Abstract
Objective:
To determine if micrometastatic disease in the sentinel lymph node is a predictor of non-sentinel lymph node (non-SLN) involvement in breast cancer.
Summary Background Data:
Sentinel lymph node biopsy (SLN) is an accepted alternative to axillary dissection in staging breast cancer. If the SLN contains metastatic foci, the standard recommendation is completion axillary node dissection (CAD). However, a large subset of patients with metastasis limited to the SLN is unnecessarily subjected to the morbidity of CAD.
Methods:
A retrospective review of prospectively gathered breast cancer patients having SLN was conducted. Patients with metastasis to the SLN were selected for analysis. Various clinicopathologic features were analyzed for association with metastasis to the non-SLN.
Results:
A total of 194 women underwent successful SLN dissection. Metastasis to the SLN was found in 48 patients (21 had micrometastases, 27 had macrometastases). Of those with micrometastases, 16 underwent CAD with 1 patient having metastasis to the non-SLN. In patients with macrometastases, 26 had CAD with 14 patients having non-SLN metastasis. Multivariable logistic regression identified only macrometastatic disease in the SLN as significantly associated with involvement of the non-SLN (P = 0.03). None of the patients with micrometastases, including those without CAD, has evidence of local recurrence to date (3–30 months).
Conclusion:
This study demonstrates that the incidence of non-SLN involvement is low in SLN that contains only micrometastatic foci and is within the accepted range of the false-negative rate of SLN. This suggests that a CAD may be omitted in patients with micrometastatic disease.
A retrospective review of breast cancer patients demonstrated that when the sentinel lymph node contains only micrometastasis to the non-sentinel lymph node is an uncommon event, occurring in only 6% of patients in our series.
Sentinel lymph node (SLN) biopsy was first applied to breast cancer just over 10 years ago.1 Since that time, it has rapidly emerged as a less invasive alternative to routine axillary dissection for the assessment of nodal status. In patients with breast cancer, if the SLN does not contain metastatic cancer, the remainder of the nodal basin should be negative for metastases2–5; thus, complete axillary dissection can be avoided. However, if the SLN is positive, the current recommendation continues to be completion axillary dissection as the degree of axillary nodal involvement remains the most important predictor of outcome in these patients.6 Fortunately, in 40% to 70% of cases with metastases to the axillary nodes, the only positive node will be the SLN.2,5–9 Given the morbidity of full level I and II axillary dissection, including lymphedema, limitations of arm mobility and sensory loss, defining a population of patients who could forego axillary dissection after SLN biopsy would be of obvious benefit. Several studies by groups, such as ACOSOG and NSABP, are currently underway to address whether or not some patients with a metastasis in the SLN could avoid completion axillary dissection. The purpose of this study was to identify a subset of patients in whom metastatic disease is confined to the SLN, and thus might avoid completion axillary dissection without compromising sound oncologic treatment.
METHODS
Patient Population
A retrospective review of prospectively gathered data was conducted of female breast cancer patients who underwent lymphatic mapping with SLN biopsy at Eastern Virginia Medical School between March 1997 and December 2002. Patients were offered lymphatic mapping with SLN if: 1) they had histologically proven invasive ductal or lobular carcinoma, 2) they were clinically node negative, and 3) they had signed an informed consent approved by our Institutional Review Board.
Surgical Technique
Patients were injected with a total of 500 μCi of filtered (0.2 μmol/L filter) 99m-technetium (99mTc) sulfur colloid injected as 4 equal doses peritumorally or around the biopsy cavity. Injections were followed by 5 minutes of breast massage, and these injections preceded any needle localization procedures of the primary tumor, if required. Early in the study, all patients were injected on the day of the planned procedure, but later in the series some patients were injected on the day prior to the planned procedure. A minimum of 2 hours between nuclear injection and operative procedure was required. Preoperative lymphoscintigraphy was performed on all patients and the drainage pattern was recorded.
Intraoperatively, 2.5 to 8 mL of Lymphazurin (Tyco/US Surgical, Norwalk, CT) was sterilely injected peritumorally or around the biopsy cavity. Five minutes of breast massage followed. During that time, the nodal basins (axillary, internal mammary, infra- and supra-clavicular, and inframammary), tumor bed and background were scanned for radioactivity and recorded. A hand-held gamma detection probe (Navigator, Tyco, CT or C-Trak, Carewise, Morgan Hill, CA) was used to identify areas of increased activity in the ipsilateral axillae. Nodal basins outside the axillae were not routinely biopsied. Once a SLN was identified, both in vivo and ex vivo counts were recorded. A SLN was defined as a blue node (or a node with a blue afferent channel) and/or a node with increased radioactivity. All nodes with counts greater than or equal to 10% of the highest ex vivo count were considered sentinel nodes and removed. If a SLN was not identified or frozen section of the SLN demonstrated metastasis, a simultaneous completion axillary node dissection (level I/II) was performed at the surgeon's discretion. Additionally, many patients received a completion axillary dissection as part of each surgeon's initial validation series.
Histopathologic Examination
Lymph nodes were marked as sentinel and a frozen section and or touch preparation was performed on the SLN. If the SLN was negative for tumor, the node was embedded in paraffin and at least 5 additional levels were examined with hematoxylin and eosin and cytokeratin antibody for confirmation. The size of the metastases was measured using an ocular micrometer. In cases where the size of the metastasis was not reported on the original pathology report, the slides were retrieved and evaluated by an independent pathologist (A.S.).
A micrometastasis was defined as a tumor deposit of ≤ 2 mm. Metastases > 2 mm were considered macrometastases. In cases where more than one SLN was positive for tumor, the patient was grouped according to the SLN with the largest metastasis. The non-SLNs were evaluated with standard hematoxylin and eosin sections at 5 μm with 3 to 5 levels and cytokeratin immunohistochemistry staining at the discretion of the pathologist. Primary tumors were evaluated by routine histologic examination. Primary tumor size, histologic type, and the presence of vascular invasion were all recorded. Estrogen receptor (ER), progesterone receptor (PR), and Her2/neu status were also determined.
Statistical analysis was performed with NCSS 2000 for windows (NCSS, Kaysville, UT) statistical software. The Fisher exact test was used to test for associations between the presence of a positive non-SLN and other factors including age, race, histologic type, and primary tumor size, presence of angiolymphatic invasion, ER, PR, Her2/neu status, and size of the SLN metastasis. Logistic regression was then used to identify independent factors that were associated with the presence of metastasis to the non-SLNs. Only the factors determined by univariate analysis to have a P value < 0.05 were used in the model.
RESULTS
Between March 1997 and December 2002, we identified 194 consecutive patients who underwent successful SLN biopsy for breast cancer at our institution. All but 47 of these patients had a standard level I and II completion axillary node dissection. A positive SLN was found in 48 (24.5%) of the 194 patients. Six (12.5%) of these 48 patients did not undergo completion axillary node dissection at the discretion of their physician or because of patient refusal.
Patient Demographics and Tumor Characteristics
The median patient age was 53 years (range 33–77 years). A total of 131 (68%) patients were white, 57 (29%) black, and 6 (3%) were of other races. The (clinical or pathologic size) median tumor was 2.1 cm (range 0.4–11 cm). The predominant histologic subtype was invasive ductal carcinoma (85%). Seventy-one percent of tumors were ER-positive and 63% were PR-positive. Of tumors evaluated for Her2/neu status, 18% were overexpressors (Table 1).
TABLE 1. Patient Demographics and Tumor Characteristics
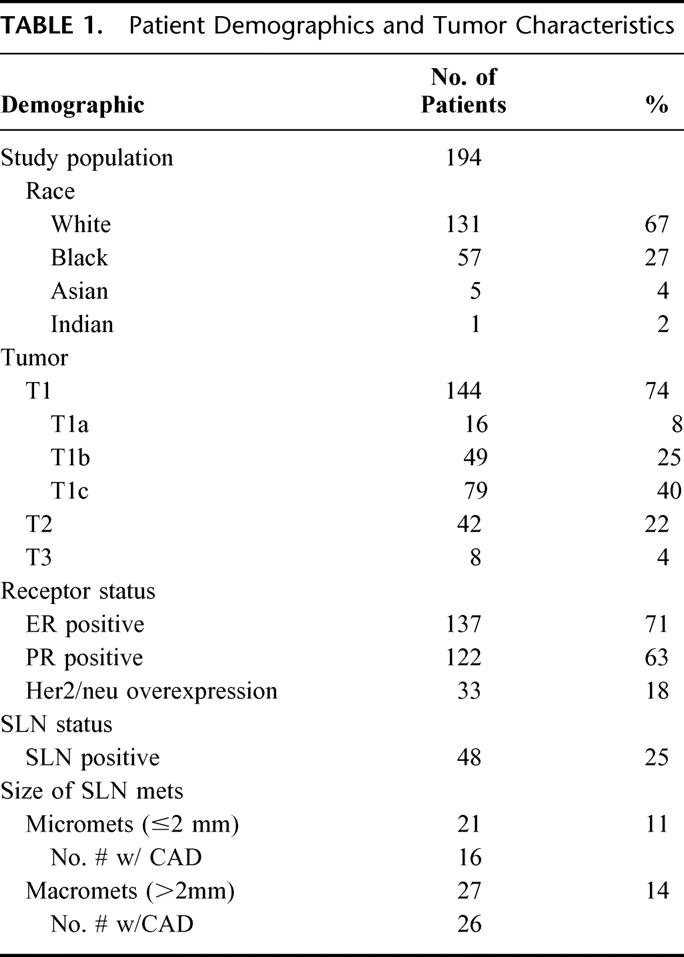
Characteristics of SLN and Non-SLN
The median number of SLNs identified was 2 (range 1–4). The median number of SLN found to be positive was 1 (range 1–3). Of the 48 patients with a positive SLN, macrometastasis was identified in 27 (56.3%) and micrometastasis was noted in 21 (43.7%) patients. Of patients with a positive SLN and completion axillary node dissection, 27 (64.3%) had disease solely in the SLN, 15 of 16 (94%) in the micrometastatic group versus 12 of 26 (46%) in the macrometastatic group. The false-negative rate (defined as the number of cases in which the SLN was found to be negative divided by the number of patients in the group with positive axillary lymph nodes) for the entire series was 8% (4 of 52).
Relationship Between Clinicopathologic Features and Positive SLN Biopsy
Table 2 summarizes the results of the statistical analysis used to determine the relationship between clinicopathologic variables and positive non-SLNs. Univariate analysis identified pathologic tumor size > 2cm (P = 0.02), ER-negative tumors (P = 0.03), Her2/neu overexpressing tumors (P = 0.04), and the presence of macrometastasis in the SLN (P = 0.002) to be associated with metastases in the non-SLN. Logistic regression identified only macrometastasis in the SLN (odds ratio, 13.9; 95% confidence interval, 1.13–170.4; P = 0.039) as significantly associated with a higher likelihood of tumor in the non-SLN.
TABLE 2. Relationship Between Clinicopathologic Features and Metastasis to Non-SLN
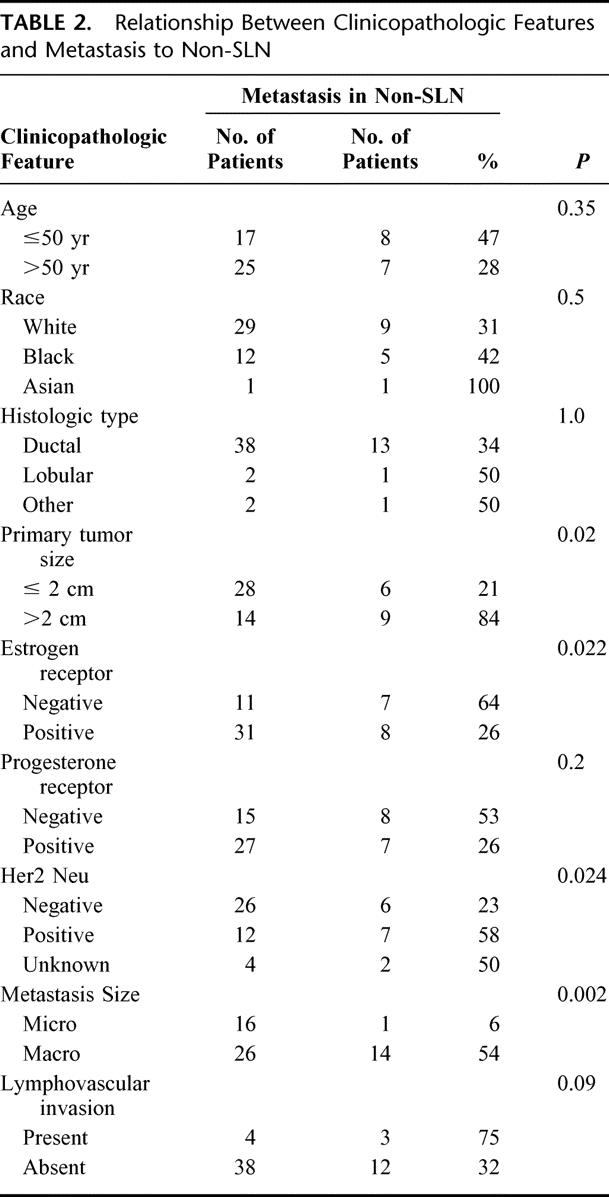
Among the 16 patients with metastases who underwent completion axillary node dissection, 1 (6%) had a metastasis found in the non-SLNs. The size of the primary tumor in the 1 patient with a positive non-SLN was 2.6 cm. Among the 6 patients with micrometastases who did not undergo completion axillary dissection, none has clinical evidence of disease in the axillae with a mean follow-up of 12 months (range 3–30 months). Of the 26 patients with macrometastases who underwent completion axillary node dissection, 14 (54.4%) had additional metastases to the nonsentinel axillary nodes.
DISCUSSION
Axillary dissection remains a major operation with potentially significant complications of seroma, shoulder dysfunction, axillary web syndrome, neuropathy, and lymphedema. As a result, over the last 10 years, SLN biopsy has emerged as a minimally invasive alternative to routine axillary node dissection to stage breast cancer. As we extend the limits of minimal invasive procedures, efforts must be made to identify patients in whom sentinel node biopsy alone is both accurate in diagnosing lymph node metastasis and reliable enough to avoid further dissection in those in whom no metastasis is observed. A number of studies have shown that 40% to 70% of breast cancer patients with lymph node metastases have the SLN as the sole site of disease2,5–9; thus, routine completion axillary dissection subjects many patients to potentially unnecessary surgery. This study supports this finding as 27 (64%) of 42 patients with a positive SLN who underwent completion axillary dissection were found to have no further disease in the axilla. Several studies (Table 3) have examined the frequency of metastases to the non-SLN in patients with micrometastases with differing results. Liang et al found no disease in the non-SLN when only micrometastases were present in the SLN.10Our study similarly shows that disease is nearly always limited to the SLN in patients who have micrometastases (≤ 2 mm). However, others, including Sachdev et al11 and Reynolds et al,12 demonstrated a higher frequency of metastases to the non-SLN in patients in this same patient population. Their studies, however, examined the non-SLNs by both hematoxylin and eosin staining and immunohistochemistry, and it is unclear how many of the positive non-SLNs were detected only by immunohistochemistry. This could account for the differences observed as we do not routinely stain non-SLNs by immunohistochemistry. In one of the largest studies to date, Viale et al found 22% of patients with the traditionally defined micrometastasis (≥ 2 mm) had additional metastases to non-SLNs versus 15.6% of patients with micrometastasis ≤ 1 mm.13 They concluded that only the subset of patients with micrometastasis ≤ 1 mm might be able to avoid completion axillary dissection.13However, the methodology in that series differed somewhat from the present study and other studies in that when more than one distinct tumor foci was present in a single SLN, they were considered multiple micrometastases and evaluated separately. Second, they examined the non-SLN in greater detail, including thinner sections and more sections than the routine pathologic evaluation of the non-SLN. The clinical significance of such evaluation remains controversial.
TABLE 3. Comparison of Frequency of Metastases to the Non-SLN in Patients With Micrometastases
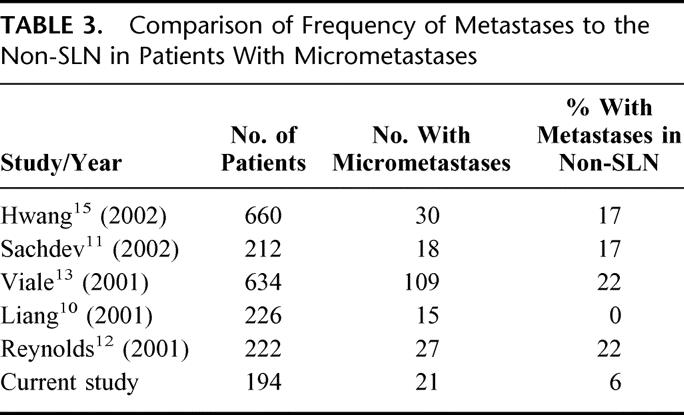
Efforts to identify a patient population who might forego completion axillary dissection without compromising oncologic treatment have been the subject of an increasing number of studies.9–16 Recently, Hwang et al evaluated the likelihood of a positive non-SLN based on clinicopathologic features and found primary tumor size > 2 cm, size of the largest SLN metastasis (> 2 mm), and the presence of angiolymphatic invasion to be predictive of finding disease in the non-SLN.15 Others,12–15 including Weisner et al16 and Sachdev et al,11 have demonstrated similar results. In our study, univariate analysis identified tumor size > 2 cm, ER-negative tumors, Her2/neu overexpressing tumors, and macrometastases (> 2 mm) in the SLN to be associated with disease in the non-SLN. However, only macrometastatic disease in the SLN was an independent predictor of spread of disease to nonsentinel nodes.
Analysis of the SLN by immunohistochemistry for cytokeratin was performed in 46 of 48 (96%) of our patients. Metastasis was detected in the SLN only by immunohistochemistry, after negative hematoxylin and eosin evaluation, in 1 of the 46 patients whose nodes were examined with immunohistochemistry analysis.
The routine use of immunohistochemistry to detect metastatic disease in the non-SLN remains controversial12,17–20 as issues of time, effort, cost, yield, and significance of a positive result continue to be addressed. In an attempt to answer these questions, Ishida et al recently examined patients with micrometastatic SLNs and examined SLNs and non-SLNs more thoroughly, including the use of immunohistochemistry, and found that the incidence of micrometastatic detection by cytokeratin immunohistochemistry occurred in ∼20% of non-SLN specimens that were negative for tumor on routine hematoxylin and eosin staining.20 The significance of these findings remains unclear. Analysis of non-SLNs by immunohistochemistry is not routinely performed at our institution and is done at the discretion of the pathologist. In our study, immunohistochemistry was performed in only 6 of 16 (37.5%) non-SLN specimens from patients with micrometastasis to the SLN. All were negative. Further clinical trials will be required to determine the significance of micrometastatic disease in SLNs and non-SLNs detected solely by cytokeratin immunohistochemistry.
The prognostic significance and management of patients with axillary micrometastasis are still under investigation. Several studies have shown that patients with axillary micrometastasis have a higher disease recurrence and lower overall survival than patients with tumor-free axillary nodes,21–23 whereas other studies demonstrated no such differences.24,25 Long-term follow-up data will be needed to evaluate this issue more thoroughly. In the 5 patients in our series with micrometastasis that did not undergo completion axillary node dissection, none has developed evidence of axillary disease with a mean follow-up of 12 months and a range of 3 to 30 months.
CONCLUSION
Our study demonstrated that the SLN was the only positive axillary node in 64% of our patients. Although univariate analysis identified tumor size > 2 cm, Her2/neu overexpression, ER-negative tumors, and macrometastasis in the SLN as predictive of a positive non-SLN, only SLN metastases > 2 mm was an independent predictor of additional disease in the axilla. Furthermore, only 1 patient with micrometastatic disease in the SLN was found to harbor metastasis in a non-SLN, which is less than the false-negative rate of SLN dissection in our series. This study suggests that completion axillary dissection may not be necessary in women who have micrometastatic disease in the SLN.
Discussions
Dr. Samuel W. Beenken (Birmingham, Alabama): President Richardson, Secretary Townsend, and fellow members of the Association. I wish to thank Dr. Perry and his colleagues for the privilege of reviewing their manuscript and I congratulate them for bringing this timely and important subject to the attention of the Association.
Most surgeons recommend completion axillary dissection when a sentinel lymph node with a patient with breast cancer is found to harbor metastatic disease. However, there are circumstances when further surgery may not be beneficial. The termination of the need for further surgery for any given patient is not a trivial matter since the type and duration of adjuvant chemotherapy or hormone therapy can depend on the extent of metastatic disease. In addition, ongoing clinical trials are determining the efficacy of adjuvant radiation therapy in women with metastases to 1, 2, or 3 axillary lymph nodes. Many of us are participating in the American College of Surgery Oncology Group's prospective multi-institutional trial, which is dealing with some of these issues.
The study presented is retrospective in nature and the authors recognize its inherent limitations. By studying the histopathology of primary breast cancer and associated regional metastases, they have determined that macrometastases are the only reliable predictor of non-sentinel lymph node metastases. Of 194 patients undergoing sentinel lymph node biopsy, 147 underwent subsequent axillary dissection. However, only 48 patients were found to have a regional metastasis after sentinel lymph node dissection, and 6 of these did not undergo completion axillary dissection.
Why were the 105 patients who underwent completion axillary dissection despite sentinel lymph nodes that were free of metastatic disease included in this study? What was the incidence of non-sentinel lymph node metastases in this group?
Since histologic grade is thought to be an important prognostic and predictive biomarker for primary breast cancer, why was it not included as a factor in the univariate analysis presented?
Would the authors recommend different surgical approaches for patients with a 1.8-mm versus a 2.1-mm metastatic focus? Did they consider analyzing the diameter of the metastatic focus as a continuous variable (ANOVA)?
How was a patient assessed during follow-up? Since clinical examination is very unreliable, did the authors consider the use of axillary ultrasonography coupled with fine needle aspiration of enlarged and/or suspicious lymph nodes?
I enjoyed reading this paper and thank the authors for their efforts. Finally, as a new member, I thank the Association for the privilege and honor of membership.
Dr. Patrick C. McGrath (Lexington, Kentucky): I appreciate having the opportunity to discuss this paper by Dr. Perry and his colleagues that addresses an important clinical question, and that is the necessity of performing a complete axillary node dissection in patients found to have only micrometastatic disease in a sentinel lymph node.
Over 25 years ago, the results of the landmark trial, the NSABP B-04 trial, indicated that there was not a survival advantage conferred to patients with clinically negative nodes who underwent a complete axillary node dissection along with their mastectomy as compared to the group that had a total mastectomy alone. Yet our current recommendations continue to be complete axillary node dissection in patients whose sentinel node is positive, whether it be a micro or macrometastases. The current study questions this recommendation.
As you know, there are 2 large randomized trials that will help sort out some of these questions, but the results of these will be several years off. Until then, we rely on single institution reports.
When asked to review this paper, I took the opportunity to look at our experience at the University of Kentucky and found that the numbers were quite similar to those reported by Dr. Perry's group. Of the 358 sentinel node procedures performed, there were 89 patients, or 25%, with positive sentinel lymph nodes. Of those 89 patients, there were 29 cases of micrometastases to the sentinel lymph node. And of those, only 1 patient had a positive non-sentinel lymph node.
These results at our institution reinforce the experience presented by Dr. Perry. So, Dr. Perry, I have 3 questions.
First of all, in your 1 case of metastasis to a non-sentinel node, was there more than one sentinel node in that patient, or was that micrometastases the only sentinel node identified? Also, was the metastasis to the non-sentinel node a micro or macrometastasis?
At our institution, we do not routinely use immunohistochemistry on sentinel nodes because the results do not alter recommendations with regard to adjuvant therapy. I realize you only had 1 case of a hematoxylin and eosin-negative but immunohistochemistry-positive sentinel lymph node. Do your medical oncologists treat those patients differently than if they were node negative?
Finally, the ultimate question is...how will this information you have reported change the management of your patients? If you are in the operating room and the immediate analysis of your sentinel node indicates the presence of metastatic disease and your pathologist says that on frozen section it appears to be a micrometastasis, will you at that time perform a completion axillary node dissection, or will you hold off?
Dr. Murray F. Brennan (New York, New York): Let me offer a constructive suggestion. These kinds of data are perfect for utilizing predictive nomograms, a situation where you take information, as has been done here, and compound that into a nomogram utilizing information that may not be significant.
What that does for the individual patient is to take some of the features that you have, like lymphovascular invasion, and weigh them such that the outcome prediction is improved. Our group at Memorial, led by Patrick Borgen, Kimberly VanZee, and Michael Kattan, have done that, and I have that nomogram on my Palm Pilot. It is not, obviously, confined to these situations that you can utilize the approach for survival analyses. I think you will see these kinds of nomograms used not only for predictive analyses here but as methods that will be more powerful than conventional staging systems. We need help in validating these nomograms, so if you have large data sets, we can offer the nomogram.
Dr. Roger R. Perry (Norfolk, Virginia): Thank you, Dr. Beenken. The majority of patients who underwent axillary dissection who were sentinel node-negative underwent that as part of each surgeon's training set. We are part of a large multi-institutional sentinel node trial.
You are absolutely right about the issue of grade. We in fact did look at grade. We have not shown our data, but we did not find it was a significant predictor in univariate analysis. Your suggestion about looking at the size of metastasis in the sentinel node as a continuous variable is certainly a good one, and we will consider doing that.
Our patients were primarily followed with clinical exam. Your point about clinical exam not being that accurate is a good one. Some of the patients have been followed with ultrasound whenever there was a question on clinical exam. But thus far we have not found any patients requiring ultrasound-guided aspiration of the remaining lymph nodes.
I thank Dr. McGrath for his good questions. The patient with the metastasis to the nonsentinel node, that patient had 1 other sentinel node that was negative in addition to the sentinel node that had the micrometastasis. The question about whether it was micrometastasis in the non-sentinel node or macrometastasis, it was a micro, a very small micrometastasis.
The question about immunohistochemistry is a very good one. Most of our patients have undergone immunohistochemistry as part of a multi-institutional clinical trial. Our medical oncologists tend not to treat patients who are immunohistochemistry positive but histologically negative.
How will this change management if the pathologist tells us in the operating room that you have a micrometastasis in the sentinel node? I would want to make sure that all the sentinel nodes that have been sent have been checked by the pathologist. Because very often when one is positive, we will find that the other nodes are not looked at. So I want to make sure that all of them have been looked at. And ultimately I want to have great confidence in the pathologist before making a decision about whether or not to proceed with axillary dissection. This decision will actually be made a little bit easier for us now because we have joined up with the Z-11 trial, and so these patients will be randomized.
I finally would like to thank Dr. Brennan for his, as usual, very important observations, and I am going to go back and talk with our statisticians about looking at developing predictive normograms with our data. So thank you for those comments.
Footnotes
Reprints: Christine Laronga, MD, Eastern Virginia Medical School, 825 Fairfax Avenue, Suite 610, Norfolk, VA 23507. E-mail: larongaC@evms.edu.
REFERENCES
- 1.Krag D, Weaver D, Alex J, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–339. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Paganelli G, Galimverti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano AE, Jones RC, Brennan M, et al. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345–2350. [DOI] [PubMed] [Google Scholar]
- 4.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer: a multi-center validation study. N Engl J Med. 1998;339:941–946. [DOI] [PubMed] [Google Scholar]
- 5.Borgstein PJ, Pijpers R, Comans EF, et al. Sentinel lymph node biopsy in breast cancer: guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. J Am Coll Surg. 1998;186:275–283. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health Consensus Conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–395.1984541 [Google Scholar]
- 7.Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;17:368–373. [DOI] [PubMed] [Google Scholar]
- 8.Cody H. Sentinel lymph node mapping in breast cancer. Breast Cancer. 1999;6:13–22. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi M. Sentinel lymph node biopsy and breast cancer. Br J Surg. 2002;89:21–34. [DOI] [PubMed] [Google Scholar]
- 10.Liang W, Sickle-Santanello B, Nims T. Is completion axillary dissection indicated for micrometastases in the sentinel lymph node. Am J Surg. 2001;182:365–368. [DOI] [PubMed] [Google Scholar]
- 11.Sachdev U, Murphy K, Derzie A, et al. Predictors of nonsentinel lymph node metastasis in breast cancer patients. Am J Surg. 2202;183:213–217. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer. J Clin Oncol. 1999;17:1720–1726. [DOI] [PubMed] [Google Scholar]
- 13.Viale G, Maiorano E, Mazzarol G, et al. Histologic detection and clinical implications of micrometastases in axillary sentinel lymph nodes for patients with breast carcinoma. Cancer. 2001;92:1378–1384. [DOI] [PubMed] [Google Scholar]
- 14.Carter CL, Allen C, Henson DC. Relation of tumor size, lymph node status and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. [DOI] [PubMed] [Google Scholar]
- 15.Hwang R, Kroshnamurthy S, Hunt K, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10:248–254. [DOI] [PubMed] [Google Scholar]
- 16.Weiser M, Montgomery L, Tan L, et al. Lymphovascular invasion enhances the prediction of non-sentinel node metastases in breast cancer patients with positive sentinel nodes. Ann Surg Oncol. 2001;8:145–149. [DOI] [PubMed] [Google Scholar]
- 17.Den Bakker, Weeszenberg A, de Kanter A, et al. Non-sentinel lymph node involvement in patients with breast cancer and sentinel node micrometastasis: too early to abandon axillary clearance. J Clin Pathol. 2002;55:932–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu K, Turner R, Hansen N, et al. Sentinel node metastasis in patients with breast carcinoma accurately predicts immunohistochemically detectable nonsentinel node metastasis. Ann Surg Oncol. 1999;6:756–761. [DOI] [PubMed] [Google Scholar]
- 19.Chu K, Turner R, Hansen N, et al. So all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection? Ann Surg. 1999;229:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida M, Kitamura K, Kinoshita J, et al. Detection of micrometastasis in the sentinel lymph nodes in breast cancer. Surgery. 2002;131:211–216. [DOI] [PubMed] [Google Scholar]
- 21.Clare S, Sener S, Wilkens W, et al. Prognostic significance of occult lymph node metastases in node-negative breast cancer. Ann Surg Oncol. 1997;4:447–451. [DOI] [PubMed] [Google Scholar]
- 22.de Mascarel I, Bonichon G, Coindre J, et al. Prognostic significance of breast cancer axillary lymph node micrometastases assessed by two special techniques: reevaluation with longer follow-up. Br J Cancer. 1992;66:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hainsworth P, Tjandra J, Stillwell R, et al. Detection and significance of occult metastases in node-negative breast cancer. Br J Surg. 1993;80:459–463. [DOI] [PubMed] [Google Scholar]
- 24.Nasser I, Lee A, Bosari S, et al. Occult axillary lymph node metastases in node-negative breast carcinoma. Hum Pathol. 1993;24:950–957. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgibbons P, Page D, Weaver D, et al. Prognostic factors in breast cancer: College of American Pathologists Consensus statement 1999. Arch Pathol Lab Med. 2000;124:966–978. [DOI] [PubMed] [Google Scholar]


