expected biology over the next several years.
The RFA-only group again appeared to have even worse biology, because although you would see a 44% recurrence in the liver your local recurrence at the edge of your ablation then is only 9% in that group. So there is something different about that group that is giving you much more significant local recurrence within the liver.
Again, I think it is a very important series that is documenting the natural history after these treatments in the real world group of patients out there. I do take issue with your last slide that there is no indication for randomized trials, and I would ask you to rethink that. I think we don't know the answer here. I think the natural history is the most important variable. I think there probably is a place for some randomized trials in the RFA versus resection arena.
You don't spell out at all, either in the presentation or in the manuscript, dealing with synchronous versus metachronous lesions. And I would submit that perhaps with a synchronous lesion there is a role for resection versus ablation. So you have no idea what the natural history is going to be for that group of patients. And I would strongly urge the RFA'ers and the resectionists to get together and conduct some appropriate randomized trials so we really know where we stand on this.
Dr. John S. Bolton (New Orleans, Louisiana): I have the impression, but no data to back it up, that over the past several years referrals for resection of colorectal cancer metastatic to the liver are decreasing, and I have wondered if some of our surgical colleagues, and probably even more importantly our medical oncology and interventional radiology colleagues, have jumped on the RFA bandwagon.
The relative ease of performance of RFA, the relative lack of complications, the allure of the possibility of a minimally invasive approach, and the intense marketing by product reps, I am afraid, have made RFA an attractive alternative to resection.
This would all be well and good if the technique delivers and proves to be equally effective. But my own experience with RFA has been that it is difficult to reliably—and this is no matter what the product rep tells you—to reliably adequately ablate lesions greater than 3 cm, especially if multiple, due to the variable and imperfect lesion shape created, due to the lack of reliable tactile or ultrasound monitoring of the lesion size, and the difficulty of overlapping ablation spheres for larger lesions.
You have to remember that with a resection technique you have 1 focal margin, the closest one to the line of resection, to worry about, and with RFA you have a 360 degree circumference to worry about. The presentation we just heard is by far, I think, the best comparison to date of resection and RFA. And it goes a long way toward putting the 2 techniques in perspective.
I think it is also worth noting the 58% 5-year survival for resected patients in this series. This is yet another in a series of 4 or 5 publications in the last 2 or 3 years showing greater than 50% survival for resection techniques for metastatic colorectal cancer, and I think that is a remarkable achievement.
The current study, although it is better than any prior study looking at RFA results, still suffers from an apples-to-oranges comparison. And that problem will probably dog this field until or unless a randomized comparison is done. So I have 3 questions for the authors.
First, are there any circumstances or study designs under which they think a randomized control trial could be done?
Second, true local recurrence as you defined it occurred in 9% of the RFA group while another 35% of the RFA group had in-liver recurrence not judged to be a local recurrence. And this compares to 2% and 11% in the resection group. That implies that the RFA patients have not only a higher risk of local failure at the treated site but a fourfold higher risk of recurrence elsewhere in the liver. And I would like to know how you explain that observation? It doesn't intuitively seem to make sense.
Lastly, if you have a patient with a 6-cm metastatic lesion in the right lobe requiring an extended right hepatic resection and a 2-cm lesion in segments 2 or 3, resection of which would leave you with a marginal future liver remnant, would you do an extended right hepatic resection and RFA of the lesion in 2 and 3, or do a 2-stage resection technique, as proposed by some authors?
I enjoyed the paper very much. And I think as a final statement, as surgeons we certainly need RFA, but I continue to feel it is more of an adjunct to resection rather than a substitute for it.
Dr. Steven C. Stain (Nashville, Tennessee): I enjoyed the paper and the manuscript. I have 3 brief questions.
First of all, surgical principles used to dictate that patients with more than 3 lesions did not get resections. In your manuscript, you have patients with as many as 8 lesions who got RFA. In your multivariate analysis, you identified that was a poor predictor of survival. Should we be performing ablation in patients with more than 3 lesions?
Secondly, is there a size cut-off in which RFA ablation will not be effective, ie, bigger than 4 or 5 cm?
And also, your median survival was only 21 months, although the study went for 11 years. How did you handle those patients in the survival analysis that were lost to follow-up?
Dr. Eddie K. Abdalla (Houston, Texas): Clearly there are 2 implications in this paper. First, based upon comparison to a control group, which has been lacking in prior studies of RFA, we demonstrated a small survival advantage for RFA over chemotherapy only; keeping in mind the fact that the control group of patients underwent laparotomy and were proven to have liver-only disease; this control group does not exist for studies that compare percutaneous treatments to chemotherapy (historical controls).
Second, we demonstrated, although admitting that we selected patients, that we have not seen survival following treatment including radiofrequency ablation that is equivalent to complete resection. We have not proposed with this analysis that we are comparing apples to apples (due to selection), but survival differences are significant, even for patients a small number of tumors.
Regarding the question: What defines resectability and what single lesions are ablated rather than resected? First of all, resectability was defined by anatomic distribution of tumors. The differences in the groups were not in the number of tumors. They were not in the patient age. They were not in the median sizes of the tumors. They were not in any of the prior factors that had been proposed to stratify the difference in survival between patients treated surgically for colorectal liver metastases.
So it brings us back to this question of resectability, and it is primarily anatomic distribution of tumors. We do have a significant group of patients who underwent extended resection, extended right or left hepatotectomy, associated with radiofrequency ablation of a contralateral lesion.
The issue of single lesions. We had lesions that were high on the hepatic veins. We are utilizing radiofrequency ablation for tumors in contact with the major hepatic veins. We do not ablate lesions that are within 2 cm of the hilum. Most of the patients with solitary tumors were thought to be at a high risk for a margin-positive resection and who could not undergo a complete resection and could not undergo a complete resection of that solitary lesion leaving sufficient residual functional hepatic parenchyma.
Why is the liver-only recurrence rate different among the groups and is this a reflection of differences in tumor biology? I don't know how specifically to determine whether the biology is different in the 3 study groups. The field at risk for recurrence in the liver is obviously smaller after an extended resection; radiofrequency ablation leaves a substantial field of risk in place. And it is a provocative question whether the anatomy of hepatic metastases is a predictor of biology because the recurrence rates were higher in the liver and the overall recurrence rates were higher following treatments including radiofrequency ablation, compared to resection only.
There has been a recent shift in our practice toward extended resection, as indicated in Dr. Vauthey's talk and a subsequent fall in the utilization of radiofrequency ablation, because we find that more patients are resectable (probably as a result of the increased use of preoperative portal vein embolization). Extended resection provides durable survival even for multiple metastases in many analysis, most recently that of Dr. Weber in which patients with up to 8 or more tumors were resected and were found to have acceptable survival.
Dr. Stain, based on the relatively small number in our study who had more than 3 lesions ablated, I don't think that we can create a definition based on number of tumors as to who should or should not be ablated. At this point, I think the best recommendation we can make is that a technique that enables complete resection is advisable.
Finally, to address the clinical scenario proposed by Dr. Bolton, in such a patient in whom an extended right resection would be required in the presence of a lesion in the left lateral section of the liver, I would first perform volume try to assess the size of the liver remnant. I would then perform a midline laparotomy, intraoperative ultrasound of the liver, and resect the lateral lesion in the left liver if I thought that a future right-sided extended operation would be possible.
I might use seprafilm in before closing with the anticipation of coming back, and then, based on the liver volume, I would or would not perform portal vein embolization, with the intercurrent chemotherapy (given that 75%–80% of the patients with Folfox or Folfiri will have stable disease or demonstrate a response to treatment). And at restaging after embolization, I would take the patient back for an extended right hepatectomy. In that scenario, I would go to significant efforts to apply the gold standard of complete resection.
Footnotes
Reprints: Steven A. Curley, MD, Department of Surgical Oncology, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 444, Houston, Texas 77030. E-mail: scurley@mdanderson.org.
REFERENCES
- 1.Moertel CG, Gunderson LL, Mailliard JA, et al. Early evaluation of combined fluorouracil and leucovorin as a radiation enhancer for locally unresectable, residual, or recurrent gastrointestinal carcinoma. J Clin Oncol. 1994;12:21–27. [DOI] [PubMed] [Google Scholar]
- 2.Matsumata T, Taketomi A, Kawahara N, et al. Morbidity and mortality after hepatic resection in the modern era. Hepatogastroenterology. 1995;42:456–460. [PubMed] [Google Scholar]
- 3.Doci R, Gennari L, Bignami P, et al. Morbidity and mortality after hepatic resection of metastases from colorectal cancer. Br J Surg. 1995;82:377–381. [DOI] [PubMed] [Google Scholar]
- 4.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–353. [DOI] [PubMed] [Google Scholar]
- 6.Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–175. [DOI] [PubMed] [Google Scholar]
- 8.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kianmanesh R, Farges O, Abdalla EK, et al. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg. 2003;164–170. [DOI] [PubMed] [Google Scholar]
- 10.Siperstein AE, Rogers SJ, Hansen PD, et al. Laparoscopic thermal ablation of hepatic neuroendocrine tumor metastases. Surgery. 1997;122:1147–1154. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg SN, Gazelle GS, Solbiati L, et al. Ablation of liver tumors using percutaneous RF therapy. AJR Am J Roentgenol. 1998;170:1023–1028. [DOI] [PubMed] [Google Scholar]
- 12.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curley SA, Izzo F, Ellis LM, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowles BJ, Machi J, Limm WM, et al. Safety and efficacy of radiofrequency thermal ablation in advanced liver tumors. Arch Surg. 2001;136:864–869. [DOI] [PubMed] [Google Scholar]
- 15.De Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619–1625. [DOI] [PubMed] [Google Scholar]
- 16.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. [DOI] [PubMed] [Google Scholar]
- 17.Scaife CL, Curley S. Complication, local recurrence, and survival rates after radiofrequency ablation for hepatic malignancies. Surg Oncol Clin North Am. 2003;12:243–255. [DOI] [PubMed] [Google Scholar]
- 18.Elias D, Goharin A, El Otmany A, et al. Usefulness of intraoperative radiofrequency thermoablation of liver tumours associated or not with hepatectomy. Eur J Surg Oncol. 2000;26:763–769. [DOI] [PubMed] [Google Scholar]
- 19.Pawlik TM, Izzo F, Cohen DS, et al. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terminology Committee of the International Hepato-Pancreato-Biliary Association. Brisbane 2000 Terminology of Liver Anatomy & Resections. Hepatobiliary Pancreat Surg. 2000;2:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist. 2001;6:14–23. [DOI] [PubMed] [Google Scholar]
- 24.Dodd GD III, Soulen MC, Kane RA, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20:9–27. [DOI] [PubMed] [Google Scholar]