Abstract
Objective:
To determine the identification of sentinel lymph node biopsy (SLNB) in breast cancer patients after intraoperative injection of unfiltered technetium-99m sulfur colloid (Tc-99) and blue dye.
Background:
SLNB guided by a combination of radioisotope and blue dye injection yields the best identification rates in breast cancer patients. Radioisotope is given preoperatively, without local anesthesia, whereas blue dye is given intraoperatively. We hypothesized that, because of the rapid drainage noted with the subareolar injection technique of radioisotope, intraoperative injection would be feasible and less painful for SLN localization in breast cancer patients.
Methods:
Intraoperative injection of Tc-99 and confirmation blue dye was performed using the subareolar technique for SLNB in patients with operable breast cancer. The time lapse between injection and axillary incision, the background count, the preincision and ex vivo counts of the hot nodes, and the axillary bed counts were documented. The identification rate was recorded.
Results:
Ninety-six SLNB procedures were done in 88 patients with breast cancer employing intraoperative subareolar injection technique for both radioisotope (all 96 procedures) and blue dye (93 procedures) injections. Ninety-three (97%) procedures had successful identification; all SLNs were hot; 91 (of 93 procedures with blue dye) were blue and hot. The mean time from radioisotope injection to incision was 19.9 minutes (SD 8.5 minutes). The mean highest 10 second count was 88,544 (SD 55,954). Three of 96 (3%) patients with failure of localization had previous excisional biopsies: 1 circumareolar and 2 upper outer quadrant incisions that may have disrupted the lymphatic flow.
Conclusion:
Intraoperative subareolar injection of radioisotope rapidly drains to the SLNs and allows immediate staging of the axilla, avoiding the need to coordinate diagnostic services and a painful preoperative procedure.
Intraoperative injection of radioisotope and blue dye successfully identified the sentinel lymph node in 93 of 96 (identification rate = 97%) procedures in breast cancer patients.
The concept of sentinel lymph node (SLN) biopsy (SLNB) for breast cancer has summarily been adopted into clinical practice. However, the technique for this procedure is not standardized as it seems almost any technique works.1 Most surgeons use both blue dye and radioisotope given variously as peritumoral, dermal, subareolar, intratumoral, or a combination of injection techniques.1–5 Whereas the blue dye is always injected in the operating room, the timing of radioisotope injection varies between immediately preoperatively and 24 hours before surgery to ensure adequate drainage of radioisotope to the SLN.1,6 These approaches have tried to ease the logistics of scheduling cases between Nuclear Medicine and Surgery, but the patients still undergo an invasive procedure prior to their main surgery and anesthesia.3,6 Local anesthesia, for the most part is not used for SLNB to avoid disturbing the lymphatic drainage properties of the radioisotope. Pain, anxiety, and vasovagal syncope related to radioisotope injection are underreported. However, rates should approximate the pain and vasovagal episodes seen with needle localization procedures (10%–20%).7
Subareolar injection drains the radioisotope from the subareolar plexus to the SLN(s) reliably and reproducibly.8 Based on the subjective observation of more rapid drainage than other techniques, we hypothesized that the intraoperative injection of subareolar radioisotope would successfully identify SLNs, omitting an extra painful preoperative procedure in an awake patient. Such an approach would also carry significant logistic advantages in terms of scheduling between the nuclear medicine department and the operating room.
METHODS
Approvals
The Institution Review Board (IRB) approval was obtained for this prospective protocol to perform the subareolar Tc-99 injection in the operating room. Radiation Safety approval of the procedure and personnel (who underwent a half-day training course) prior to beginning the study was similar to what is involved in using radioactivity in a laboratory setting. All personnel involved in any given procedure, including surgeons, surgical technicians, nurses and data managers, underwent radiation safety education. This involved gloving, handling without opening the lead container until the time of injection, and returning the empty syringes in the lead container. As with preoperative injection, all personnel directly handling the radioactivity wore the dosimeters at all times during the operating day. Dosimeters were returned to the Radiation Safety Department every month for monitoring radiation exposure of the personnel.
Radiation Safety
The study procedures and personnel involved were identified to obtain approval by the Radiation Safety Department. All cases were booked from the clinic notifying the Nuclear Medicine Department of approximate time of surgery so that the dose decay could be planned appropriately to have 1 mCi at time of injection. Authorized personnel from the breast surgery team carried the dose to the operating room in lead containers, which were promptly returned after injection. Dose disposition and container receipt were documented.
A Geiger counter was used by the breast team personnel to scan the operating room after each procedure including the surgical table and trays, instruments, trash, and linens. Any material with higher than background count was collected in a radiation hazard bag and disposed by standardized protocol by the Nuclear Medicine Department.
Study Procedure
After informed consent, patients with breast cancer were prospectively enrolled in the study to compute the success rate of SLNB following subareolar injection Tc-99 and blue dye intraoperatively, after induction of general anesthesia. Tc-99 was injected variably from 5 minutes prior to incision prior to routine scrub, prep, and drape. Blue dye was always injected 5 minutes prior to incision. All injections were performed into the subareolar lymphatic plexus inserting the needle at the limbus of the areola at 45 degrees to instill just beneath the nipple.9 Radioactivity counts in the axilla were recorded prior to the incision. The hand-held gamma probe (Neoprobe, Dublin, OH) was used to localize radioactivity. The time from injection of radioisotope to the incision was also recorded. All hot, blue, and palpable nodes were submitted for pathology as SLNs. Patients then underwent excision of the tumor via lumpectomy or mastectomy. Dissection of the axilla was performed if SLNs were positive and the patient was not on a study protocol.
Pathology
The SLN(s) greater than 5 mm in size were sectioned at 3-mm intervals along the long axis. Intraoperative touch prep cytology was performed followed by routine hematoxylin and eosin staining. Complete axillary dissection specimens were submitted for pathology as nonsentinel axillary nodes. The nonsentinel axillary lymph nodes were bisected along the long axis and 1 section from each node was submitted for hematoxylin and eosin staining. Immunohistochemical staining for SLNs was only used for lobular cancers.
Statistics
Data regarding patient demographics, details of surgery and pathology including number and radioactivity counts of SLNs, and results of axillary dissection were recorded on Microsoft Excel software. Excel Stats software was used to calculate identification rates computation of means and standard deviations.
RESULTS
Radiation Safety
Ninety-six intraoperative radioisotope injection SLNB procedures were performed with blue dye confirmation between October 18, 2002 and November 7, 2003. On 3 occasions, the blood-stained sponges had to be sent Nuclear Medicine Department for decay monitoring and disposal. Maximum exposure to surgeon in this study was 100 microSv/mo.
Study Patients
Eighty-eight patients were enrolled during the study period, 8 of which had bilateral procedures making a total of 96 SLNB procedures. Of 8 patients with bilateral procedures, 7 had a prophylactic contralateral mastectomy while 1 had bilateral cancer. Thus, a total of 89 SLNB procedures were done for cancer, 20 of which were ductal carcinoma in situ. Mean age of the patients was 58 years (SD 13 years); 48 of 89 cancers (54%) were located in upper outer quadrant (Fig. 1); 11 (12%) patients had prior excisional biopsies, and 13 (15%) patients had multicentric disease. Five (6%) of the cancers were infiltrating lobular carcinoma while 84 (94%) were ductal cancers: 20 (22%) in situ and 64 (72%) invasive. The mean tumor size was 1.8 cm (SD 1.7 cm; range 0.1–7 cm). Tumor staging distribution was as follows: Tis = 20, T1a = 7, T1b = 11, T1c = 28, T2 = 20, T3 = 3.
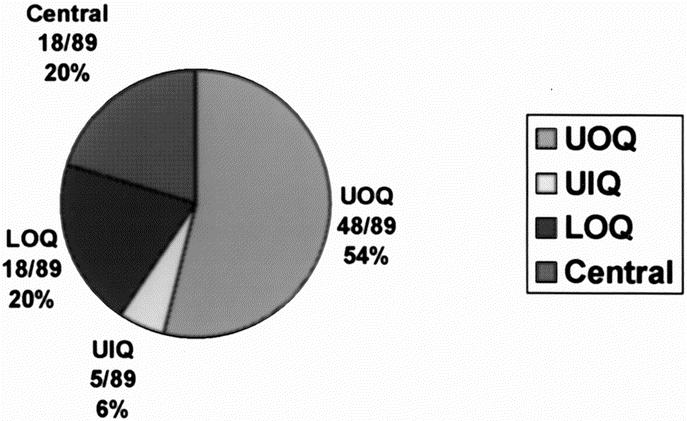
FIGURE 1. Distribution of tumors among various quadrants of the breast.
Study Procedure
The mean dose of Tc-99 injected intraoperatively was 1.07 mCi (SD 0.18 mCi; range 0.76–1.6 mCi. Ninety of 96 (94%) SLNBs were done as first procedure, while 6 (6%) SLNBs were performed after completion of another procedure in the same setting. Three of these 6 patients had contralateral mastectomies, 2 had insertion of indwelling vascular catheters, and 1 had an umbilical hernia repair as first procedure. Eighty-seven of 90 first SLNB procedures underwent successful localization of SLNs (identification rate = 97%). Blue dye was not used in 3 patients with history of dye allergies. All 87 patients had hot nodes; of 84 patients injected with blue dye, blue lymph nodes were identified in 82 patients; 9 patients had suspicious palpable nodes. The mean localization time (time lapse between injection and axillary incision) in 87 patients was 19.9 minutes (SD 8.5 minutes). Mean highest ex vivo 10-second count was 88,544 (SD 55,954). The mean background count was 102.6 per 10 seconds (SD 96.3 per 10 seconds). The ex vivo to background count ratio was 863 (SD 581). Three patients with failure of localization had prior excisional biopsies (1 circumareolar and 2 upper outer quadrants). The patient with a circumareolar incision should have been screened out of the study; the other 2 patients had upper outer quadrant incisions, with one of them explored twice through the same incision. All 6 patients who had SLNB following another surgical procedure prior to SLNB underwent successful localization; all had hot and blue nodes. The mean localization time in these patients was 109 minutes (SD 58 minutes). Mean highest ex vivo 10-second count in these patients was 52,757 (SD 49,991). Mean background count was 46.6 per 10 seconds (SD 10.3 per 10 seconds). The ex vivo to background count ratio was 1132 (SD 4853).
Pathology
A total of 160 SLNs were identified (mean 1.7 per patient [SD 0.78]; range 1–6). Fifteen of 69 invasive cancers (22%) had SLNs positive for metastatic disease. Complete axillary dissection revealed additional positive non-SLNs in 7 of the 15 patients (47%). The rate of lymph node positivity was related to the tumor size; 11% of T1, 40% of T2, and 67% of T3 lesions had positive lymph nodes (Fig. 2).
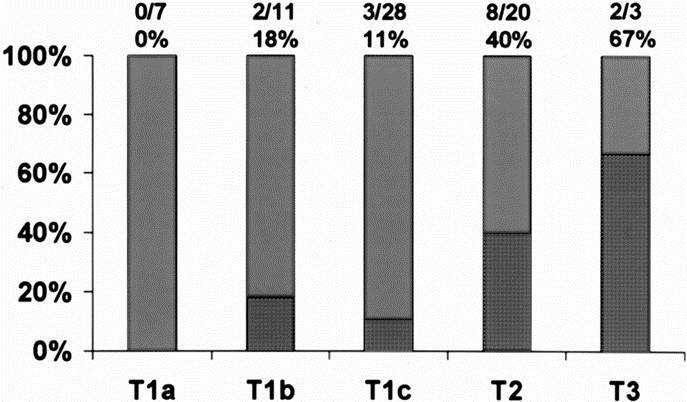
FIGURE 2. Rate of lymph node positivity according to tumor size (P = 0.01).
DISCUSSION
The objective of SLNB in breast cancer patients is twofold: to accurately stage the axilla and to minimize the morbidity and discomfort to the patient. The technique of SLNB has been evolving to identify how best to achieve these objectives. It is generally accepted that the use of blue dye and radioisotope in combination is more accurate than either approach alone.10 Variations in the technique include type of carrier particle for the radioisotope (sulfur colloid, albumin, antimony, or dextran), dose of radioactivity (1–10 mCi), and route of administration (peritumoral, intradermal, subdermal, and subareolar); we initially reported 100% confirmation of subareolar Tc-99 using peritumoral blue dye in patients with invasive breast cancer.11 Similarly, volume (0.05–16 mL) and timing of injection have been extensively scrutinized with reports of injection anywhere from immediately preoperatively to 24 hours in advance.1,6 This report represents the first intraoperative injection of radioisotope for SLN localization. We suspect that dermal injection would locate just as quickly although the false-negative rate of this technique on average is greater that 5%.
The pattern of short-term morbidity reported after SLNB procedure delineates problems of arm function, pain, and numbness, which is similar for both SLNB and axillary lymph node dissection procedures.12,13 However, patient discomfort and anxiety related to the procedure itself are not reported. In addition to complaint of pain with the injection of Tc-99, some patients had vasovagal episodes during the procedure. Since there are no data on rates of these complications and anxiety related to radioisotope injection prior to anesthesia, we think that it should be somewhat similar to that reported for needle localization procedures. Kelly and Winslow reported the mean anxiety score of 5.3 (scale 0–10) in women undergoing needle localization procedures.7 Nine percent of patients fainted and other reported complications included pain, stinging, embarrassment, and dizziness. Subareolar radioisotope injection drains rapidly to the SLNs localizing within 2 to 15 minutes confirmed via lymphoscintigraphy.14 We therefore hypothesized that all the morbidity and discomfort of preoperative Tc-99 injection could be avoided if injection after general anesthesia demonstrated successful identification of SLNs. In the present study, we successfully identified the SLNs in 97% of patients with intraoperative radioisotope injection with blue dye confirmation. Three failures were in patients with previous incisions (2 upper outer quadrants and 1 circumareolar) that could have disrupted the normal lymphatic flow. Since patients with negative SLNs did not undergo axillary dissection and we cannot compute the false-negative rate, the mean number of SLNs identified per patient was 1.7, which is similar to our previous reported series in which the radioisotope was injected 30 minutes to 8 hours prior to surgery.11 In the current study, 22% of SLNs were positive for cancer, and lymph node positivity was related to the size of the tumor (Fig. 2) as expected.
The level of radiation exposure has been a cause for concern among surgeons, surgical technicians, and pathologists. One argument is that using a day-before or 2-day protocol may lower the exposure to ionizing radiation by allowing more time to decay since the half-life of Tc-99 is 6 hours. Annual allowable radiation exposure limits are set by the Occupational Safety and Health administration (OSHA).15 Dose limits are determined for 3 groups: exposed workers, nonexposed workers, and members of the public. The radiation dose for the exposed workers is measured every 4 weeks with dosimeters and quantified in effective dose. The special SI unit of effective dose is the sievert (Sv); 1 sievert is equal to 1 J/kg. The average dose of natural radiation is 1.4 mSv/year. Surgeons, scrubbed personnel, and pathologists qualify to be nonexposed worker by the OSHA criteria and their exposure dose limit is 1 mSv/year (approximately equal to 20 chest x-rays). deKanter et al showed that in the worst case scenario the highest measured dose is at the abdominal wall of the surgeon, which is 8.2 Sv/ procedure.16 Miner et al showed that the exposure to surgeon's hands during SLNB procedure is 98 micro Sv/procedure.17 Thus, surgeons could perform 1000 procedures per year before reaching the dose limit for nonexposed workers set forth by OSHA. Since radioactivity lasts for up to 48 hours after the injection,17 2-day or 1-day protocols are unlikely to curtail the already minimal radiation exposure to the involved personnel. On the contrary, intraoperative injection has the significant advantages of alleviating patient anxiety and vasovagal episodes. Since intraoperative injections are performed after anesthesia, the unscheduled delays in surgery do not cause any untoward effect or technical problems with the procedure. Intraoperative injection is also very convenient for the surgeon because the injection can be performed before or simultaneously with the blue dye injection.
Injection of radioisotope several hours before surgery in the Nuclear Medicine Department requires cocoordinated scheduling of cases between 2 hospital areas and limits flexibility in the operating room scheduling. Similar results in terms of accuracy have been reported with same-day versus 2-day protocol of injection by other authors.3,4,18,19 Winchester et al studied 180 patients with breast cancer who underwent SLNB.20 Eighty patients received injection of radioisotope 1 to 4 hours before surgery, while 100 patients were injected 16 to 20 hours before surgery. There was no significant difference between the 2 groups in the mean number of SLNs identified per patient. Vargas et al hypothesized that all scheduling problems could be avoided by injecting radioisotope immediately preoperatively.6 They reported a success rate of 97%; however, this approach does not alleviate patient anxiety and morbidity associated with injection. Some authors think that same-day injection may be associated with technical difficulties in SLN localization because of low ratio of SLN-to-background radioactivity.21 We did not encounter these problems in the current study. Moreover, the hot node counts using subareolar injections are quite high as compared with the background (88,544 [SD 55,954] vs. 102.59 [SD 96.3] in this study), giving an ex vivo to background ratio of 863. In a report by Sato et al, the hot node counts with peritumoral injections were studied by dividing the patients in low and high uptake group; the mean ex vivo counts were 39 and 1003 per second, respectively.22 Yoshida et al reported that radioisotope counts are higher with subareolar than with subdermal injections.23 Borgstein et al reported a mean ex vivo SLN count of 385 per 10 seconds with peritumoral injections.24 In the current study, only 8% of SLNs had a 10 second count of less than 1000 (Fig. 3).
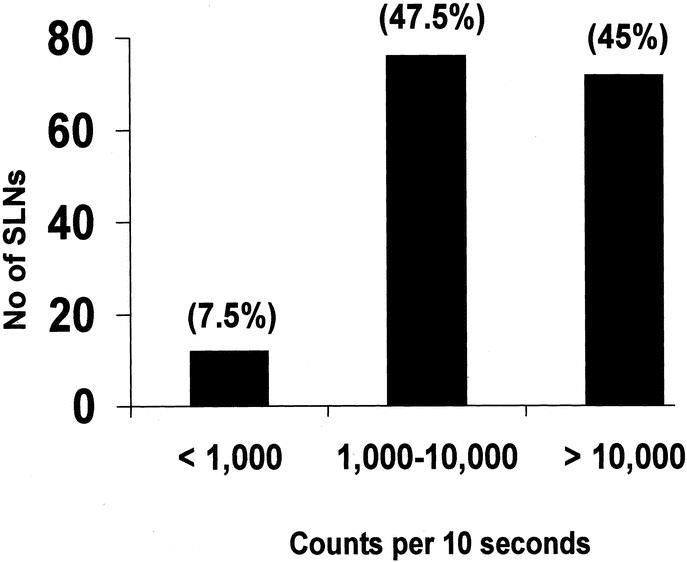
FIGURE 3. Radioactivity in sentinel nodes.
CONCLUSION
Intraoperative subareolar Tc-99 and blue dye injection is feasible and successfully identified the SLNs in 97% of patients in this study. The subareolar technique facilitates rapid SLN identification and is superior in terms of patient comfort and procedure scheduling.
Discussions
Dr. Kelly M. McMasters (Louisville, Kentucky): Dr. Klimberg and her associates at the University of Arkansas have pioneered the technique of subareolar injection of radioactive colloid for sentinel node biopsy. In this paper, they take it 1 step further by telling us that excellent results can be obtained by injecting the radioactive colloids right in the operating room.
Those of you who have followed this field of sentinel node biopsy realize that there have been many different injection techniques described, and it can be very confusing to figure out which technique is best. The problem with the subareolar injection technique, and the reason that we have been somewhat reluctant to recommend it in the past, has been that there have been relatively few studies in which completion axillary dissection was performed in order to substantiate a low false-negative rate.
Dr. Klimberg and colleagues will be happy to know that we recently reported at the Western Surgical this year the results of such a study using subareolar injection of radioactive colloid in 148 patients and periareolar injection (at the edge of the areola) in 183 patients. Subareolar and periareolar injection was associated with 99% and 96% identification rates, respectively. And both techniques had just over an 8% false-negative rate, which was no different than peritumoral, dermal, or subdermal injection techniques.
While our data do not support the idea that subareolar injection is in any way more accurate than other techniques, I do think our data support the notion that the entire breast drains to the same few sentinel nodes regardless of injection site. I would like your thoughts on this issue, please.
If the false-negative rates are similar for all techniques, then the choice of injection technique really comes down to factors of simplicity, convenience, efficiency, reliability, and patient satisfaction. Certainly, the intraoperative subareolar injection technique fulfills all these requirements. Dermal injection of radioactive colloid, I might add, will work equally as well, since we currently have a 100% identification rate using this technique. So I have a few questions.
In the manuscript, some technical details were not clearly defined. You use 1 mCi of technetium sulfur colloid. Is this filtered or unfiltered colloid? What volume is injected? How much blue dye is injected? And do you use massage of the breast to facilitate transport of these tracer agents?
Second, you blamed the failure to identify the sentinel node in a few cases on prior excisional biopsy. What are your contraindications for subareolar injection? What is your preferred injection technique for patients who have had a circumareolar or an upper outer quadrant excisional biopsy?
Third, some people are interested in identifying internal mammary sentinel nodes. Will the subareolar injection technique identify internal mammary nodes ever?
Finally, I have heard from many surgeons that they are not personally allowed to perform the radioactive injections themselves; it must be done by the radiologists or nuclear medicine physicians. What is your understanding of the regulations regarding this issue? Does it vary from state to state, or are there some national guidelines?
Dr. David Berger (Houston, Texas): I want to thank Dr. Klimberg and her collaborators for the opportunity to read their manuscript ahead of time and for allowing me to comment on their work. Since the first reported use of sentinel lymph node (SLN) biopsy for breast cancer in 1994, numerous studies have documented that the findings in the sentinel node accurately predict the status of other axillary nodes. However, the false-negative rate, as Dr. Klimberg and Dr. McMasters have mentioned, has been reported to be up to 9% in some randomized prospective trials.
Additionally, no study reported to date has been sufficiently powered to address the issue of whether SLN biopsy alone provides equivalent survival to axillary node dissection. Despite these facts, SLN biopsy has become the standard of care in many institutions. Some caution should be observed until the results of the large randomized multi-institutional trials from the NSABP and the College of Surgeon's Oncology Group addressing the issue of survival with this procedure are completed and reported.
I want to congratulate Dr. Klimberg and her colleagues for pioneering the use of subareolar injection for the detection of the sentinel node(s). These authors have demonstrated this technique to be equivalent to peritumoral and intradermal injection while appearing to be simpler to perform. I also want to congratulate them for trying to improve the patient experience by bringing the radioisotope injection to the operating room. Over the years, we as surgeons have been too willing to abrogate portions of our patients’ care to radiologists and other interventionalists. It is good to see that general surgeons and surgical oncologists are following the lead of our vascular colleagues and taking back surgically related patient care.
I would like to ask the following questions: First, regarding surgeons doing the injection, was there any difficulty in credentialing surgeons to perform the nuclear injections? What was the reaction from your colleagues in nuclear medicine? Can this procedure be billed for separately and what is the reimbursement?
As you showed, all sentinel lymph nodes were hot. Why then bother using the blue dye at all, since allergic reactions and anaphylactic shock have been reported with blue dye?
You have stated that your goal is to simplify the patient experience and try to perform all procedures at one sitting under one anesthetic. However, in your study, evaluation of the SLN is done intraoperatively by a single touch prep and hematoxylin and eosin staining. You reported a sentinel lymph node positivity rate of 22% below that reported in the literature for similarly staged patients. Additionally, some authors have reported up to a 30% negative rate for intraoperative frozen section. What additional evaluation do you do on permanent sections and what percentage of patients who have negative intraoperative staging subsequently have a positive SLN on more thorough evaluation, requiring them to come back to the OR for axillary lymph node dissection?
It has been reported that the learning curve for SLN biopsy is 20 to 30 cases with dissection of the axilla required to validate each surgeon's experience. This number is becoming harder to achieve as more patients demand the SLN biopsy procedure. Does subareolar injection change the learning curve for SLN biopsy?
Finally, we are going to hear a paper tomorrow telling us that we don't need to dissect the axilla if only micrometastases are found in the SLN. How do you currently handle this issue?
Dr. Henry Kuerer (Houston, Texas): When a positive axillary sentinel node is identified, there has been a move by radiation oncologists in this country to actually treat the internal mammary nodes when dual drainage to the axilla internal mammary nodes are demonstrated by preoperative lymphoscintigraphy, mostly based on the experience of Urban and Veronesi with combined dissection of the axillary and internal mammary nodes. How do you handle this situation in Arkansas?
Dr. Rakhshanda Layeeque (Little Rock, Arkansas): First of all, Dr. McMasters brought up the point about the false-negative in their own experience. Presented data are what we had accumulated from the world literature to look at the technique. And cumulatively, we have 268 node-negative patients with follow-up dissections, to give us a final rate of 1%, which is still, to our knowledge, the best and most accurate.
Now if we add, if I heard you correctly, your data with 148 patients, assuming about 25% node-positive patients, which gives us about 30 more cases of node positives, and an 8% false-negative would be 3 more patients. So about 6 false-negative patients out of 300, which would be still about 2% of false-negative rate, which still looks the best among the reported techniques.
Secondly, of course, as you mentioned, the subareolar technique is a much simpler, convenient, and efficient way to go ahead. Certainly, it does not require a big learning curve, as Dr. Klimberg mentioned; specifically our first study was funded toward involving community surgeons who were starting to do sentinel node biopsies; and that study compared 19 patients with peritumoral with 19 with the subareolar technique, and we had zero percent false-negative with subareolar versus 20% with peritumoral. So it does seem to have a lower and sharper learning curve to it.
And of course the applicability to the nonpalpable tumors, the image guidance required for peritumoral injection, is not needed for subareolar technique, which is certainly very convenient. Moreover, we also reported on 40 patients with multicentric tumors, which is another whole area where subareolar technique becomes much more applicable as compared to peritumoral, which was first reported by Schrenk, and we had reported on 40 of our patients also. So yes, all those points kind of took our balance and bias toward the subareolar injection.
About some details that were requested. The technetium we are using is unfiltered, 4 cc was used in the subareolar plexus and the technique of which was described during the presentation. The blue dye we are injecting is somewhere between 2 cc and 5 cc. If we see an immediate blue flush, we don't necessarily go up to 5 cc. But that is really the maximum, 5 cc of blue dye, that we will use.
At this point we do kind of a gentle breast massage after the blue dye injection. But most often the technetium has already localized into the axillary nodal basin. So the massage is really for the blue dye. We don't believe that it is necessary, but that is the way the technique was initially developed. So it is just a matter of protocol that we are still doing it. And it does comply with B-32 protocol as well.
About the failure that we have 3 cases of. Certainly, 1 patient with the periareolar incision had resected subareolar plexus. And that certainly should not be the patient you would want to choose for subareolar injection. But since this exclusion criteria, frankly, was not defined in the protocol, we had to report on this patient. The 2 other patients had upper outer quadrant incisions. Generally, we have not had identification or false-negative problems with upper outer quadrant incisions, but these were particularly large incisions and 1 patient had a couple of explorations through the same wound. So we believe that the transection of major lymphatics could have been responsible. That has been identified in Kern's paper, for example, which is listed in the table.
There was a question about internal mammary nodes. We have not in this study or in the previous study reported identification of internal mammary nodes with subareolar injections, but some authors have reported that. The identification rate of internal mammary is certainly much lower with the subareolar injection. But we believe that it has to do more with the physiology of the lymphatic drainage rather than true drainage patterns because the larger the volume and the speedier the injection is, the more likely we are to disturb the volume pressure mechanics of the low pressure, low-volume system. So the fact that the internal mammary is identified more often may be a technical issue rather than a true lymphatic drainage of the breast.
And most importantly, we do not know the clinical implications of internal mammary identification as of yet. The Consensus Conference, the latest statement, says that we do not really have to dissect internal mammary nodes. So that is our stand on the internal mammary issue.
There was a question about the radiation safety and whether our colleagues were happy with the switch from nuclear medicine to the injections in the OR. Certainly, we had great cooperation by our radiation team. Credentialing at this point requires a 4-hour or half-day training of everybody who is to be involved in such procedures and some practice sessions to get acquainted with institution protocol, especially color coding of disposal bags, identifying by labels, etc., requires half a day for credentialing.
There are guidelines, of course, to answer your question regarding regulations of radiation safety; the Office of Safety and Health Administration guideline, which is the U.S. national guideline, does not in any form prohibit surgeons to perform radioisotope injection in OR. It really is not true that nuclear medicine personnel have to do it and we cannot do it. The guidelines by the International Commission on Radiation Protection are a bit more strict, but even they do not define that surgeons cannot inject radioisotope in the OR. Their exposure limits are a bit different, but both allow us to do it. And we do have examples of intrathoracic and intra-abdominal malignancies, which have been using radioisotope injections in the OR without a problem. So that has not been the issue.
The procedure is certainly billable under the Injection of Contrast Code, which is CPT Code 38792. It is about $100 billable charge, which the surgeons can do.
The question of intraoperative assessment of the sentinel node. We are using touch prep cytology (TPC) and we have reported that previously. Our false-negative with the TPC was about 5% and we believe that if you give 95% of patients a chance of not having to come back to the OR, it is certainly a good situation for them. But even at a 30% false-egative with frozen section, you are still saving 70% of patients another surgical procedure. So we believe that attempting to know the intraoperative pathology in the sentinel lymph node is certainly worthwhile.
Our stand is actually the opposite. We do not want to unnecessarily dissect someone's axilla who eventually is found to have no disease. So in fact what I am saying is...are more worried about false positives which have been reported with frozen sections as opposed to TPC which we rely on.
Then the issue of micrometastatic disease. Certainly, as we will hear tomorrow, probably micrometastatic disease does not need to be addressed further. However, at this point we do address it further; we try to sign these patients up for the Z-11 trial, or we go back and perform the axillary dissection. And that is kind of a mutual decision, presenting them with all the risks.
About the blue dye. It was mentioned why use the blue dye? There are really 2 reasons. The identification rates so far have been the best with combination, although some of the authors rely on radioisotope alone. But the best results across the world are with combination.
Secondly, when we are testing 1 technique, it is always wiser to have the other as a standard so that you can confirm. In the previous paper, we do have confirmation of subareolar technique with the blue dye. From this study also, we have confirmation of our radioactive technique with the blue dye.
Footnotes
Dr. Layeeque, Dr. Kepple, and Dr. Kass were supported by the Susan G. Komen Breast Cancer Clinical Fellowship and the Virginia Clinton Kelley/Fashion Footwear Association of New York Breast Cancer Research Fellowship.
Reprints: V. Suzanne Klimberg, MD, Department of Surgery, Division of Breast Surgical Oncology, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 725, Little Rock, AR 72205. E-mail: klimbergsuzanne@uams.edu.
REFERENCES
- 1.Schwartz GF, Guiliano AE, Veronesi U. Proceeding of the consensus conference of the role of sentinel lymph node biopsy in carcinoma or the breast April 19–22, 2001, Philadelphia, PA, USA [Review]. Breast J. 2002;8:124–138. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albertini JJ, Lyman GH, Cox C, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276:1818–1822. [PubMed] [Google Scholar]
- 4.Pijpers R, Meijer S, Hoekstra OS, et al. Impact of lymphoscintigraphy on sentinel node identification with technetium-99-m-colloidal albumin in breast cancer. J Nucl Med. 1997;38:366–368. [PubMed] [Google Scholar]
- 5.Cox CE, Pendas S, Cox JM, et al. Guidelines for sentinel biopsy and lymphatic mapping of patients with breast cancer. Ann Surg. 1998;227:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas HI, Vargas MP, Gonzalez KD, et al. Immediate preoperative injection of 99m-Tc sulfur colloid is effective in the detection of breast sentinel lymph nodes. Am Surg. 2002;68:1083–1087. [PubMed] [Google Scholar]
- 7.Kelly P, Winslow EH. Needle wire localization for nonpalpable breast lesions: sensations anxiety levels, and informational needs. Oncol Nurs Forum. 1996;23:639–645. [PubMed] [Google Scholar]
- 8.Cody HS. Clinical aspects of sentinel node biopsy. Breast Cancer Res. 2001;3:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith LF, Cross MJ, Klimberg VS. Subareolar injection is a better technique for sentinel lymph node biopsy. Am J Surg. 2000;180:434–438. [DOI] [PubMed] [Google Scholar]
- 10.Badwe RA, Thorat MA, Parmar VV, et al. Sentinel-node biopsy in breast cancer. N Eng J Med. 2003;349:1968–1971. [DOI] [PubMed] [Google Scholar]
- 11.Klimberg VS, Rubio IT, Henry-Tillman RS, et al. Subareolar versus peritumoral injection for location of the sentinel lymph node. Ann Surg. 1999;229:860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peintinger F, Reitsamer R, Stranzl H, et al. Comparison of quality of life and arm complaints after axillary lymph node dissection vs. sentinel lymph node biopsy in breast cancer patients. Br J Cancer. 2003;89:648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reitman JS, Dijkstra PU, Geertzen JHB, et al. Short-term morbidity of the upper limb after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast carcinoma. Cancer. 2003;98:690–696. [DOI] [PubMed] [Google Scholar]
- 14.Maza S, Valencia R, Geworski L, et al. Peritumoral versus subareolar administration of technetium-99m nanocolloid for sentinel lymph node detection in breast cancer: preliminary results of a prospective intra-individual comparative study. Eur J Nucl Med Mol Imaging. 2003;30:651–656. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Labor Occupational Safety and Health Administration. Ionizing radiation. In: Toxic and Hazardous Substances. Washington, DC: U.S. Department of Labor Occupational Safety and Health Administration, 1998:1–9. [Google Scholar]
- 16.deKanter AY, Arends PPAM, Eggermont AMM, et al. Radiation protection for the sentinel node procedure in breast cancer. Eur J Surg Oncol. 2003;29:396–399. [DOI] [PubMed] [Google Scholar]
- 17.Miner TJ, Shriver CD, Flicek PR, et al. Guidelines for the safe use of radioactive materials during localization and resection of sentinel lymph node. Ann Surg Oncol. 1999;6:75–82. [DOI] [PubMed] [Google Scholar]
- 18.McCarter MD, Yeung H, Yeh S, et al. Localization of sentinel lymph node in breast cancer: identical results with same-day and day-before isotope injection. Ann Surg Oncol. 2001;8:682–686. [DOI] [PubMed] [Google Scholar]
- 19.Chok S, Chow LWC, Wong K, et al. Breast sentinel lymph node biopsy using radioisotope injection: is one-day better than two-day protocol? Am Surg. 2003;69:358–361. [PubMed] [Google Scholar]
- 20.Winchester DJ, Sener SF, Winchester DP, et al. Sentinel lymphadenectomy for breast cancer: Experience with 180 consecutive patients. Efficacy of filtered technetium 99m sulfur colloid with overnight migration time. J Am Coll Surg. 1999;188:597–603. [DOI] [PubMed] [Google Scholar]
- 21.Solorzano CC, Ross MI, Delpassand E, et al. Utility of breast sentinel lymph node biopsy using day-before-surgery injection of high dose 99m Tc-labelled sulfur colloid. Ann Surg Oncol. 2001;8:821–827. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Tamaki K, Shigekawa T, et al. Clinicopathologic and technical factors associated with the uptake of radiocolloid by sentinel nodes in patients with breast cancer. Surg Today. 2003;33:403–407. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida K, Yamamoto N, Imanaka N, et al. Will subareolar injection be a standard technique for sentinel lymph node biopsy? Breast Cancer. 2002;9:319–322. [DOI] [PubMed] [Google Scholar]
- 24.Borgstein PJ, Meijer S, Pijpers RJ, et al. Functional lymphatic anatomy for sentinel lymph node biopsy in breast cancer: echoes from the past and the periareolar blue method. Ann Surg. 2000;232:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


