Abstract
Introduction:
Radiation therapy is increasingly used as adjuvant treatment of many childhood and adult malignancies. Radiation-induced sarcoma is a well recognized if uncommon event. The objective of this study is to determine the prevalence and long-term outcome for patients who develop radiation-induced sarcomas.
Methods:
From July 1982 to December 2001, 4884 adult patients with sarcoma were admitted and treated at our institution and recorded in a prospective database. There were 123 (2.5%) patients who had radiation-induced soft tissue sarcomas. Survival was determined by Kaplan-Meier analysis. Patient, tumor, and treatment characteristics were tested for their prognostic significance by log rank and the Cox proportional hazards model.
Results:
The median interval between radiation and development of sarcoma was 103 (6 to 534) months. In 114 patients with radiation-induced sarcoma who underwent curative resection, the 5-year actuarial survival was 41%, with a median survival of 48 months at a median follow-up of 36 months for survivors. The most common malignancy for which radiation was used was breast cancer (29%), followed by lymphoma (16%) and prostate cancer (15%). Malignant fibrous histiocytoma (23%) was the most common histologic diagnosis, followed by fibrosarcoma (15%) and angiosarcoma (15%). High-grade tumors (n = 85; 79%), age > 60 years (n = 61; 50%), and gross positive resection margin (n = 36; 32%) were predictive of poor sarcoma-specific survival on univariate and multivariate analysis.
Conclusions:
The increasing utilization of adjuvant radiation therapy, especially for early-stage breast cancer mandates long-term follow-up to detect radiation-induced sarcoma. Surgical resection remains the primary therapy, but 5-year survival remains ∼40%.
Radiation therapy is increasingly used as adjuvant treatment for many childhood and adult malignancies. Between July 1982 to December 2001, there were 123 patients who had radiation-induced soft tissue sarcomas. The increasing utilization of adjuvant radiation therapy for cancer mandates long-term follow-up to detect radiation-induced sarcoma. Surgical resection remains the primary therapy, and 5-year survival remains ∼40%.
Radiation therapy (RT) has become an integral part of overall cancer therapy, and the majority of cancer patients will receive RT at some point during the course of their disease.1 Exposure to radiation has long been recognized to induce sarcomas, with published reports from the 1920s documenting the development of sarcomas in workers manufacturing radium watch dials.2 Since that time, few discoveries have been made toward elucidating the molecular pathogenesis, and the majority of evidence that radiation induces sarcoma formation is based on the increased incidence of sarcoma seen in populations receiving radiation. This risk has been estimated to be up to 0.8% overall in exposed populations.3
The diagnosis of RT-induced sarcoma is still largely a clinical one and is based on criteria proposed in 1948 by Cahan et al.4 In general, patients are considered to have RT-induced sarcoma if they have had an antecedent history of radiation exposure before the development of the sarcoma, occurrence of the sarcoma in or near the field of radiation, and pathologic confirmation of sarcoma that is histologically unique from the primary cancer. Using these criteria and a prospective database, we report our institutional experience with resection of radiation-induced sarcomas of the soft tissue and we evaluate specific clinicopathologic factors that influence survival.
METHODS
Between 1982 and 2001, 4884 adult patients were admitted and treated at Memorial Sloan-Kettering Cancer Center (MSKCC) and identified in our prospective database. There were 123 patients (2.5%) who met the criteria for radiation-induced soft tissue sarcomas. Bony sarcomas were excluded in this analysis. The criteria for the diagnosis were (1) a history of radiation exposure at least 6 months before the development of the sarcoma; (2) occurrence of the sarcoma in the field of radiation; and (3) pathologic confirmation at MSKCC of a sarcoma that was histologically unique from the primary cancer.
The following variables were analyzed with relation to survival: (1) patient factors: age and sex; (2) tumor factors: anatomic site, size, depth, and grade of sarcoma; and (3) pathologic factors: histologic subtype, histologic grade, and status of surgical margins. Locally recurrent disease was defined as tumor recurrence at a site previously treated for a soft tissue sarcoma. Tumors were defined as high-grade or low-grade on the basis of the degree of differentiation, degree of cellularity, number of mitoses per high-powered field, amount of stroma necrosis, and degree of vascularity.5 A microscopically positive surgical margin was defined as tumor seen within < 1 mm of the inked margin.
Patients resected for curative intent were treated with wide excision, limb-sparing surgery or amputation. End points for multivariate analysis were overall survival and disease-specific survival. The rate of death was estimated using the Kaplan-Meier method, and the effect of each prognostic factor was examined using the log-rank test. Cox proportional hazards model and regression analysis was performed using SPSS software (SPSS, Inc., Chicago, IL). A P value less than 0.05 was considered significant.
RESULTS
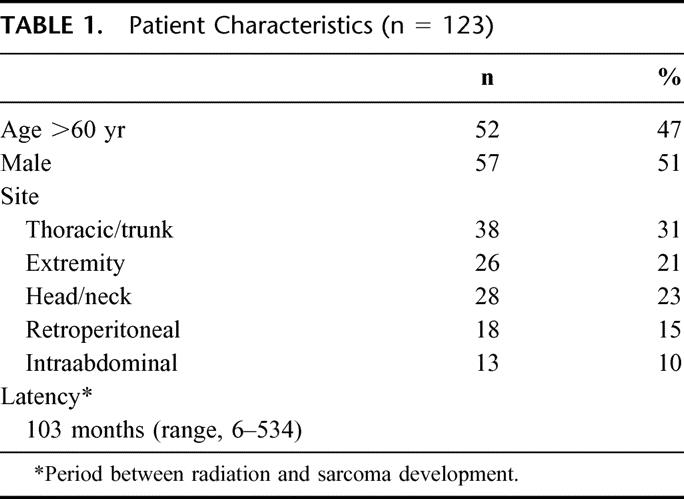
The clinical characteristics of all 123 patients with RT-induced sarcomas are listed in Table 1. The median age of patients was 62 years (range, 22 to 88 years). Males and female patients were evenly divided, even though a truncal site after breast irradiation was most common site for RT sarcoma. Other sites, including extremity, head/neck, retroperitoneum, and intraabdominal sarcomas, were relatively evenly divided. The median latency period between radiation exposure and sarcoma development was 8.4 years, with a range of 6 to 534 months at a median follow-up of 12 years (0.5 to 57) after radiation exposure.
TABLE 1. Patient Characteristics (n = 123)
Of the 123 patients who had RT-induced sarcomas, 114 patients (93%) were resected with curative intent. Three patients with desmoid tumors were excluded from the survival analysis due to the indolent nature of the disease, and survival for the remaining 111 patients was analyzed. Pathologic characteristic of tumors from the 111 patients who underwent resection with curative intent are seen in Table 2. The majority (84%) of lesions were discovered at a size < 10 cm. The majority of patients (68%) could be resected with gross clear margins, and an additional 14% of patients had a microscopically positive margin, for an overall 46% of patients having either a gross or microscopic positive margin. In the overall population of 123 patients, the radiation-induced sarcomas were high grade and were histopathologically varied (Fig. 1). Malignant fibrous histiocytoma (23%) was the most common diagnosis followed by angiosarcoma (15%), fibrosarcoma (15%), and leiomyosarcoma (12%). Two patients had epithelioid variants of their angiosarcoma and synovial sarcomas, respectively. A variety of histopathologic diagnoses, including undifferentiated sarcomas (3%), rhabdomyosarcoma (1%), primitive neuroectodermal tumors (1%), and nonosseous osteosarcoma (1%), were classified as “other.”
TABLE 2. Pathologic Characteristics (n = 111)
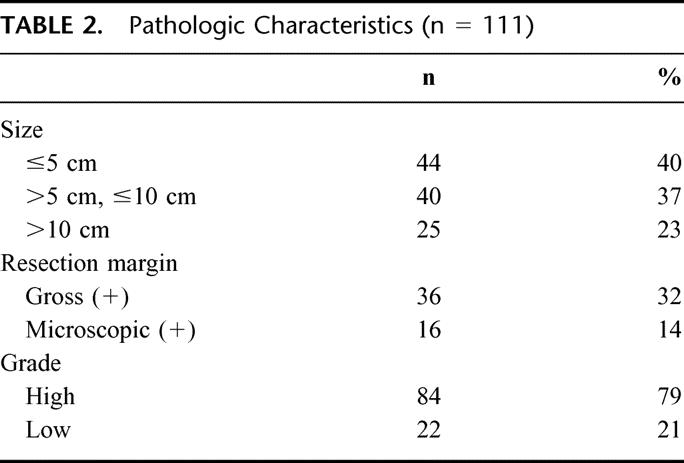
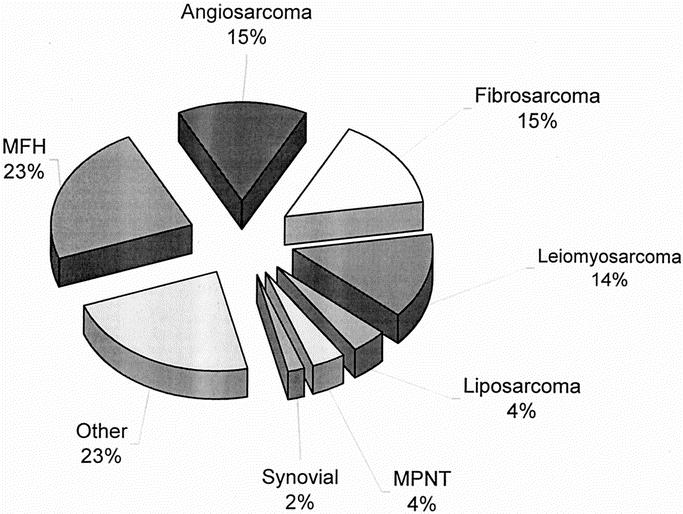
FIGURE 1. Histology of radiation-induced sarcomas (n = 123). MFH, malignant fibrous histiocytoma; MPNT, malignant peripheral nerve tumor.
At a median follow-up of 38 months for patients who were alive, the 1-year, 3-year, and 5-year overall actuarial survival rates were 78%, 58%, and 41%, respectively. Median survival was 48 months (Fig. 2). The exact cause of death could be determined in 102 (91%) of the 111 patients, and the 1-year, 3-year, and 5-year disease-specific survival rates in that subset of patients were 80%, 59%, and 44% in this population with a median disease-specific survival of 55 months (Fig. 3). Overall morbidity was 8%, and mortality was < 1% after resection (Table 3). There were no long-term morbidities, and the 1 death occurred in a patient with a history of Chronic obstructive pulmonary disease (COPD) who died postoperatively due to adult respiratory distress syndrome (ARDS) after a forequarter amputation and chest wall reconstruction.
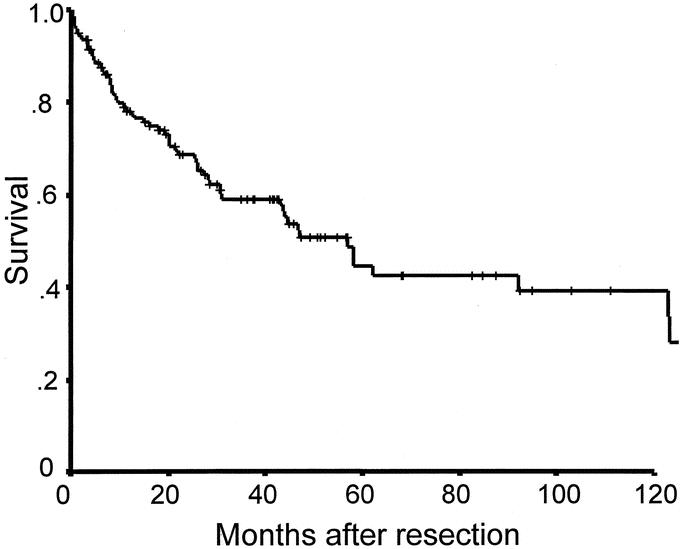
FIGURE 2. Overall survival of radiation-induced sarcomas after resection (n = 111).
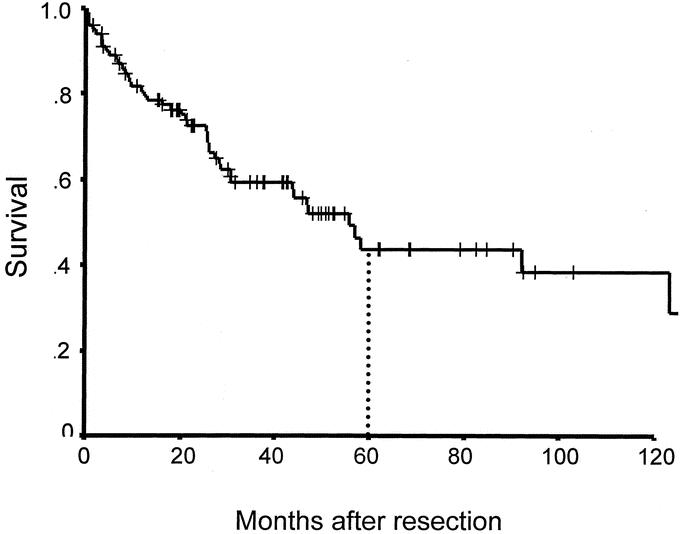
FIGURE 3. Disease-specific survival after resection for radiation-induced sarcoma (n = 102).
TABLE 3. Perioperative Outcome
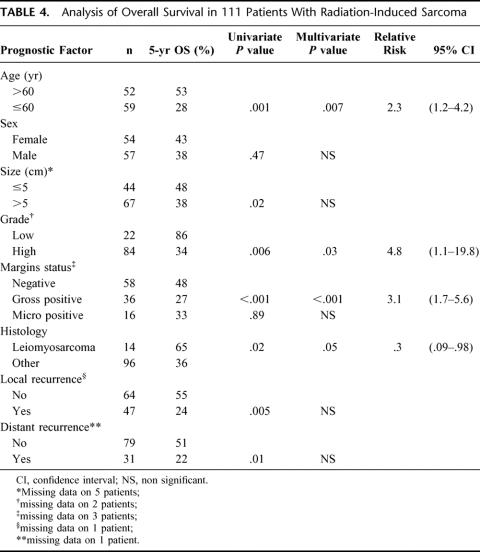
Clincopathologic factors predicting survival on univariate and multivariate analysis are seen in Table 4. In the multivariate analysis, patient age > 60 years (RR, 2.3; 95% CI, 1.2 to 4.2), high-grade tumors (RR, 4.8; 95% CI, 1.1 to 19.8), and gross positive margins (RR, 3.1; 95% CI, 1.7 to 5.6) were adverse prognostic factors. An age of 60 years was chosen as a cutoff because it is the median for the population. Leiomyosarcoma (RR, 0.3, 95% CI, 0.09 to 0.98) was associated with improved outcome. As would be expected, both local recurrence and distant recurrence were predictive of poor survival on univariate analysis. The lack of significance on multivariate analysis suggests that age, grade, or microscopic margin positivity are surrogates of recurrence. Figure 4 demonstrates survival according to resection margin status. In patients who underwent a complete gross resection at the time of surgery, the 5-year survival was 44% versus 27% for patients who have a gross positive margin at the time of surgery (P = 0.0001). Additionally, in the patients with microscopically negative resections, 5-year survival increases to 54% versus 32% in patients with a microscopically positive margin (P = 0.0004). However, in patients (n = 16, 14%) with a microscopically positive resection without gross positive disease at the time of resection, the 5-year survival was not statistically different compared with patients who had a complete resection (n = 55, 50%, P = 0.14).
TABLE 4. Analysis of Overall Survival in 111 Patients With Radiation-Induced Sarcoma
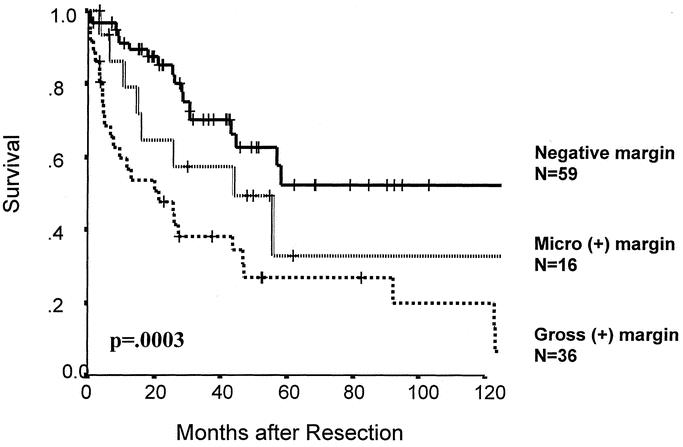
FIGURE 4. Survival according to resection margin status (n = 111).
Analysis of survival according to site of disease (head/neck, trunk, extremities, abdominal, retroperitoneal) did not show any statistically significant differences. Similarly, analysis of survival according to patient's primary diagnosis for which radiation was given did not demonstrate any statistical differences. The most common malignancy for which radiation was used was breast cancer (32 patients, 29%), followed by lymphoma (17 patients, 16%) and prostate cancer (15 patients, 14%). In patients with radiation for breast cancer, the majority (56%) received radiation for early-stage disease, either ductal carcinoma in situ (DCIS) or a T1 or smaller tumor. After resection of the subsequent RT-induced sarcomas, the 5-year survival was 49% with a median survival of 55 months. Angiosarcoma (18 patients, 44%) was the most common diagnosis in these patients.
At the time of our analysis, 50% of patients are alive at a median follow-up of 38 months. There are 36 patients (32%) who show no evidence of disease and 20 patients (18%) who are alive with recurrent disease. Recurrence occurred in 59 patients (53%) with 28 patients (47%) having a local recurrence, 13 patients (23%) with a distant recurrence, and 18 patients (30%) having both local and distant disease (Fig. 5). The majority of distant recurrences involved the lungs (68%), followed by the liver (3%).
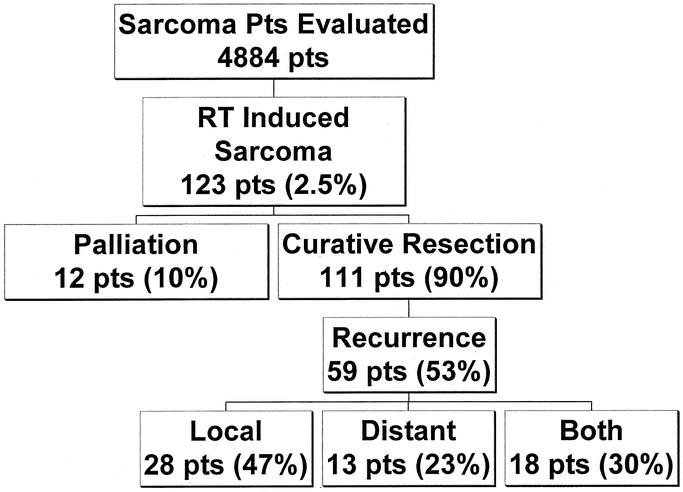
FIGURE 5. Summary of sarcoma patients evaluated between 1982 and 2001.
DISCUSSION
RT plays an integral part of the modern treatment of cancer. The utilization of RT has increased over the past few decades as technology has evolved allowing for organ conservation as well as curative roles for radiation. Newer modalities such as intensity-modulated RT (IMRT) can target tumors more precisely but actually increase the overall amount of normal tissue exposed to low-dose ionizing radiation.6 These moderate to low doses of radiation are actually thought to induce carcinogenesis more efficiently than high-dose radiation, which paradoxically prevents the formation of tumors. Furthermore, as more patients with early-stage cancer are treated with radiation and live longer, the incidence of RT-induced sarcomas will increase as well.7
The definition of RT-induced sarcomas has never been well established, and the clinical definition put forth in 1948 by Cahan et al remains essentially unchanged. The length of time required between radiation exposure and sarcoma formation is the 1 criterion that has been modified by most investigators from the several year requirement originally proposed. This latency period is necessary to differentiate a RT-induced sarcoma from a second primary that may predate the radiation; because no accurate molecular or pathologic markers exist, any spontaneous sarcomas that may appear in a radiation field cannot reliably be excluded from analysis. A minimum latency as short as 1 month has been suggested by some authors;1 in the current study, a cutoff of 6 months was chosen, and our median interval between radiation exposure and sarcoma formation was 8.4 years (0.5 to 44 years).
Current estimates suggest that RT-induced sarcomas account for between 0.5 to 5.5% of all sarcomas;8–10 in our current study, we found RT-induced sarcomas represented 2.5% of the 4884 sarcomas seen at our institution during the concurrent time period. The true incidence of radiation-induced sarcomas during the study time period is a difficult number to establish because the denominator of all patients receiving RT is impossible to determine at our tertiary referral center. Some authors, however, have suggested a 0.03 to 0.8% incidence of RT-induced sarcomas using large population-based studies following patients after radiation treatment.3,11
Radiation-induced sarcomas have traditionally been viewed as aggressive, high-grade tumors with poor prognosis. In our current study, 90% of patients evaluated were able to undergo resection for curative intent; however, nearly half of patients had either a gross or microscopically positive margin (46%), indicating that many of these tumors were diffuse and insinuating. This is despite the fact that the majority of tumors were < 5 cm in size. Previous irradiation may have played a role in obscuring anatomic and tumor planes, preventing surgeons from appreciating true tumor margins. This underscores the necessity for aggressive and wide resections, especially considering that a positive surgical margin (gross or microscopic) will reduce survival by nearly half. In addition, the majority (80%) of resected patients had high-grade tumors, resulting in a 5-year survival of 34% for this subset of patients. Survival in the patients with low-grade tumors (excluding desmoids) was 86% at 5 years, and this survival is sustained long-term (Fig. 6). Despite the aggressive nature of these tumors, which are resected from an irradiated field, we found that excision of RT-induced sarcomas can be performed safely, with low morbidity and mortality and with reasonable long-term survival.
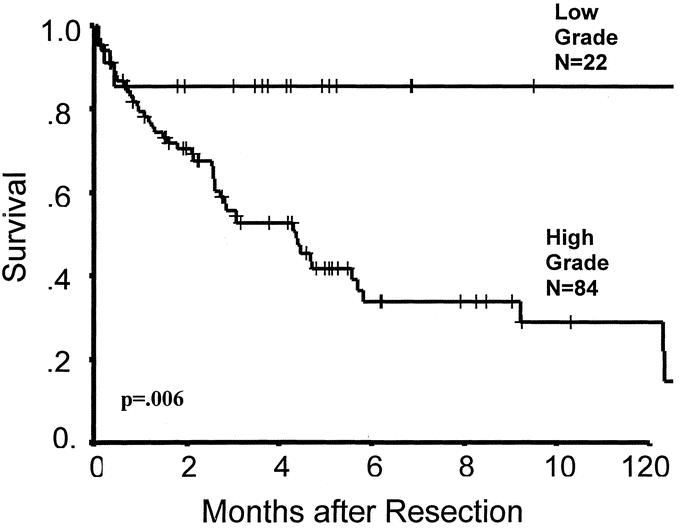
FIGURE 6. Survival according to grade (n = 106, information unavailable on 5 patients).
Prior analyses of patients with RT-induced sarcomas have not differentiated those patients undergoing palliative management versus curative resection; in those studies, 5-year survival rates of 15 to 30% have been reported.1,8,11–18 In the current study, when only curative resections are analyzed, we found a 5-year survival rate of 41%. This is compared with sarcoma data published from our institution, where the 5-year actuarial survival rate was 76% for all extremity soft tissue sarcomas and 55% for all retroperitoneal sarcomas.19 In contrast, the 5-year survival rates in the RT sarcoma patients were 43% and 36% for extremity (n = 26) and retroperitoneal sarcomas (n = 18), respectively.20–22
Multivariate analysis confirmed that age > 60 years, high-grade and a microscopically positive margin were all independent predictors of poor outcome; of these, only surgical margin is a variable that can potentially be influenced by surgeons. However, a grossly positive resection still appears to allow for a 5-year survival rate of 30%, suggesting a role for aggressive surgical management in these patients. We have previously demonstrated that histology plays an important role in predicting outcome for sarcoma.19,23 In our current study, the diagnosis of leiomyosarcoma, found in 12% of patients, was an independent predictor of good outcome on multivariate analysis, and leiomyosarcoma was the only histologic subtype to have predictive value with regards to survival. The improved survival seen in leiomyosarcoma may be due to its decreased propensity to locally recur; however, it is generally not considered to be an indolent histologic subtype.23 The role of adjuvant therapy in radiation-induced sarcomas is unknown; in this study, 24 patients received a variety of chemotherapy for their sarcoma, and there was no difference in their survival compared with those not receiving chemotherapy.
Interestingly, tumor site was not predictive of survival, either on multivariate or univariate analysis. Truncal sarcoma was the most frequent site for tumors, due mostly to adjuvant radiation received for breast cancer. The trend over the last few decades has been to treat breast cancer patients at earlier stages with radiation to reduce local recurrences and allow breast conservation. Although large randomized trials such as the National Surgical Adjuvant Breast and Bowel Project B-06 demonstrate a decreased local recurrence with radiation compared with surgery alone, they do not show a significant improvement in survival with radiation. This has been confirmed in a large meta-analysis of over 28,000 patients with early-stage breast cancer, where survival was found to be equivalent between patients receiving surgery and radiation versus those receiving surgery alone.24 In our study, there were 32 patients with sarcomas following radiation for breast cancer, and the majority (56%) had radiation for either T1 disease or DCIS. As more patients with early-stage tumors are treated with radiation, it is presumed that the incidence of RT sarcoma will increase, particularly when sarcomas were observed more than 40 years after exposure to radiation in the current study.
RT-induced sarcomas are a significant complication after RT, and radiation should be used judiciously when there is a clear benefit. Survival for radiation-induced sarcomas remains around 40% at 5 years, but the outcome in patients with low-grade tumors after complete resection is excellent. Long-term, careful follow-up is essential for patients who have been treated with RT, and any abnormality should be aggressively biopsied. If a sarcoma is detected, the treatment of choice for radiation-induced sarcomas is surgical resection with negative margins.
Discussions
Dr. J. Bradley Aust (San Antonio, Texas): I am pleased to have an opportunity to discuss this paper by Drs. Cha et al from Memorial Sloan-Kettering. I received it in time to review it completely. As is typical of most papers coming from this large cancer center, rare tumors, which are ordinarily published as case reports, appear here as a relatively large series.
The paper is a retrospective analysis of 123 cases culled from an experience of almost 5000 sarcoma patients. Even in this large center, it is important to recognize that each of the sarcomas discussed is a separate disease entity, but, because of the numbers involved, we forced to evaluate sarcomas as a group, that is we “lump” all sarcomas. This is possible because the majority of sarcomas have comparable growth characteristics and metastatic potential. Leiomyosarcomas in this group happened to be the least aggressive of the radiation-induced sarcomas.
This retrospective series was analyzed primarily to determine those characteristics that influenced recurrence and survival. Not surprisingly, the findings revealed that patients with grossly positive margins faired more poorly than microscopically positive margins or negative margins. Also statistically evident was the worse prognosis for high-grade tumors compared with low-grade tumors. There was also a statistically significant detrimental prognosis for those over 60 years of age compared with those under 60. I was surprised to find that size was not a significant feature distinguishing recurrence or poor survivorship. These findings clearly suggest that improving the wide local excision is of paramount importance in improving the results, since one half of the patients developed either local recurrent and/or distant metastasis and eventually succumbed from this disease.
Since radiation-induced sarcomas occur in about 1% of the patients, the selection of patients to receive such therapy is important, especially in breast cancer. One of 8 women has a lifetime risk of developing breast cancer. The most common accepted form of therapy is lumpectomy followed by RT. Multiple thousands of patients are currently being treated under this protocol. Because of this large number at risk, 1% constitutes a relatively large number of cases who will be at risk for sarcoma. As we improve our diagnostic accuracy, the incidence of sarcoma will increase, as will the numbers of radiation-induced sarcomas to which half the patients will succumb. This is especially tragic in breast cancer patients, because the latent period of 8.5 years would indicate that the patients most likely to develop radiation-induced sarcomas are those who are already potentially cured of their breast cancer.
Surgical treatment is a localized form of treatment; success or failure should be judged by local recurrence, not by survival or distant metastases. Surgical recurrence is therefore prima-facie evidence of inadequate surgery. We accept local recurrence after lumpectomy for cosmetic reasons and employ RT, also a localized form of treatment, to sterilize the field of residual cancer cells. However, if the local recurrence after lumpectomy is 30%, then 70% of the patients did not need RT. It is important to identify the characteristics of that 70% who do not need RT. The authors suggest that it may very well be important to consider wide local excision definitive treatment for those patients with early cancer of low-grade whose wide local excision has clearly free margins and who have low levels of adverse genetic and hormonal indicators. I think that this approach should be quite feasible and would avoid both the low morbidity of postoperative RT as well as the long-term risk of radiation-induced sarcomas and should be a goal worth achieving.
Dr. Martin J. Heslin (Birmingham, Alabama): I too rise to congratulate Drs. Cha and Brennan on a fine presentation about soft tissue sarcoma, and I echo Dr. Aust's comments about a large database making important advances in a relatively uncommon disease. The paper is well written, and the conclusions I think are sound. I have a few questions I would like to ask; 1 is definitional, and the other 3 are management questions.
The first question I would like to ask is always an important question for surgeons, the issue of microscopic margins. The manuscript defines the microscopic margin as positive within 1 mm of the margin of resection, where I think previous definitions have defined tumor at the margin of resection. I was wondering if you could comment on the management of a positive microscopic margin in this setting and how your institution currently defines it.
The second question I would like to ask is about leiomyosarcoma and if you could comment on why in this series it is a good prognostic factor while it was an adverse prognostic factor in other large series, especially in extremity sarcoma? Do you think it is actually a different disease or simply different classification?
The third question deals with its pattern of recurrence. The pattern of recurrence here mimics that of a retroperitoneal sarcoma. I was wondering if you could comment on the extent of operation in the presence of a radiated field; in the manuscript, 1 of the recommendations is for more aggressive, wider surgery to treat these tumors. I think one must be cautious when making that recommendation, especially with the anatomic constraints that are often seen in the chest, retroperitoneum, or pelvis.
Lastly, I would like to ask the question of follow-up. The final recommendation was for aggressive long-term follow-up. What are your recommendations, if different than the follow-up recommendations for early breast cancer? Who should do it? How long?
Thank you for a very interesting and important study.
Dr. Lynn H. Harrison, Jr. (New Orleans, Louisiana): Recently, a number of different institutions have advocated a very aggressive approach to pulmonary metastases, notably with osteogenic sarcoma, which was not one of the cell types included in your group, but more recently even in carcinomas, particularly adenocarcinoma of the colon when the primary has been controlled and there are no demonstrable hepatic mets. What is your institutional policy with regard to approaching pulmonary metastases, and what would you require of a patient who has undergone apparently successful pulmonary metastasectomy before subjecting him to a second attempt down the road?
Dr. Murray F. Brennan (New York, New York): Closing the last paper of the last day of the meeting should imply that I would have an extraordinary opportunity to say something totally apocryphal that no one could respond to! But, let me try to address the issues.
Dr. Aust, thank you for your insightful commentary. You addressed the issues, I think. If we do the mathematics and say that conservatively 3 in 10,000 women will get a sarcoma after breast irradiation, and less conservatively 80 in 10,000, then you are talking about somewhere between 30 and 800 new sarcoma cases a year, at least 50% of whom will die of sarcoma. Because the half-life of development is 10 years, my angst is that this generation is creating a problem for the next generation! Like Medicare benefits, we are making decisions now and someone else will pay for them later! In the California registry, if you develop a sarcoma of the anterior chest wall, you are 50 times more likely to have received prior RT than not.
Dr. Heslin had a number of important questions. The first addresses the issue of margins. And it is correct that the definition of margin has always been difficult. It is a little bit like class; it is easy to recognize, but hard to define.
The positive margin we are taking is less than 1 mm, and we do this so nobody is missed. The technical problem, if you are operating on a sarcoma in a previously radiated field, is the definition of your actual surgical margin. This extends over 20 years. We began these studies in the early 1980s. Now we are much more liberal and much more cognizant of the need to have a more extended resection, but that requires free tissue resection.
We have learned over and over again the problems with trying to minimize the resection so that you could obtain primary closure of these lesions and then watch the entire wound fall apart—and this is where our plastic and reconstructive colleagues have made a major contribution.
What do we do about the management of a positive microscopic margin now? Nowadays, that should be a margin that comes about because you have maximized the resection but are on the brachial plexus.
That is very difficult; if we have a positive microscopic margin in a patient who develops a primary sarcoma, we turn to the patient and say we know we can minimize your local recurrence rate with RT, and the patient rightfully says, “Didn't we have a discussion about the fact that this is the reason I am here in the first place?”
I think the leiomyosarcoma issue is an issue of small numbers. I would not believe that the histology is much different.
The recurrence is site-specific. Some of the chest wall lesions were invasive, so that slight increase in visceral as opposed to lung metastasis is not too surprising.
We could spend the rest of the day talking about follow-up. There is no definable follow-up program, essentially, in any malignancy that is conclusively proven to make a difference in subsequent outcome. And 50% of all patients who develop a recurrence of their malignancy come to see the doctor between their planned visits! I think it is incorrect when organizations define what is appropriate follow-up because that has medico-legal implications in a situation where you cannot demonstrate difference. We have been very reluctant to say, “This is our plan,” because we do not have a plan that is supported by the data.
It is very hard to do a randomized trial of follow-up length. We tried to do that and failed miserably.
Finally, Dr. Harrison, you asked me a generic question about pulmonary metastasis. About 30% of all patients with soft tissue sarcoma will get pulmonary metastasis only. Of those patients, about 80% have a curative resection, predominantly solitary lesions, but not always. Of those, 30% would be alive at 3 years. The historically claimed data that 30% to 40% of people are alive at 5 years after pulmonary resection are a consequence of selection bias; when you take all comers, that number is consistently less.
There is no proven benefit of adjuvant chemotherapy after the resection of pulmonary metastasis, so our approach would be to try to use new therapies. The most promising scientifically is directed therapy at now identified fusion genes, currently purely a research topic for which there are no data to prove efficacy.
Footnotes
Supported by CA #47179 from the National Institutes of Health, Bethesda, MD (to MFB).
Reprints: Murray F. Brennan, MD, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: brennanm@mskcc.org.
REFERENCES
- 1.Mark RJ, Poen J, Tran LM, et al. Postirradiation sarcomas. A single-institution study and review of the literature. Cancer. 1994;73:2653–2662. [DOI] [PubMed] [Google Scholar]
- 2.Martland HS. Occupational poisoning in manufacture of luminous watch dials. JAMA. 1929;92:466–473. [Google Scholar]
- 3.Pierce SM, Recht A, Lingos TI, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:915–923. [DOI] [PubMed] [Google Scholar]
- 4.Cahan W, Woodward H, Higinbotham N, et al. Sarcoma arising in irradiated bone: report of eleven cases. Cancer. 1948;1:3–29. [DOI] [PubMed] [Google Scholar]
- 5.Hajdu SI, Shiu MH, Brennan MF. The role of the pathologist in the management of soft tissue sarcomas. World J Surg. 1988;12:326–331. [DOI] [PubMed] [Google Scholar]
- 6.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–88. [DOI] [PubMed] [Google Scholar]
- 7.Taghian A, de Vathaire F, Terrier P, et al. Long-term risk of sarcoma following radiation treatment for breast cancer. Int J Radiat Oncol Biol Phys. 1991;21:361–367. [DOI] [PubMed] [Google Scholar]
- 8.Davidson T, Westbury G, Harmer CL. Radiation-induced soft-tissue sarcoma. Br J Surg. 1986;73:308–309. [DOI] [PubMed] [Google Scholar]
- 9.Huvos AG, Woodard HQ, Cahan WG, et al. Postradiation osteogenic sarcoma of bone and soft tissues. A clinicopathologic study of 66 patients. Cancer. 1985;55:1244–1255. [DOI] [PubMed] [Google Scholar]
- 10.Souba WW, McKenna RJ, Meis J, et al. Radiation-induced sarcomas of the chest wall. Cancer. 1986;57:610–615. [DOI] [PubMed] [Google Scholar]
- 11.Wiklund TA, Blomqvist CP, Raty J, et al. Postirradiation sarcoma. Analysis of a nationwide cancer registry material. Cancer. 1991;68:524–531. [DOI] [PubMed] [Google Scholar]
- 12.Amendola BE, Amendola MA, McClatchey KD, et al. Radiation-associated sarcoma: a review of 23 patients with postradiation sarcoma over a 50-year period. Am J Clin Oncol. 1989;12:411–415. [PubMed] [Google Scholar]
- 13.Brady MS, Gaynor JJ, Brennan MF. Radiation-associated sarcoma of bone and soft tissue. Arch Surg. 1992;127:1379–1385. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Chu FC, Woodard HQ, et al. Radiation-induced soft-tissue and bone sarcoma. Radiology. 1978;129:501–508. [DOI] [PubMed] [Google Scholar]
- 15.Lagrange JL, Ramaioli A, Chateau MC, Marchal C, Resbeut M, Richaud P, Lagarde P, Rambert P, Tortechaux J, Seng SH, de l, Reme S, Bof J, Ghnassia JP, Coindre JM. Sarcoma after radiation therapy: retrospective multiinstitutional study of 80 histologically confirmed cases. Radiation Therapist and Pathologist Groups of the Federation Nationale des Centres de Lutte Contre le Cancer. Radiology. 2000;216:197–205. [DOI] [PubMed] [Google Scholar]
- 16.Laskin WB, Silverman TA, Enzinger FM. Postradiation soft tissue sarcomas. An analysis of 53 cases. Cancer. 1988;62:2330–2340. [DOI] [PubMed] [Google Scholar]
- 17.Murray EM, Werner D, Greeff EA, et al. Postradiation sarcomas: 20 cases and a literature review. Int J Radiat Oncol Biol Phys. 1999;45:951–961. [DOI] [PubMed] [Google Scholar]
- 18.Sheppard DG, Libshitz HI. Post-radiation sarcomas: a review of the clinical and imaging features in 63 cases. Clin Radiol. 2001;56:22–29. [DOI] [PubMed] [Google Scholar]
- 19.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. [DOI] [PubMed] [Google Scholar]
- 20.Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832–2839. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koea JB, Leung D, Lewis JJ, et al. Histopathologic type: an independent prognostic factor in primary soft tissue sarcoma of the extremity? Ann Surg Oncol. 10:432–440. [DOI] [PubMed]
- 24.Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. Early Breast Cancer Trialists’ Collaborative Group. N Engl J Med. 1995;333:1444–1455. [DOI] [PubMed]