Abstract
Objective:
Cytokines may be a mechanism by which surgical delay can increase flap survival. We previously found that preoperative vascular endothelium growth factor (VEGF) administration in the rat transverse rectus abdominis myocutaneous (TRAM) flap could improve skin paddle survival. In this study, we used partial elevation of the rat TRAM flap as a surgical delay to assess endogenous cytokine expression and tissue survival comparable to undelayed TRAM flaps.
Methods:
In Part I, TRAM flaps underwent surgical delay procedures; 7 days later, the flaps were completely elevated and reinset. At the same time, other flaps were raised and reinset without delay. Skin paddle survival in both groups was evaluated at 7 days. In Part II, skin biopsies from TRAM zones I to IV were taken at the time of delay and at intervals of 12, 24, 48, and 72 hours. Specimens were assessed for selected cytokine gene expression by reverse transcription-polymerase chain reaction analysis (TR-PCR).
Results:
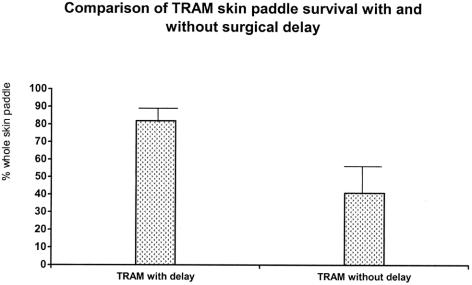
Surgical delay significantly (P < 0.001) increased skin paddle survival in the delayed TRAM flaps (16.14 ± 1.53 cm, 81.9%) compared with undelayed flaps (7.68 ± 3.16 cm, 40.9%). TGF-β and PDGF expressions were not changed by surgical delay, but basic fibroblast growth factor (bFGF) and VEGF expressions increased significantly (P < 0.05 and P < 0.01) after delay.
Conclusions:
In the rat TRAM model, surgical delay resulted in increased VEGF expression and increased skin paddle survival. These results correlate with previous studies showing the preoperative injection of VEGF increases skin paddle survival. VEGF may be an important element in the delay phenomenon and may be an agent for pharmacological delay.
Surgical delay is a clinical strategy that can improve flap survival by prior partial flap elevation. Cytokine up-regulation may play roles in initiating vasodilation and angiogenesis in the delay phenomenon. In a rat myocutaneous flap model, surgical delay was associated with increased expression of vascular endothelium growth factor (VEGF) and increased flap survival. Cytokine manipulation may offer routes of pharmacological delay.
Necrosis of skin flaps, either partial or complete, has been a complication of reconstructive surgery for centuries and has stimulated research throughout the history of plastic surgery. The study of flap necrosis has led investigators into studies of anatomy, physiology, and surgical technique, all of which have profoundly changed flap surgery.1,2
The technique of surgical delay has been used for centuries as a strategy for making the survival of flap tissue more reliable. Briefly stated, surgical delay consists of partially elevating and undermining a cutaneous flap at some time interval (often 7 to 14 days) prior to full elevation and inset of the flap. As early as the sixteenth century, surgeons recognized that this maneuver could convert flap designs from unpredictable amounts of necrosis to reliable survival.3–6
The use of flap delay has decreased considerably over the last 50 years as flap designs have become more refined and anatomically based and as improved surgical techniques (including microsurgery) have led to more reliably successful dissection and handling of critical structures. The delay technique, however, remains of some value in procedures frequently complicated by unpredictable cutaneous necrosis such as the transverse rectus abdominis myocutaneous (TRAM) flap in breast reconstruction.6
The mechanisms of the delay phenomenon, however, continue to challenge investigators. The delay phenomenon has always held the potential for secrets that could lead to pharmacological or biochemical strategies to manipulate vascular territories. Investigators have attempted to define adrenergic responses, angiogenesis, vascular reorganization, and changes in tissue metabolism as factors underlying the delay phenomenon.7–14 Identification of a critical factor in the delay phenomenon could lead to use of the factor to produce the phenomenon without performing a surgical procedure. Such a so-called “pharmacological delay” has never been reliably demonstrated.6
Cytokines are small proteins that mediate many of the processes of inflammation, scarring, and blood vessel formation.15,16 Cytokines have opened up new dimensions in the investigation of tissue responses and healing. Transforming growth factor (TGF) in its multiple isoforms, fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF) play critical roles in many phases of cell activation and differentiation.17–19 Vascular endothelium growth factor (VEGF) affects microcirculation acutely by change in vascular permeability and in a sustained manner by promoting angiogenesis.20–23 Improved flap survival in many models has been observed following manipulation of TGF isoforms, basic fibroblast growth factor (bFGF), PDGF, and VEGF.24–33 We have conducted this series of experiments in a rat TRAM model to identify cytokine changes during surgical delay. We have previously described VEGF as a means of extending the skin paddle survival of the rat TRAM.34 The experiments in this report specifically attempt to define a role of VEGF in the delay phenomenon.
MATERIALS AND METHODS
Fifty male Sprague-Dawley rats weighing between 380 to 420 g were used in this study. The National Research Council’s guidelines for the care and use of laboratory animals were followed. The rats were anesthetized using pentobarbital administered by intraperitoneal injection (50 mg/kg).
Delay TRAM Flap Model, a model of the caudally based TRAM flap, based on the right abdominis rectus muscle as carrier and inferior epigastric vessels as vascular pedicle, was used in the study.35 Following induction of general anesthesia, the abdominal regions were shaved, and a rectangle measuring 3 × 8 cm was drawn onto the upper abdominal area of the rats. The borders of the skin paddle of the proposed TRAM were cut down to deep fascia. The branches of both superficial epigastric vessels joining the flap were ligated and cut (Fig. 1). Both rectus abdominis muscles were divided at the superior border of the skin paddle. The superior deep epigastric vessels of both rectus abdominis muscles were divided. The skin incision was then closed using 4-0 nylon sutures. The study was divided into 3 parts.
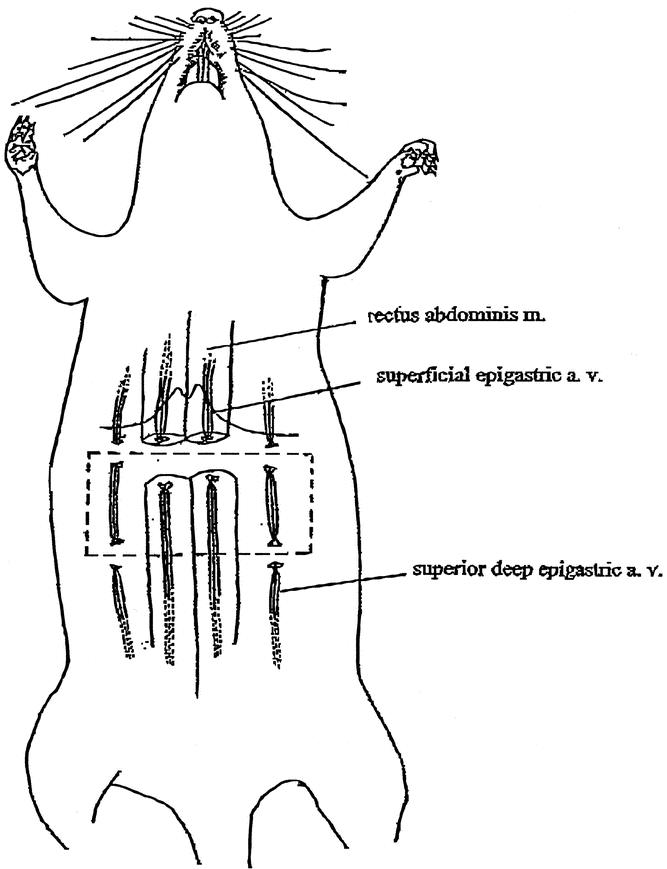
FIGURE 1. Surgical delay of the TRAM flap in the rat model.
Effect of Surgical Delay on Flap Survival
Twenty rats were divided into 2 groups. In the experimental group (n = 10), the flaps underwent delay procedures as described above. One week later, using the same skin incision, the right caudally based TRAM flap, based on the right rectus abdominis muscle as a carrier, was elevated (Fig. 2). In this flap model, the right side of the flap is an axial flap and the left a random flap based on subcutaneous vessels crossing the midline from the axial portion. The skin paddle can be divided into zones I, II, III, and IV on the basis of the design of human TRAM flap. A silastic sheet the size as the skin paddle was placed under the skin paddle. The flap was then sutured to its original bed. In the control group, the TRAM flap was elevated and replaced without the delay procedures.
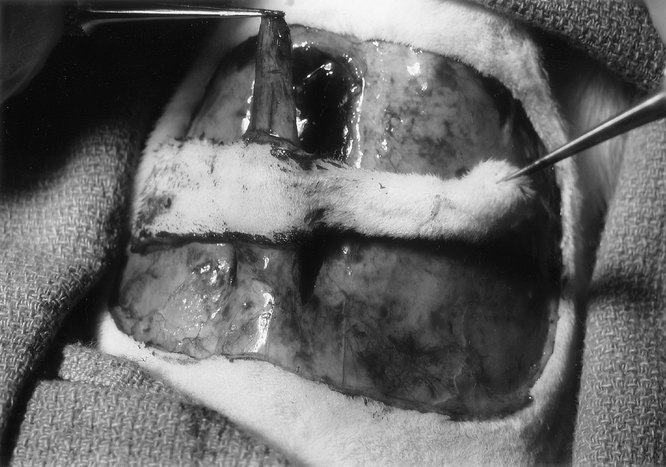
FIGURE 2. The right caudally based TRAM flap based on the right rectus abdominis muscle as a carrier.
The animals were returned to their individual cages for a period of 7 days, after which they were reanesthetized as previously described. The survival area of the skin paddle of each flap was grossly determined on the basis of its appearance, color, and texture. It was then marked on the template. With the aid of an M2 Image Analysis System (Imaging Research Inc., St. Catharines, Ontario), the template was photographed by a high-resolution video camera and scanned on a computer. The survival area of the skin paddle was measured as a percentage of the total skin paddle and calculated by the M2 software.
Gene Expression
Thirty rats underwent TRAM flap delay procedures. After the flaps were sutured, the animals were divided into 5 groups (n = 6 for each group) according to the time of biopsies: immediately and 12, 24, 48, and 72 hours postoperatively. Two full-thickness 6-mm punch biopsies were taken from the TRAM flap at the center of the skin paddle from zones I, II, III, and IV, respectively. Expression of TGF-β, bFGF, PDGF, and VEGF mRNAs from tissue samples of each group at various time intervals was tested by reverse transcription-polymerase chain reaction assay (RT-PCR).
RT-PCR
The biopsied tissues were homogenized. Total RNA was isolated from the skin using Trizol reagent (GIBCO BRL, Gaithersburg, MD). The extracted RNA was reverse transcribed into cDNA. The reaction was performed in a final volume of 25 μL, consisting of 2.5 μL of 10 PCR buffer, 1.5 μL of 25 mM MgCl2, 2-μL mixture of 1 mM dNTP, 0.125 μL of TaqDNA polymerase (5 U/μL), 1.5 μL of each primer 50 μM, 1 μL of cDNA. The typical PCR reaction conditions were 2 minutes at 94°C, 35 cycles at 94°C for 50 seconds, 60°C for 50 seconds, and 72°C for 50 seconds, and after the cycles at 72°C for 10 minutes and then remaining at 4°C. The primers were obtained commercially (The Midland Certified Reagent Company, Midland, TX). PCR products were subjected to 10% polyacrylamide gel electrophoresis. The gel with the separated DNA bands was photographed and scanned. To ensure that equal amounts of reverse-transcribed cDNA were applied to the PCR reaction, the primer pairs for β-actin, a constitutively expressed gene, were also included in the PCR reaction. Quantitative gene expression was expressed as fraction of growth factors to β-actin.
Histology
Tissue samples were placed in 10% formalin and sectioned with hematoxylin-eosin staining for histologic analysis.
After flap survival examination and tissue biopsies, the animals were killed by an overdose of pentobarbital. The data on flap survival from different groups were compared using t test. The data on gene expression from different locations and intervals were compared using one-way analysis of variance (ANOVA) for multiple testing. Statistical significance was assumed at P < 0.05.
RESULTS
Flap Survival With Surgical Delay
At postoperative day 7, the regions of survival and necrosis on the TRAM skin paddle were clearly demarcated in every flap. The surviving skin appeared pink-white, tender, and normal in its texture. In contrast, the necrotic skin was black, rigid, and did not bleed when cut. In Part I, the mean and standard deviation of the viable area of the skin paddles with surgical delay was 16.14 ± 1.53 cm2 (81.9% of whole skin paddle at the time of measurement). The mean and standard deviation of the viable area in the control group was 7.68 ± 3.16 cm2 (40.9%). The difference in the mean percentage survival area between the 2 groups is statistical significant (P < 0.001). A comparison of the mean percentage of the surviving area between the 2 groups is shown in Figure 3.
FIGURE 3. Comparison of the surviving area between the skin paddles of TRAM flaps with and without surgical delay.
Gene Expression
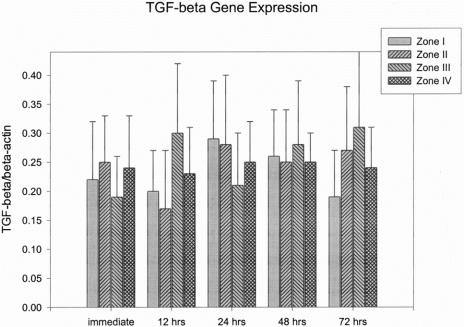
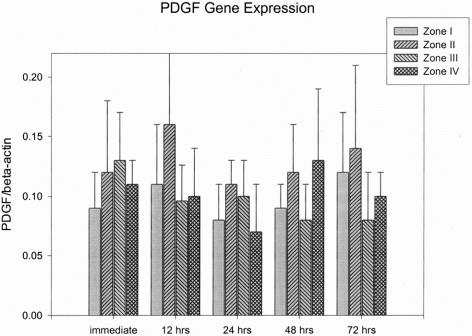
Gene expressions were expressed as a fraction of growth factors to β-actin (mean ± SEM). TGF-β, bFGF, PDGF, and VEGF mRNA expressions were all detected from the skin paddle that underwent surgical delay procedures. The values of TGF-β and PDGF mRNA expressions from skin paddles did not show statistically significant differences between different zones and time intervals after surgical delay (Fig. 4 and 5).
FIGURE 4. Results of TGF-β gene expression.
FIGURE 5. Results of PDGF gene expression.
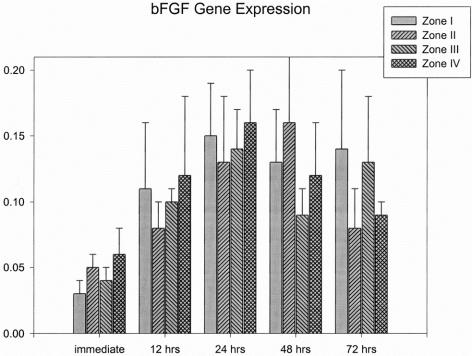
bFGF expression from the skin paddles were slightly increased after the flap underwent surgical delay. At 24 hours postoperatively, the values of bFGF expressions ranged from 0.13 ± 0.05 to 0.16 ± 0.04 from zone I to IV, which were significantly higher than in immediately biopsied tissues (0.03 ± 0.01 to 0.06 ± 0.02; P < 0.05). However, there were no significant differences in the bFGF expression among the 4 zones at each time interval after surgical delay. The bFGF expressions from different parts of the flap and time interval are shown in Figure 6.
FIGURE 6. Results of bFGF gene expression.
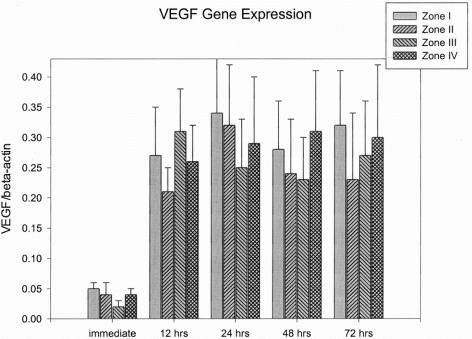
The VEGF expressions in the immediately biopsied tissues (without delay) ranged from 0.02 ± 0.01 to 0.05 ± 0.01. When the skin paddle underwent the surgical delay procedure, VEGF expressions were significantly increased (0.21 ± 0.04 to 0.34 ± 0.09) compared with the VEGF expressions in the skin samples without surgical delay (P < 0.01). There were also no significant differences in the VEGF expressions among the 4 zones at each time interval after surgical delay. The VEGF expressions remained elevated until 72 hours after delay. The VEGF expressions from different parts of the flap and time intervals are shown in Figure 7.
FIGURE 7. Results of VEGF gene expression.
Histology
Skin biopsies were taken from different zones of the skin paddle. No evidence of neovascularization was observed from the tissue biopsied at 12 and 24 hours after surgical delay. At 48 and 72 hours after delay, sections taken from zones III and IV of the skin paddle revealed an exaggerated amount of capillaries in the dermal and subdermal layers (Fig. 8). However, a section taken from zones I and II of the skin paddle did not show an increased in angiogenesis.
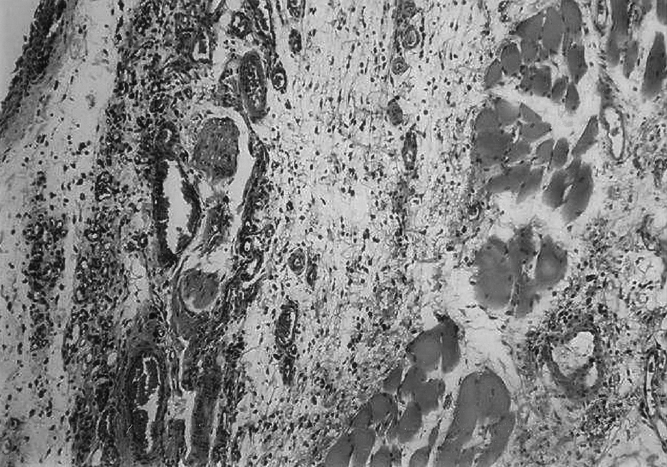
FIGURE 8. An exaggerated amount of capillaries in the dermal and subdermal layers of zones III and IV was observed in the skin paddle undergoing surgical delay.
DISCUSSION
Surgical delay is clinically used most frequently today in TRAM flap procedures and head and neck flap cases.36–41 Despite its use as a reliable method for preventing flap necrosis, the mechanisms by which surgical delay acts to improve survival are poorly understood. “Sublethal ischemia” is considered the initiator of the delay phenomenon. Insights into the mechanisms of surgical delay could help us to develop pharmacological manipulations to augment skin flap survival and forego surgical delay procedure.
In this study, we used a rat TRAM flap model in which the unilateral rectus abdominis muscle was used as a carrier and inferior epigastric vessels as a vascular pedicle. The average survival area of the skin paddles was significantly improved when the flap underwent the delay procedure compared with the flap without surgical delay. This result confirms reports by Ozgentas et al of surgical delay in rat TRAM flaps,35 and it provides a clinically analogous model to study the surgical delay phenomenon in the skin paddle of a myocutaneous flap.
The hypotheses that have been proposed to explain the mechanisms of the delay phenomenon include closing of the arteriovenous shunts,8 conditioning of tissues to survive hypoxia due to ischemia,7 and improving blood flow to the flap by vasodilatation of pre-existing vessels.11–13 These hypotheses were based on the findings of circulating catecholamines at the end of the delay period, which cause closure of the A-V shunts,8,14 arachidonic acid metabolites that play important roles in the progressive ischemia of the flap and inflammation,10,42 and sympathectomy and denervation hypersensitivity during delay procedure,8,14,43 which cause vasodilatation. However, the factors that mediate these metabolic changes from ischemia and surgical manipulation in the delay phenomenon remain undescribed.
Cytokines, which are small proteins or glycoproteins secreted to influence the biologic activities of responsive cells and mediators of the inflammatory and traumatic responses have been extensively studied. Administration of exogenous growth factors has been demonstrated to improve the viability of ischemic skin flaps in many models.24–29 Investigation in molecular markers in flap physiology may offer possibilities for a better understanding of the role of cytokines in enhancing flap survival.
In this study, we found that there were no significant change in TGF-β and PDGF mRNA expressions in the surgically delayed flaps. However, bFGF and VEGF expressions were found to be significantly increased in the tissue with surgical delay. This observation indicates that some growth factors, especially VEGF, may play an important role in mediating the surgical delay phenomenon. The up-regulation of VEGF may be induced by partial ischemia caused from surgical delay procedure.44
VEGF is a heparin-binding glycoprotein. This cytokine and its receptor system have been shown to be the fundamental regulator in cell signaling of angiogenesis.20–22 bFGF also has a strong affinity for the glycosaminoglycan heparan sulfate and is a potent mitogen for proliferation of capillary endothelial cells.18,45 Both VEGF and bFGF action in angiogenesis and flap survival and have been well described in the literature.26,27,30–33
Whether augmentation of blood flow in the delay phenomenon is attributable to angiogenesis remains controversial. Pang et al studied the density of arteries in a pig random skin flap within 2 to 3 days of surgical delay and found no significant neovascularization.13 They concluded that the delay phenomenon does not depend on angiogenesis. They believed that the local release of neurohumoral substances, which cause vasodilatation, are responsible for flap survival.13 In contrast, Ortega’s group found significant signs of angiogenesis in the skin within 3 to 7 days of surgical delay in a rabbit skin flap model using an immunohistochemical technique.46,47 In the current study, we histologically demonstrated neovascularization in the skin paddle in the late period of surgical delay. This angiogenesis could be triggered by an up-regulation of VEGF and bFGF. The microcirculatory changes in a surgical delay procedure may also be attributed to the vasodilatation action of VEGF, which has been demonstrated to cause vasodilatation partly through stimulation of nitric oxide synthase in endothelial cells.48
The use of growth factors to induce a delay phenomenon has been reported. Im et al demonstrated that intradermal administration of bFGF to pedicle skin flaps before flap elevation produced an increase in viability that approximates the increase obtained by the surgical delay procedure.49 By using an arterial skin flap model on the mouse ear, Uhl et al also reported that an appropriate dose of bFGF injected 5 to 6 days before flap creation resulted in a significant reduction of nonperfused tissue postoperatively in an arterial skin flap model on the mouse ear.50
We have previously demonstrated that preoperative treatment using exogenous VEGF significantly increased the area of skin paddle survival in the rat TRAM flap.34 VEGF was injected into the skin paddle of the TRAM flap, and the flap was elevated 7 days later. The skin paddle survival percentage was 74.4%, which was significantly higher than acute VEGF injection (25.1%) or preoperative saline injection (28.4%). The skin paddle survival with preoperative VEGF administration was equivalent to surgical delay (81.9%) in the current study. Angiogenic effect by exogenous VEGF may be the mechanism resulting in enhancement of vascular communication between different zones of the skin paddle and the improvement of the flap survival.
In conclusion, surgical delay can significantly improve survival of the skin paddle of a rat TRAM flap. VEGF and bFGF were found to be up-regulated in the skin paddle with surgical delay, and these cytokines may play an important role in initiating vasodilation and angiogenesis in the delay phenomenon. Preoperative subcutaneous injections of VEGF have previously been shown to increase skin paddle survival in this model. These studies add up to identifying VEGF as an important element in the delay phenomenon and a potential agent of pharmacological delay.
Discussions
Dr. Maurice J. Jurkiewicz (Atlanta, Georgia): At the 33rd Annual Meeting of this Association in 1920, Vilray Papin Blair of St. Louis and Washington University presented a paper in this venue, The Homestead, entitled “The Delayed Transfer of Pedicle Flaps in Plastic Surgery.” To my knowledge, this was the first time the term “flap delay” appeared in the surgical literature. Subsequently, the term, as Bill Lineaweaver has pointed out, has had a life of its own, and there is a whole body of literature on the various suppositions, theories, hypotheses of how the delay phenomenon works.
Now, 83 years later in this same venue comes another paper on this surgical delay. The only other paper that even remotely relates to the delay phenomenon was a paper by Bert Myers of Tulane back in the 1970s. He discussed the use of intraoperative intravenous fluorescein to identify impending necrosis in skin flaps in radical mastectomy. And he used fluorescein to demonstrate. Now, these are clearly related, but the second one is only remotely related. This hiatus then of 83 years seems to me to be a bit long for an important subject like this.
Bill Lineaweaver was kind enough to send me a copy of their paper. As he stated, the authors have previously found that preoperative administration of VEGF improved survival in this model. This present study defines the endogenous cytokine expression and survival in the delay of the TRAM flap. VEGF becomes the dominant player, and the minor player is bFGF.
Now, the rat model that he described in the paper is a rectangular flap, and the TRAM flap that we know is used in breast reconstruction is actually an elliptical flap that goes well out into the flank. In the actual presentation, the rectangular flap does go out in the flank, but there are some differences in this model as opposed to the human model. Moreover, he pointed out that it is caudally based rather than superiorly based.
At 48 and 72 hours after delay, biopsies from zone III and IV, which are out in the periphery, disclosed vigorous angiogenesis. However, zones I and II did not, which suggests to me that the robust blood supply from the inferior epigastric just masks that, so that there is no true ischemia stimulus there for increased angiogenesis.
Now, as you all know, acute ischemic preconditioning has become extensively studied in 2 organ systems, the myocardium and also the liver. I have long had an interest in getting our unit at Emory interested in acute ischemic preconditioning, and they have not yet responded. I guess I have lost my influence.
So my question: What about acute ischemic preconditioning of the TRAM flap and prior injection of VEGF? Another way of asking this is: What are the next steps in your pursuit of pharmacologic delay?
I want to congratulate Bill Lineaweaver. He and I have a history going back almost 50 years. He was a high school student in Gainesville when I left Barnes Hospital and went to Gainesville. At that time he was a merit scholar. We hired him in our laboratory, and I guess he was unduly influenced to become a plastic surgeon at that time. Dr. Lineaweaver, thanks very much for bringing this very significant material to the attention of the members of this Association. I think it goes a long way in unraveling the delay phenomenon and may help us a great deal not only in skin flap delay but in other organ systems.
Dr. Leonard T. Furlow, Jr. (Gainesville, Florida): I want to thank you for the opportunity to discuss Dr. Lineaweaver’s paper. When he asked me to discuss what I interpreted as a paper on VEGF, I knew that he had published other papers on VEGF, and that I knew nothing about growth factors. I read a review article on them and was even more confused, so I wrote Dr. Lineaweaver back and said, “There has got to be somebody else in the Southern Surgical who knows a whole lot more about VEGF than I do, so I don’t think I ought to discuss it.” He wrote and said, “Okay, that’s all right.” But he sent me a copy of the manuscript. I brought it with me but didn’t read it until we got here. With Dr. Jurkiewicz’s gentle help, I realized that this is maybe more a paper on the delay phenomenon than it is on VEGF.
Now, I do know something about the delay phenomenon, because for the first 10 years that I was involved in plastic surgery we were in a major way interested in and using tube pedicles, worrying about length-width ratios, and flap delay was our main weapon against producing a flap which became “black flag dead.”
But the very interesting discussions and investigations into the phenomenon of flap delay sort of trailed off without resolution, because in the early 1970s the entire practice of plastic surgery was changed when John McCraw, a member of this organization, introduced the myocutaneous flap, and then axial flaps and microvascular-free flaps came in, and we properly turned our attention to designing flaps based on their vascular anatomy instead of their geometric shape.
The young plastic surgeon today likely has never seen a tube pedicle flap; if you mention delay to him, he is liable to think that this is what the insurance company does with his applications for payment of his fees.
But the flap delay obviously is still a very intriguing and unexplained phenomenon. I think that the previous paper from Dr. Lineaweaver’s group and this paper have shown that injected VEGF begets delay and that surgical delay begets VEGF. My first question is, how does the surgical delay call forth the VEGF? How else can we get the tissues of the flap to produce their own VEGF supplies so that we don’t have to inject the material?
Is ischemia responsible for calling forth the VEGF? If so, why was the VEGF distributed the same in both sides of the flap all the way across? If ischemia were responsible, you would think that there would be more VEGF produced on the left side that has been converted to a random flap attached to the side that is still an axial pedicle flap based on the inferior epigastrics of the right side of the flap.
In the manuscript, Dr. Lineaweaver describes that the neovascularization that they saw occurred on the random side of the flap—if I have interpreted this correctly—zones III and IV. Was this due to ischemia? Was the neovascularization a response to ischemia and the presence of the VEGF? Or was it a response to the reorientation of the blood supply, which used to come from the rectus pedicle upward into subcutaneous tissue but now has to come across the midline superficially in the subcutaneous or fascial plane into the other side of the flap, reversing the blood flow through those vessels? Is that perhaps responsible for the neovascularization?
If ischemia is responsible, would daily temporary clamping of the pedicles before they were divided improve the flap survival further, perhaps to 100%? Or maybe you could do that more easily by injecting epinephrine into the pedicles to induce vasospasm. If ischemia is responsible, is VEGF produced in an upper extremity that is subjected to 2 hours of tourniquet time without an operation or to daily repetitive episodes of tourniquet time? Or is trauma responsible for the production of the VEGF? If you took the area that you wanted to make into a flap and beat it like Lionel Hampton playing his vibraphone, to the point of edema, would VEGF be produced without ischemia?
These are all questions that I am not sure Dr. Lineaweaver can answer right now, but I hope that his future endeavors will enlighten us further on this intriguing and important subject.
Dr. John I. Hollenbeck (Charlotte, North Carolina): I would like to ask Dr. Lineaweaver, my former medical student of many years back, 1 question. Sometimes in the trauma venue, especially with an exposed vascular injury, an acute flap is needed to cover that. Would it be of any value to use VEGF in that patient?
Dr. Yuman Fong (New York, New York): I very much enjoyed the paper. Two quick questions. First, have you performed blockade studies using various antibodies to see which of the growth factors or which combinations of growth factors are most important? The second is a practical question. When you move into the clinics with administration of VEGF or other growth factors, many of the patients will be having flap reconstructions for major cancers. What safeguards and what biologic intermediate end points are going to put in order to look at tumor growth during growth factor administration?
Dr. Blair A. Jobe (Portland, Oregon): I very much enjoyed your presentation. We have recently applied the delay phenomenon to the GI tract in an attempt to improve anastomotic healing after esophageal resection in an opossum model. My question to you is, did you measure blood flow to the pedicle? And as a corollary to that, what role do you think venous drainage has on this effect?
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): Help us put this in physiologic perspective. Did the histologic evidence of angiogenesis correlate with tissue oxygen levels? Secondly, ischemia will evoke a heat shock protein response. Did the tissue loss correlate with heat shock protein levels?
Dr. William C. Lineaweaver (Jackson, Mississippi): In response to Dr. Jurkiewicz’s comments, the model itself acts a lot like the clinical TRAM in terms of its zones of necrosis. It is probably the only true myocutaneous flap in the rat and has demonstrated perforators. We have gotten a lot of mileage out of this as a reasonable analogue.
Does VEGF work in acute ischemic preconditioning? This question is a really excellent one. The studies of VEGF have to struggle with the reality that VEGF has an acute and a sustained effect. The acute effect appears to be one of increased capillary permeability and perhaps vasodilation and the sustained one is the development of new vessels. In certain conditions such as cardiac surgery and major trauma, the use of VEGF has had massive complications because of tissue edema, especially in areas of administration. So there are phasic events in the use of VEGF that are just being sorted out but are very much a reality in attempting to use this compound. VEGF does not appear to underlie ischemic preconditioning in any simple way.
Moving on to Dr. Furlow’s comments, one of the structural pleasures of this paper was bringing new analyses to a classic problem. VEGF appears to be stimulated both by ischemia and by direct trauma. So the trauma probably added to the overall effect that we saw VEGF distributed across the flap, not just in the ischemic areas. In neovascularization in the areas of 3 and 4, in the most ischemic areas, we have not quantified this observation. We are just peeking at some histologic slides. But it does seem to be more pronounced in the most ischemic areas.
Would intermittent clamping be more effective? In effect this is another way of asking about the preconditioning question. I don’t know if VEGF is a substitute for preconditioning. Preconditioning seems to be relatively harmless, and we see the situation every time we do a hand case with the tourniquet and release it. We do not have major problems with that situation, whereas simply administering VEGF in localized trauma areas usually results in tremendous edema. So there seems to be some difference between preconditioning and VEGF effect per se.
Would flap abuse do the same thing? There was an old technique of actually hitting the donor site for a skin graft to try to make the graft pink up a little better. I would have to develop an entire new protocol for this. It would be very hard to hit rats, they are very small, and the IRB I think might give us trouble.
Dr. Hollenbeck, again the use of VEGF in acute trauma would be deleterious. At least in the cardiac data, VEGF seems to generate a lot of edema in immediate tissue injury.
Dr. Jobe’s questions. Using the delay phenomenon in GI cases is an extremely interesting area to look at. We have not done blood flow measurements in these studies. We do have a laser Doppler in the lab, but we simply haven’t applied it to these particular studies. In most flap models, and certainly in the rat TRAM and probably in the human TRAM, venous drainage seems to be an absolutely critical element; at least in this particular rat TRAM, we showed that of all the different things you can do to the circulation, including arterial ligation over periods of time, adequate venous drainage gave us our best survival. I think that happens in a lot of flaps.
Whether or not VEGF predominantly neovascularizes venous channels is a particularly interesting thing to look at.
Dr. Pruitt, we have not measured tissue O2, and we have not measured heat shock protein. What we are looking at with VEGF, I think, is 1 dot in a large cascade that is kind of vaguely analogous to the clotting factor inflammatory cascade, and factor 1 through 13 is at some point going to be factor 1 through 13,000 in terms of the way we are looking at inflammatory response. We hope to start connecting the VEGF dots to other dots. The biologic activity of VEGF is about 10 or 15 minutes. So whatever happens when we inject VEGF, it is not the VEGF acting directly, especially when looking a week or more down the line. What we need to understand about VEGF is how it interacts with the other inflammatory cascades.
Footnotes
Reprints: William C. Lineaweaver, MD, Division of Plastic Surgery, The University of Mississippi Medical Center, 2500 North State Street, Jackson, MS. E-mail: wlineaweaver@surgery.umsmed.edu.
REFERENCES
- 1.Myers MB, Cherry G. Causes of necrosis in pedicle flaps. Plast Reconstr Surg. 1968;42:43–50. [PubMed] [Google Scholar]
- 2.Kerrigan CL. Skin flap failure: pathophysiology. Plast Reconstr Surg. 1983;72:766–777. [DOI] [PubMed] [Google Scholar]
- 3.Haughey BH, Panje WR. Extension of the musculocutaneous flap by surgical delay. Arch Otolaryngol. 1985;111:234–240. [DOI] [PubMed] [Google Scholar]
- 4.Taylor GI, Corlett RJ, Caddy CM, et al. An anatomical review of the delay phenomenon, II: clinical applications. Plast Reconstr Surg. 1992;89:408–416. [PubMed] [Google Scholar]
- 5.Attinger CE, Picken CA, Troost TR, et al. Minimizing pectoralis myocutaneous flap loss with the delay principle. Otolaryngol Head Neck Surg. 1996;114:148–157. [DOI] [PubMed] [Google Scholar]
- 6.Smith J, Pribaz JJ. Flaps. In: Achauer B, Eriksson E (eds.): Plastic Surgery. St. Louis: Mosby; 2000:285–287. [Google Scholar]
- 7.McFarlane RM, Heagy FC, Radin S, et al. A study of the delay phenomenon in experimental pedicle flaps. Plast Reconstr Surg. 1965;35:245–262. [DOI] [PubMed] [Google Scholar]
- 8.Reinisch JF. The pathophysiology of skin flap circulation. The delay phenomenon. Plast Reconstr Surg. 1974;54:585–598. [DOI] [PubMed] [Google Scholar]
- 9.Cutting CB, Bardach J, Finseth F. Haemodynamics of the delayed skin flap: a total blood-flow study. Br J Plast Surg. 1981;34:133–135. [DOI] [PubMed] [Google Scholar]
- 10.Murphy RC, Lawrence WT, Robson MC, et al. Surgical delay and arachidonic acid metabolites: evidence for an inflammatory mechanism: an experimental study in rats. Br J Plast Surg. 1985;38:272–277. [DOI] [PubMed] [Google Scholar]
- 11.Hendel PM, Lilien DL, Buncke HJ. A study of the pharmacologic control of blood flow to delayed skin flaps using xenon washout. Part II. Plast Reconstr Surg. 1983;71:399–407. [DOI] [PubMed] [Google Scholar]
- 12.Barker JH, Frank J, Bidiwala SB, et al. An animal model to study microcirculatory changes associated with vascular delay. Br J Plast Surg. 1999;52:133–142. [DOI] [PubMed] [Google Scholar]
- 13.Pang CY, Forrest CR, Neligan PC, et al. Augmentation of blood flow in delayed random skin flaps in the pig: effect of length of delay period and angiogenesis. Plast Reconstr Surg. 1986;78:68–74. [DOI] [PubMed] [Google Scholar]
- 14.Pearl RM. A unifying theory of the delay phenomenon–recovery from the hyperadrenergic state. Ann Plast Surg. 1981;7:102–112. [DOI] [PubMed] [Google Scholar]
- 15.Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamic s of chronic cutaneous wounds. Am J Surg. 1998;176:26S–38S. [DOI] [PubMed] [Google Scholar]
- 16.Arnold F, West DC. Angiogenesis in wound healing. Pharmacol Ther. 1991;52:407–422. [DOI] [PubMed] [Google Scholar]
- 17.Burt DW. Evolutionary grouping of the transforming growth factor-beta superfamily. Biochem Biophys Res Commun. 1992;184:590–595. [DOI] [PubMed] [Google Scholar]
- 18.Rifkin DB, Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989;109:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Moon A, Kim HR. Both platelet-derived growth factor receptor (PDGFR)-alpha and PDGFR-beta promote murine fibroblast cell migration. Biochem Biophys Res Commun. 2001;282:697–700. [DOI] [PubMed] [Google Scholar]
- 20.Flamme I, von Reutern M, Drexler HC, et al. Overexpression of vascular endothelial growth factor in the avian embryo induces hypervascularization and increased vascular permeability without alterations of embryonic pattern formation. Dev Biol. 1995;171:399–414. [DOI] [PubMed] [Google Scholar]
- 21.Hippenstiel S, Krull M, Ikemann A, et al. VEGF induces hyperpermeability by a direct action on endothelial cells. Am J Physiol. 1998;274:L678–84. [DOI] [PubMed] [Google Scholar]
- 22.Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem. 1999;274:31047–1054. [DOI] [PubMed] [Google Scholar]
- 23.Lineaweaver W, Zhang F. Vascular endothelial growth factor and the biological bases of reconstructive surgery. Proc. II Congress, World Society of Reconstructive Microsurgery. Bologna: Monduzzi Editore; 2003:69–71. [Google Scholar]
- 24.Nall AV, Brownlee RE, Colvin CP, et al. Transforming growth factor beta 1 improves wound healing and random flap survival in normal and irradiated rats. Arch Otolaryngol Head Neck Surg. 1996;122:171–177. [DOI] [PubMed] [Google Scholar]
- 25.Iwasawa M. Accelerated maturation in prefabricated flaps by transforming growth factor-beta: an experimental study in the rabbit. Ann Plast Surg. 1993;31:72–75. [PubMed] [Google Scholar]
- 26.Quirinia A, Viidik A. The effect of recombinant basic fibroblast growth factor (bFGF) in fibrin adhesive vehicle on the healing of ischaemic and normal incisional skin wounds. Scand J Plast Reconstr Surg Hand Surg. 1998;32:9–18. [DOI] [PubMed] [Google Scholar]
- 27.Carroll SM, Carroll CM, Stremel RW, et al. Vascular delay and administration of basic fibroblast growth factor augment latissimus dorsi muscle flap perfusion and function. Plast Reconstr Surg. 2000;105:964–971. [DOI] [PubMed] [Google Scholar]
- 28.Carroll CM, Carroll SM, Schuschke DA, et al. Augmentation of skeletal muscle flap survival using platelet derived growth factor. Plast Reconstr Surg. 1998;102:407–415. [DOI] [PubMed] [Google Scholar]
- 29.Ishiguro N, Yabe Y, Shimizu T, et al. Basic fibroblast growth factor has a beneficial effect on the viability of random skin flaps in rats. Ann Plast Surg. 1994;32:356–360. [DOI] [PubMed] [Google Scholar]
- 30.Kryger Z, Zhang F, Dogan T, et al. The effects of VEGF on survival of a random flap in the rat: examination of various routes of administration. Br J Plast Surg. 2000;53:234–239. [DOI] [PubMed] [Google Scholar]
- 31.Padubidri A, Browne E. Effect of vascular endothelial growth factor (VEGF) on survival of random extension of axial pattern skin flaps in the rat. Ann Plast Surg. 1996;37:604–611. [DOI] [PubMed] [Google Scholar]
- 32.Li QF, Reis ED, Zhang WX, et al. Accelerated flap prefabrication with vascular endothelial growth factor. J Reconstr Microsurg. 2000;16:45–49. [DOI] [PubMed] [Google Scholar]
- 33.Corral CJ, Siddiqui A, Wu L, et al. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg. 1999;134:200–205. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Fischer K, Komorowska-Timek E, et al. Improvement of skin paddle survival by application of vascular endothelial growth factor in a rat TRAM flap model. Ann Plast Surg. 2001;46:314–319. [DOI] [PubMed] [Google Scholar]
- 35.Ozgentas HE, Shenaq S, Spira M. Study of the delay phenomenon in the rat TRAM flap model. Plast Reconstr Surg. 1994;94:1018–1024. [DOI] [PubMed] [Google Scholar]
- 36.Kroll SS, Reece GP, Miller MJ, et al. Comparison of the rectus abdominis free flap with the pectoralis major myocutaneous flap for reconstructions of the head and neck. Am J Surg. 1992;164:615–618. [DOI] [PubMed] [Google Scholar]
- 37.Shrotria S, Webster DJ, Mansel RE, et al. Complications of rectus abdominis myocutaneous flaps in breast surgery. Eur J Surg Oncol. 1993;19:80–83. [PubMed] [Google Scholar]
- 38.Carlson GW. Breast reconstruction: surgical options and patient selection. Cancer. 1994;74:436–439. [DOI] [PubMed] [Google Scholar]
- 39.Ribuffo D, Muratori L, Antoniadou K, et al. A hemodynamic approach to clinical results in the TRAM flap after selective delay. Plast Reconstr Surg. 1997;99:1706–1714. [PubMed] [Google Scholar]
- 40.Hudson DA. The surgically delayed unipedicled TRAM flap for breast reconstruction. Ann Plast Surg. 1996;36:238–245. [DOI] [PubMed] [Google Scholar]
- 41.Erdmann D, Sundin BM, Moquin KJ, et al. Delay in unipedicled TRAM flap reconstruction of the breast: a review of 76 consecutive cases. Plast Reconstr Surg. 2002;110:762–767. [DOI] [PubMed] [Google Scholar]
- 42.Eriksson E, Robson MC. Experimental flap delay with formic acid. Br J Plast Surg. 1978;31:238–241. [DOI] [PubMed] [Google Scholar]
- 43.Hynes W. The blood vessels in skin tubes and flaps. Br J Plast Surg. 1950;3:165–175. [DOI] [PubMed] [Google Scholar]
- 44.Shima DT, Deutsch U, D’Amore PA. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett. 1995;370:203–208. [DOI] [PubMed] [Google Scholar]
- 45.Folkman J, Klagsburn M. Angiogenic factors. Science. 1987;235:442–447. [DOI] [PubMed] [Google Scholar]
- 46.Arranz Lopez JL, Suarez Nieto C, Barthe Garcia P, et al. Evaluation of angiogenesis in delayed skin flaps using a monoclonal antibody for the vascular endothelium. Br J Plast Surg. 1995;48:479–486. [DOI] [PubMed] [Google Scholar]
- 47.Barthe Garcia P, Suarez Nieto C, Rojo Ortega JM. Morphological changes in the vascularisation of delayed flaps in rabbits. Br J Plast Surg. 1991;44:285–290. [DOI] [PubMed] [Google Scholar]
- 48.Yang R, Thomas GR, Bunting S, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996;27:838–844. [DOI] [PubMed] [Google Scholar]
- 49.Im MJ, Kim YS, Edwards RJ, et al. The effect of bovine basic fibroblast growth factor on skin flap survival in rats. Ann Plast Surg. 1992;28:242–245. [DOI] [PubMed] [Google Scholar]
- 50.Uhl E, Barker JH, Bondar I, et al. Improvement of skin flap perfusion by subdermal injection of recombinant human basic fibroblast growth factor. Ann Plast Surg. 1994;32:361–365. [DOI] [PubMed] [Google Scholar]