Abstract
ERBB2 is a receptor tyrosine kinase present on the basolateral membrane of polarized epithelia and has important functions in organ development and tumorigenesis. Using mutagenic analyses and Madin-Darby canine kidney (MDCK) cells, we have investigated the signals that regulate basolateral targeting of ERBB2. We show that basolateral delivery of ERBB2 is dependent on a novel bipartite juxtamembrane sorting signal residing between Gln-692 and Thr-701. The signal shows only limited sequence homology to known basolateral targeting signals and is both necessary and sufficient for correct sorting of ERBB2. In addition we demonstrate that this motif can function as a dominant basolateral targeting signal by its ability to redirect the apically localized P75 neurotrophin receptor to the basolateral membrane domain of polarized epithelial cells. Interestingly, LLC-PK1 cells, which are deficient for the μ1B subunit of the AP1B adaptor complex, missort a large proportion of ERBB2 to the apical membrane domain. This missorting can be partially corrected by the introduction of μ1B, suggesting a possible role for AP1B in ERBB2 endosomal trafficking. Furthermore, we find that the C-terminal ERBIN binding domain of ERBB2 is not necessary for its basolateral targeting in MDCK cells.
The formation of many organs and tissues involves a complex series of reciprocal interactions between the epithelia and underlying mesenchyme. Growth factors and their cognate receptors have been implicated in the signaling between these two cell lineages (reviewed in reference 15). Epithelial cells can be divided into two morphologically and functionally distinct domains: an apical domain that usually faces the organ lumen and a basolateral domain that rests on the basement membrane which separates the epithelium from the underlying mesenchyme. Tight junctions separate the apical and basolateral membranes, generating an epithelial asymmetry (polarity) that is maintained by the continuous synthesis and delivery of new protein and lipid components to the respective membrane domains. Furthermore, epithelial polarity requires the efficient recycling and delivery of endocytosed products back to the appropriate apical or basolateral membrane (for reviews see references 21 and 30). This physical separation of apical and basolateral domains means that growth factors and their receptors must be targeted to the appropriate membrane to ensure correct intercellular signaling.
The selective targeting of proteins from the trans-Golgi network (TGN) to the basolateral membrane requires the presence of cis-acting signals. For the most part, basolateral sorting or targeting signals have been identified as minimal amino acid motifs in the juxtamembrane cytoplasmic region of membrane proteins. Several cell surface receptors possess signals similar to those involved in clathrin-mediated endocytosis, which rely either on a critical tyrosine residue, as found in the low-density lipoprotein (LDL) receptor (27) and the vesicular stomatitis virus glycoprotein (35), or on a dihydrophobic motif, as found in E-cadherin (29) and the Fc receptor (16). Other basolateral sorting motifs, unrelated to clathrin-mediated endocytic signals, have been described for the neural cell adhesion molecule (N-CAM) (23), and epidermal growth factor receptor (14). The similarity between these basolateral sorting signals and clathrin-coated pit determinants has suggested that the adaptor proteins might also be implicated in basolateral sorting (reviewed in reference 26). In particular, epithelial cells deficient in the heteromeric adaptor complex AP1B or AP4 have been shown to missort several basolateral proteins to the apical membrane (10, 31, 33).
In addition to cis-acting signals, PDZ domain-containing proteins can act in trans to correctly target or to retain receptors at either the apical or basolateral domain of epithelial cells, as well as the pre- or postsynaptic membranes of neurons. These PDZ domain-containing proteins usually interact with a short amino acid sequence at the extreme C terminus of the targeted membrane protein (for reviews see references 9 and 19).
The ERBB/HER family of receptor tyrosine kinases (RTKs) consists of four members in mammals: the epidermal growth factor receptor (also called ERBB1, or HER1 for the human allele), ERBB2/HER2, ERBB3/HER3, and ERBB4/HER4. These receptors play crucial roles in animal development, and their aberrant expression has been associated with tumorigenesis (reviewed in reference 1). In epithelia, ERBB1 and ERBB2 are predominantly localized to the basolateral membrane (3, 5, 7, 13), where they are able to mediate signals between the epithelium and the adjacent mesenchyme. Although structurally homologous to other members in the family, ERBB2 is unique in that ligand-mediated homodimers have not been identified, indicating that normal signal transduction occurs through the formation of heterodimers (20). Moreover, ERBB2 seems to be the preferred heterodimerization partner for other family members (12).
Despite a high degree of homology between ERBB family members, the mechanisms that govern receptor localization appear to differ. Whereas ERBB1 has been shown to contain an autonomous basolateral sorting signal in the juxtamembrane region (13, 14), a trans-acting mechanism of localization through an interaction with the PDZ domain-containing protein ERBIN appears to control ERBB2 localization (3). We have undertaken extensive mutagenic analyses of ERBB2 to investigate the signals involved in its targeting. In this report we identify and characterize a novel bipartite juxtamembrane basolateral sorting signal that is both necessary and sufficient for the targeting of ERBB2. Moreover, we find no evidence that the C-terminal ERBIN binding domain of ERBB2 plays a role in basolateral localization. These studies extend the repertoire of basolateral sorting signals and provide further insights into the role of C-terminal PDZ protein binding domains in membrane trafficking.
MATERIALS AND METHODS
Antibodies.
Primary antibodies were the mouse monoclonal anti-ERBB2 clone 42, anti-E-cadherin, anti-β-catenin (all from Transduction Laboratories), anti-P75NTR clone ME20.4 (Upstate Biotechnology), anti-ERBB2 9G6.10 (Neomarkers), anti-transferrin receptor B325, and rat monoclonal anti-ZO1 (Chemicon International). Secondary antibodies were horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulins (IgG) (Dako), fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG and tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-rat IgG (Jackson ImmunoResearch), and infrared (IR)-goat anti-mouse IgG (LI COR Biosciences).
DNA plasmids and mutagenesis.
A 4-kb fragment of cDNA encoding the entire human ERBB2 sequence was excised from vector pSV2ERBB2 (a kind gift from Helen Hurst) (36) by digestion with HindIII and cloned into pcDNA4/TO (Invitrogen) to form plasmid pc4-B2. Four ERBB2 truncation mutants, Δ690, Δ695, Δ701, and Δ706, were generated by creating sequential C-terminal truncations at amino acids 690, 695, 701, and 706, respectively (numbering based on reference 6). PCR products were generated with a 5′ HindIII site and a 3′ stop codon followed by an XbaI site and cloned into pcDNA4/TO. Internal deletion mutants Δ684-690, Δ684-695, Δ684-706, Δ696-701, and Δ702-706 were created by deleting the indicated amino acids using a modified version of a PCR-based strategy (28). These deletions were generated by using full-length ERBB2 cDNA as a template and were then cloned by use of HindIII into pcDNA4/TO. Mutations V1255A and Δ1249 contain a valine-to-alanine mutation at position 1255 and a deletion of the last 6 C-terminal amino acids, respectively. Mutations were generated by using a forward primer annealing to sequences 5′ of the Eco47III site and a reverse mutagenic primer containing the appropriate mutation, followed by a 3′ stop codon and an XbaI site. Mutated ERBB2 fragments were then cloned into pc4-B2 by using Eco47III and XbaI. All other alanine mutations were generated with the QuikChange XL site-directed mutagenesis kit (Stratagene) using pc4-B2 as a template.
Chimeric P75 neurotrophin receptor (P75NTR)/ERBB2 receptors were created by generating a truncated P75NTR product with a HindIII site 5′ to the initiation codon and an in-frame EcoRV site at position 930 (numbering based on reference 18). This was cloned into pcDNA4/TO by using HindIII/EcoRV to generate plasmid pP75-TrFu. A similar vector containing a double stop codon 5′ to the EcoRV site (pP75-Tr) was constructed. EcoRV/NotI digestion allowed the ligation of ERBB2 fragments corresponding to amino acids 680 to 690 [P75B2(680-690)], 680 to 706 [P75B2(680-706)], 1241 to 1255 [P75B2(C15)], and 680 to 1255 [P75B2(680-1255)] into pP75-TrFu. Two further chimeric receptors based on P75B2(C15) were generated by mutating the C-terminal valine [P75B2(C15)VA] or tyrosine [P75B2(C15)YA] to an alanine residue. All ERBB2 mutants and chimeric receptor constructs were verified by DNA sequencing.
μ1B was amplified from reverse-transcribed cDNA obtained from MCF10A cells, verified by DNA sequencing, and subsequently cloned into pcDNA3 (Invitrogen) by using HindIII and NotI. A transferrin receptor (TFr) expression vector was kindly provided by Colin Hopkins (Imperial College, London, United Kingdom).
Cell culture.
Madin-Darby canine kidney (MDCK) and LLC-PK1 (American Type Culture Collection) cells were grown and passaged in Dulbecco's modified Eagle's medium (DMEM) (Cancer Research UK Cell Services) supplemented with 10% fetal bovine serum (Gibco-BRL) and maintained under 10% CO2 at 37°C. Cells were transfected by using Lipofectamine reagent (Invitrogen) according to the manufacturer's instructions. Stable cell lines were generated by cotransfecting an FspI-linearized expression plasmid and pcDNA6/TR (Invitrogen) at a ratio of 1:5. Individual clones were selected, maintained in medium as above supplemented with Zeocin (400 μg/ml) and blasticidin S HCl (5 μg/ml) (Invitrogen), and screened for tetracycline-inducible expression by immunoblotting.
Immunoblotting.
Stable cell clones grown to subconfluence in 6-well plates were induced overnight with tetracycline (1 μg/ml) (Invitrogen), washed twice with phosphate-buffered saline (PBS), and harvested using Laemmli buffer. Cell lysates were separated by electrophoresis through SDS-8% polyacrylamide gels and transferred to Protran nitrocellulose membranes (Schleicher & Schuell) using a Bio-Rad Trans-blot transfer cell. After being blocked in 5% fat-free milk powder in PBS containing 0.1% Tween 20, membranes were probed with anti-ERBB2 clone 42 (1:2,500). Bound antibodies were revealed with HRP-conjugated goat anti-mouse IgGs (1:5,000) by using the ECL detection system (Amersham).
Indirect immunofluorescence.
Stable or transiently transfected cells were grown on 0.4-μm-pore size polyethylene tetraphthalate (PET) track-etched membrane cell culture inserts (Falcon) to establish a polarized monolayer. If appropriate, the cells were induced overnight with tetracycline (1 μg/ml). MDCK cells were fixed in −20°C methanol for 5 to 10 min and blocked in 0.2% fish gelatin (Sigma) in PBS for 1 h. LLC-PK1 cells were fixed in 4% paraformaldehyde in PBS and were blocked and permeabilized in PBS containing 3% bovine serum albumin (BSA) and 0.2% Triton X-100. Double immunofluorescent labeling was achieved by first incubating cells with either anti-ERBB2 9G6.10 (1:200), anti-P75NTR ME20.4 (1:1,000), anti-transferrin receptor B325 (1:100), or anti-β-catenin (1:1,000) and anti-ZO1 (1:1,000) for 1 h and then incubating them with FITC-conjugated goat anti-mouse IgG (1:100) and TRITC-conjugated goat anti-rat IgG (1:100) for 1 h. Then cells were mounted on glass slides in Mowiol (Calbiochem) containing 2.5% 1,4-diazabicyclo[2,2,2]octane (DABCO) (Sigma). Indirect immunofluorescence was visualized by using a Zeiss LSM510 laser scanning confocal microscope.
Quantification of cell surface ERBB2.
MDCK cell clones were grown to confluence on membranes as described above, induced overnight with 1 μg of tetracycline/ml, and then washed twice in cold PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS++). Surface biotinylation of apical or basolateral proteins was performed twice at 4°C for 20 min by using freshly prepared 0.5-mg/ml EZ-Link Sulfo-NHS-Biotin (Pierce) in PBS++. Reactions were quenched by using 50 mM NH4Cl. After several washes, cells were lysed in 1% Triton X-100-20 mM Tris-HCl (pH 8.0)-50 mM NaCl-5 mM EDTA-0.2% BSA containing a cocktail of protease inhibitors and were then cleared by centrifugation at 16,000 × g. Labeled proteins were precipitated by using immobilized streptavidin (Pierce) and were prepared for SDS-polyacrylamide gel electrophoresis (PAGE). Bound antigens were revealed with anti-ERBB2 clone 42 (1:2,500) and IR-labeled goat anti-mouse IgG (1:2,000) and were visualized and quantified by using an Odyssey IR scanner (LI COR Biosciences).
RESULTS
Basolateral targeting of human ERBB2.
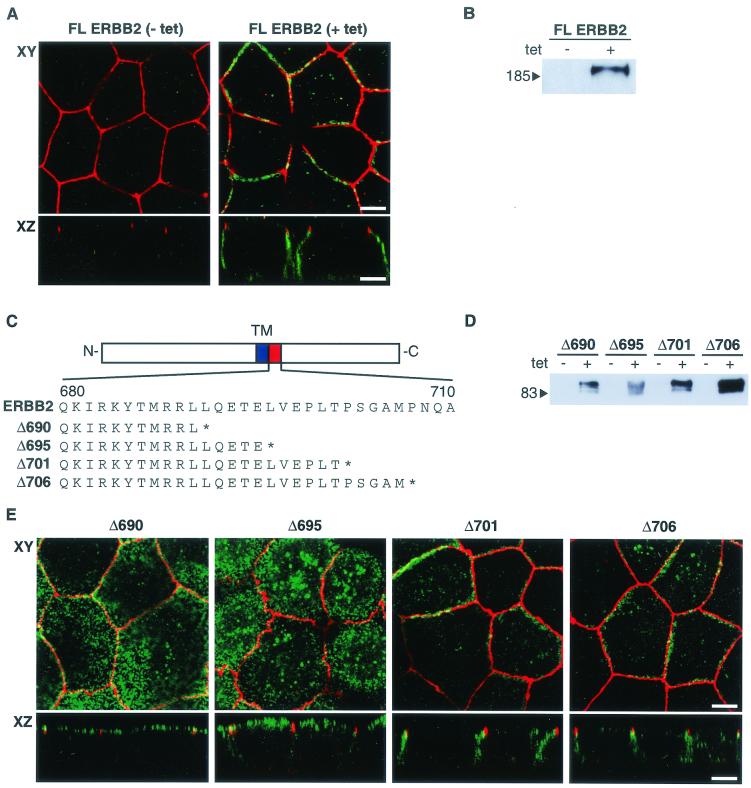
MDCK cells were grown to confluence on a permeable support, and their polarization was assessed by indirect immunofluorescent staining of marker proteins. As expected for polarized epithelial cells, E-cadherin and β-catenin were distributed on the lateral membranes with additional weak staining of the basement membrane, while the zona occludens protein ZO-1 was detected exclusively in the tight junctions at the interface between the apical and basolateral domains (data not shown). To confirm the basolateral localization of ERBB2, stable MDCK cell clones were generated that express the full-length ERBB2 receptor under the transcriptional regulation of a tetracycline-inducible cytomegalovirus promoter. This inducible system allowed ERBB2 expression while minimizing the risk of cellular transformation that could indirectly affect cell polarization (8). Furthermore, ERBB2 expression could be induced after the cells had become confluent, avoiding possible nonpolarized localization of protein that might occur prior to establishment of a polarized monolayer. In agreement with previous reports, we found that human ERBB2 was localized to the basolateral domain of MDCK cells (Fig. 1A) (3, 7). Under the same conditions, ERBB2 expression was not detected in uninduced cells by immunoblotting or immunofluorescence, providing confirmation of the specificity of the antibodies (Fig. 1A and B).
FIG. 1.
ERBB2 contains a juxtamembrane basolateral sorting signal. (A) MDCK cells containing an inducible ERBB2 expression vector were grown on transwell filters to confluence before overnight induction with tetracycline (1 μg/ml). After induction, cells were fixed in methanol, incubated with a rat antibody to ZO-1 and a mouse monoclonal antibody against ERBB2, and subsequently stained with FITC-conjugated goat anti-mouse IgG and TRITC-conjugated goat anti-rat IgG. Panels show confocal images of the XY sections through the tight junctions, and XZ sections with the apical membrane uppermost. (B) Immunoblot of an MDCK cell clone showing induced and uninduced ERBB2 expression. tet, tetracycline. (C) Schematic depiction of ERBB2 vectors showing the transmembrane domain (TM) and the extent of the C-terminal truncations in the juxtamembrane domain (amino acids 680 to 710). The asterisk shows the position of the introduced termination codon. (D) Immunoblot analysis of truncation mutants analyzed by confocal microscopy in panel E. (E) Confocal microscopy images of XY sections and XZ sections prepared and stained as for panel A. Bars, 5 μm.
The juxtamembrane region of ERBB2 contains a basolateral targeting signal.
Previous studies have indicated that basolateral targeting of ERBB2 is governed by interaction of the C terminus with the PDZ domain-containing protein ERBIN (3). However, contrary to this report, we found that a C-terminally truncated ERBB2 receptor lacking the tyrosine kinase domain and the ERBIN binding site at the C terminus (GLDVPV) was still correctly targeted to the basolateral membrane (data not shown). To further investigate basolateral targeting signals in ERBB2, we generated cDNAs encoding proteins with C-terminal truncations at residue 690, 695, 701, or 706 (Fig. 1C). MDCK cell clones were selected that stably expressed truncated ERBB2 proteins of the predicted size, as determined by immunoblotting (Fig. 1D). Examination of several independent clones by confocal microscopy showed that truncation of ERBB2 at amino acid 690 or 695 caused the protein to locate predominantly to apical membranes, whereas basolateral sorting was retained for the truncated ERBB2 proteins that terminated at amino acid 701 or 706 (Fig. 1E). Noninduced cell clones showed no detectable ERBB2 protein by immunoblotting or immunofluorescence (Fig. 1D; also data not shown). A similar distribution of ERBB2 protein was found when the same truncated proteins were expressed in CACO-2 cells by use of a transient transfection assay (data not shown).
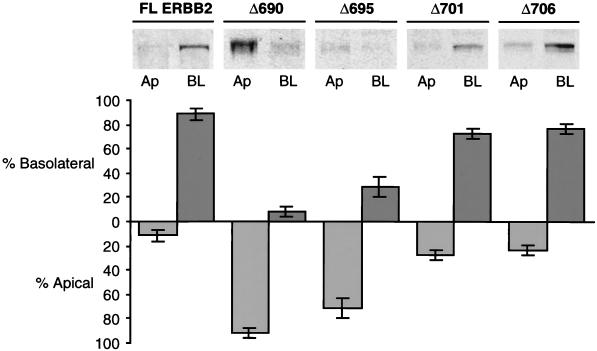
An assessment of the distribution of the receptor between basolateral and apical membranes was obtained by immunoblotting of biotinylated basolateral or apical membrane proteins after their selection on streptavidin beads (Fig. 2) (see Materials and Methods). The results show that full-length ERBB2 and the truncated proteins Δ701 and Δ706 were located predominantly on the basolateral membrane, while the Δ690 and Δ695 proteins were predominantly apical. However, the Δ695 protein also showed a small degree of basolateral expression, which is consistent with a partially compromised basolateral targeting signal. These findings are in agreement with the immunofluorescence results (Fig. 1E). Taken together, these data demonstrate the presence of a signal for basolateral sorting in the juxtamembrane region of ERBB2 between amino acids 690 and 706.
FIG. 2.
Quantification of steady-state cell surface ERBB2. Stable clones of MDCK cells expressing full-length ERBB2 and truncation mutants Δ690, Δ695, Δ701, and Δ706 were grown as described in the legend to Fig. 1. To quantitate the surface expression of ERBB2, apical (Ap) or basolateral (BL) membrane proteins were biotinylated, captured on immobilized streptavidin beads, and then prepared for immunoblotting (see Materials and Methods). Error bars, standard errors.
The juxtamembrane targeting motif of ERBB2 is required for basolateral targeting.
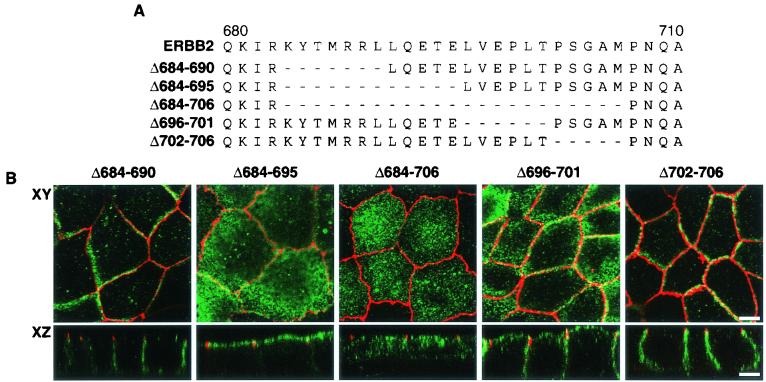
To determine whether the juxtamembrane signal is necessary for basolateral targeting in the presence of the ERBIN binding domain, we introduced limited internal deletions, within the context of the full-length receptor, in and around the region containing the putative sorting signal (Fig. 3A). Clones of MDCK cells containing stable mutant cDNAs were induced to express ERBB2 proteins of the predicted size as revealed by immunoblot analysis (data not shown). Full-length receptor proteins with deletions close to the proposed signal (mutants Δ684-690 and Δ702-706) were shown to localize to the basolateral membrane similarly to the full-length receptor (Fig. 3B). By contrast, the deletion of amino acids 684 to 695 or 684 to 706 resulted in an apical location, although the larger deletion also showed a very significant punctate distribution in the cytoplasm, suggesting a perturbation of endosomal trafficking. A deletion encompassing amino acids 696 to 701 resulted in a shared apical-basolateral localization (Fig. 3B). These results establish the presence of a signal between amino acids 690 to 701 (LLQETELVEPLT) that is necessary for ERBB2 basolateral delivery even in the presence of the C-terminal PDZ protein binding domain.
FIG. 3.
The basolateral and apical localization of ERBB2 proteins containing juxtamembrane internal deletions. (A) Schematic depiction of ERBB2 juxtamembrane domain showing the extents of internal cytoplasmic deletions for mutations Δ684-690, Δ684-695, Δ684-706, Δ696-701, and Δ702-706. (B) Clones of MDCK cells expressing the mutations shown in panel A. Cells were processed and stained as described in the legend to Fig. 1. Bars, 5 μm.
Characterization of the juxtamembrane targeting sequence.
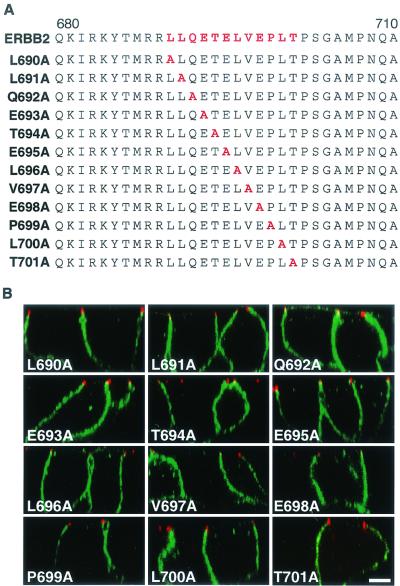
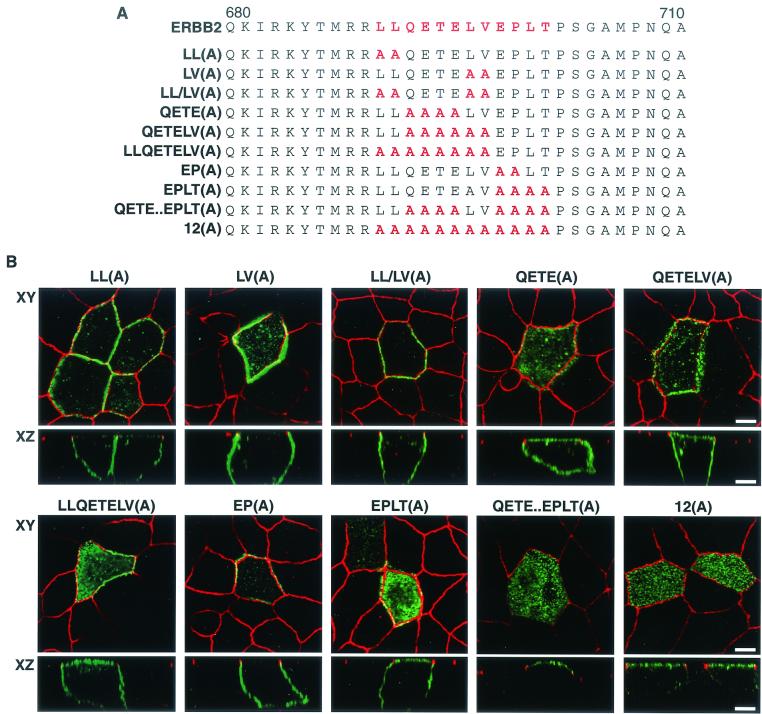
To identify the key residues that constitute the basolateral sorting motif, each amino acid in the candidate region was individually changed to alanine by site-directed mutagenesis (Fig. 4A). All proteins containing a single amino acid substitution were expressed in MDCK cells by transient transfection with the appropriate vector, and the location of the encoded protein was assessed by indirect immunofluorescence and confocal microscopy. Surprisingly, all the ERBB2 proteins containing missense point mutations were correctly localized to the basolateral membrane (Fig. 4B), suggesting a functional redundancy or plasticity of the targeting motif. As several membrane receptors have been shown to contain basolateral sorting signals with essential dihydrophobic residues, the two pairs of hydrophobic residues at positions 690 and 691 (LL) and 696 and 697 (LV) were replaced with alanines (Fig. 5A). After expression in MDCK cells, the two mutated proteins were both shown to locate to the basolateral membrane, suggesting that neither was essential for basolateral sorting (Fig. 5B). To determine whether these dihydrophobic residues might be acting as a redundant signal, an ERBB2 receptor with all four hydrophobic residues (LL and LV) replaced with alanine was expressed in MDCK cells. This protein was also located basolaterally (Fig. 5B), suggesting that neither pair of hydrophobic residues is essential to the sorting signal. We next replaced the cluster QETE, which contains two acidic residues, with alanines; clusters of acidic residues are a feature of some basolateral targeting signals, such as those found in the LDL receptor (27). Expression of this mutated receptor showed both apical and basolateral localization, suggesting either a nonpolar distribution or a partial impairment of the basolateral sorting signal (Fig. 5B).
FIG. 4.
Membrane distribution of ERBB2 proteins containing single amino acids replaced with alanine in polarized MDCK cells. (A) Sequence of the ERBB2 juxtamembrane region showing the positions of the alanine substitutions within the described basolateral sorting motif (red). (B) MDCK cells were transiently transfected with expression vectors containing the mutations shown in panel A, and then the cells were processed and stained as described in the legend to Fig. 1. Panels show vertical XZ sections with FITC staining of ERBB2 and TRITC staining of ZO-1. Bar, 5 μm.
FIG. 5.
Apical and basolateral distribution of ERBB2 proteins containing multiple amino acid substitutions in polarized MDCK cells. (A) Sequence of the ERBB2 juxtamembrane targeting signal (red) showing positions of multiple alanine substitutions. (B) MDCK cells were transiently transfected with expression vectors containing the mutations shown in panel A, and then the cells were processed and stained as described in the legend to Fig. 1. Panels show confocal images of XY sections through the tight junctions and XZ sections with the apical membrane uppermost. Bars, 5 μm.
Introduction of further alanine substitutions of the downstream LV [QETELV(A)] and the upstream LL [LLQETELV(A)] did not significantly enhance the level of apical staining, again indicating no role for the dihydrophobic residues in the sorting signal. However, alanine substitution of the next four C-terminal residues [EPLT(A)] also resulted in an apical and basolateral distribution of ERBB2, identifying a second motif forming part of the sorting signal. Interestingly, a mutated ERBB2 with just the EP replaced with alanines [EP(A)] was found in a basolateral distribution, suggesting that LT might constitute the more important residues. When all 12 amino acids were replaced with alanines, an essentially apical distribution was obtained [Fig. 5B, 12(A)]. A similar result was obtained if the two dihydrophobic pairs were left intact [QETE..EPLT(A)]. To determine whether the ERBIN binding domain might be contributing to basolateral localization when the mutated proteins showed a partial apical relocation [e.g., QETE(A) and EPLT(A)], we generated and tested these mutant receptors with an additional deletion of the last 6 C-terminal amino acids. We did not observe any significant change in apical or basolateral membrane distribution compared to the analogous constructs containing an intact PDZ binding domain (data not shown). Hence, these results indicate a bipartite basolateral sorting signal composed of QETE and EPLT, and they agree with the initial finding that the C-terminal PDZ binding domain of ERBB2 is not required for ERBB2 basolateral targeting in MDCK cells.
The ERBB2 juxtamembrane targeting motif is a dominant basolateral sorting signal.
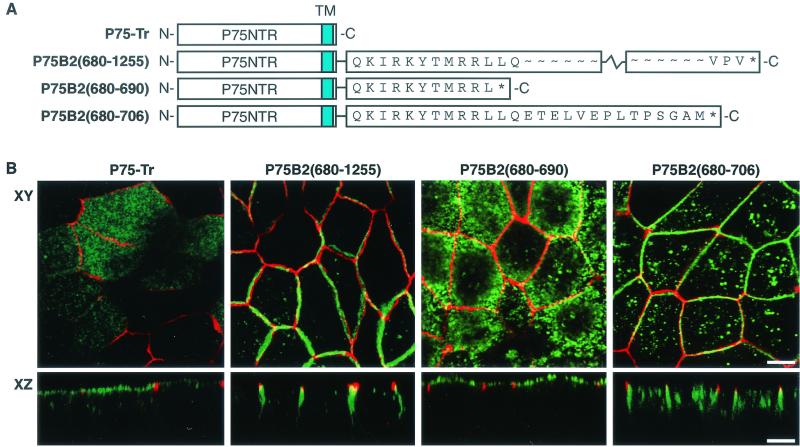
To determine whether the ERBB2 juxtamembrane sequence could act as a dominant basolateral targeting signal, its ability to direct a heterologous protein to the basolateral membrane was examined. P75NTR is normally directed to the apical membrane when it is expressed in MDCK cells. Delivery of P75NTR to the apical membrane was previously shown to involve O-glycosylation sites in the stalk domain of the receptor (4, 37). P75NTR/ERBB2 chimeric receptors were generated by fusing the extracellular and transmembrane domains of P75NTR to either the complete cytoplasmic domain of ERBB2 [P75B2(680-1255)] or the cytoplasmic region of ERBB2 truncated at position 690 [P75B2(680-690)] or position 706 [P75B2(680-706)] (Fig. 6A). Stable MDCK cell clones expressing inducible forms of the chimeric receptors were selected, and their subcellular distribution was analyzed. Chimeric receptors of the predicted sizes were expressed as determined by immunoblot analysis using an antibody directed against the extracellular domain of P75NTR.
FIG. 6.
Apical and basolateral location of P75NTR/ERBB2 chimeras in MDCK cells. (A) Depiction of the P75NTR/ERBB2 chimeras tested in polarized MDCK cells. The extracellular and transmembrane domains of P75NTR were fused in frame either to the full-length cytoplasmic domain of ERBB2 or to truncations similar to those shown in Fig. 1. (B) MDCK cell clones containing inducible P75NTR/ERBB2 chimeras were grown on transwell filters to confluence before induction with tetracycline (1 μg/ml). Induced cells were processed and stained as described in the legend to Fig. 1, except that the ERBB2 antibody was replaced by an antibody against P75NTR. Panels show confocal images of XY sections through the tight junctions and XZ sections with the apical membrane uppermost. Bars, 5 μm.
As expected, expression of P75NTR with a cytoplasmic truncation resulted in sorting to the apical membrane, with a small amount of vesicular intracytoplasmic staining (Fig. 6B) (22). By contrast, the chimeric receptor containing the whole cytoplasmic domain of ERBB2 was directed to the basolateral membrane, demonstrating the presence of a dominant basolateral sorting signal in the cytoplasmic domain of ERBB2 (Fig. 6B). Expression of a P75NTR chimera containing amino acids 680 to 690 of ERBB2 was localized to the apical membrane, whereas a similar chimera containing ERBB2 amino acids 680 to 706 localized to the basolateral membrane, although a significant amount of intracellular staining was also detected. These results indicate the autonomous or dominant nature of the sorting motif between amino acids 692 and 701. Moreover, the ERBB2 basolateral sorting signal was dominant over the apical targeting signal present in the extracellular region of P75NTR.
Missorting of ERBB2 in LLC-PK1 cells.
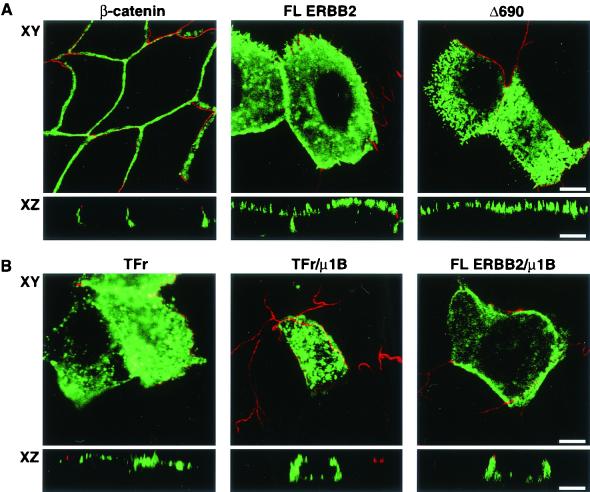
The AP1B complex associated with clathrin-coated vesicles has been implicated in the trafficking of proteins from the TGN to the endocytic compartment, as well as in the targeting of membrane proteins with a tyrosine-containing basolateral sorting signal (2, 10). The LLC-PK1 cell line has been shown to be deficient in the μ1B subunit of the AP1B adaptor complex and missorts basolateral proteins containing a tyrosine-based sorting motif (31). Although the ERBB2 juxtamembrane basolateral sorting signal does not contain a tyrosine, we examined its distribution in LLC-PK1 cells. Surprisingly, significant amounts of full-length ERBB2 were missorted to the apical membrane in this cell line, although the apical localization was not as complete as that of the truncation mutant Δ690 lacking the basolateral targeting signal (Fig. 7A). These results suggest that μ1B might bind other types of basolateral sorting motifs in addition to those containing a tyrosine or, alternatively, that LLC-PK1 cells may possess other deficiencies in protein trafficking.
FIG. 7.
Apical localization of ERBB2 in LLC-PK1 cells can be partially rescued with μ1B. (A) LLC-PK1 cells were transiently transfected with a full-length ERBB2 expression vector or a similar vector containing the C-terminal truncation Δ690 (Fig. 1C). After 2 days, cells were fixed in paraformaldehyde and were incubated with a rat monoclonal antibody to ZO-1 and mouse monoclonal antibodies to either β-catenin or ERBB2 as indicated. The markers for tight junctions and lateral staining, ZO1 and β-catenin, respectively, confirm that the LLC-PK1 cells were polarized. The Δ690 mutant shows an exclusively apical localization similar to that in MDCK cells (Fig. 1E). (B) LLC-PK1 cells were transiently transfected with a transferrin receptor (TFr) or a full-length ERBB2 expression vector with or without a μ1B expression vector. Panels show confocal images of basolaterally localized transferrin receptor or ERBB2 in the presence of μ1B, although some cells still show a mixed localization. Bars, 5 μm.
To test the involvement of μ1B in ERBB2 sorting, we cotransfected a human μ1B expression vector with full-length ERBB2 (pc4-B2) and assessed whether expression of μ1B could redirect ERBB2 to the basolateral membrane. As a control for functional μ1B expression, we separately expressed a human transferrin receptor shown to be mistargeted in LLC-PK1 cells and previously demonstrated to be rescued by μ1B expression (10). As shown in Fig. 7B, both the transferrin receptor and ERBB2 were partially rescued by coexpression with μ1B. While cells expressing ERBB2 showed mainly apical expression and a mixed apical and basolateral expression, those receiving μ1B cDNA showed many more cells expressing ERBB2 basolaterally. A similar change in localization was seen with the transferrin receptor control when it was expressed with μ1B.
Basolateral localization of ERBB2 is independent of the C-terminal PDZ binding domain.
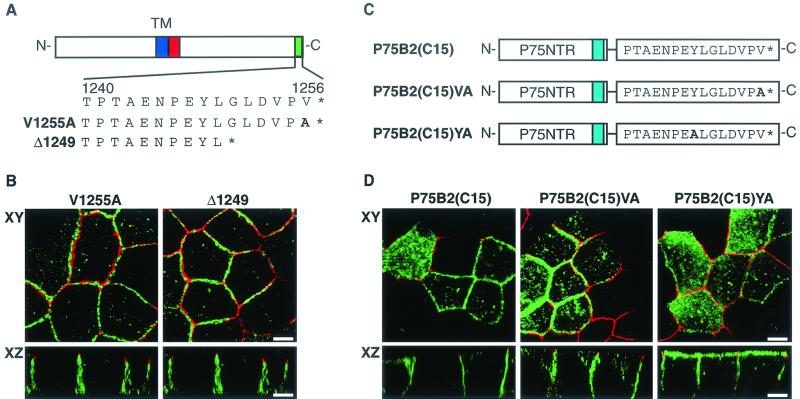
The presence of the ERBB2 juxtamembrane sorting sequence presents an interesting conundrum concerning the role of ERBIN in ERBB2 basolateral targeting. In the experiments described by Borg et al. (3), either loss of the last 6 C-terminal amino acids or a valine-to-alanine C-terminal substitution was sufficient to redirect a chimeric receptor composed of ERBB1 and ERBB2 from the basolateral to the apical membranes of polarized MDCK cells. These mutations correlated with the loss of binding for a PDZ domain-containing protein designated ERBIN, suggesting that ERBIN is responsible for the basolateral targeting of ERBB2. In an attempt to resolve this paradox, we created the same mutations within ERBB2 and analyzed the subcellular localizations of the mutants. In contrast to the findings of Borg et al., the mutant ERBB2 proteins, either minus the last 6 C-terminal amino acids (Δ1249) or with a C-terminal V-to-A substitution at position 1255 (V1255A), showed a basolateral localization that was indistinguishable from that of full-length ERBB2 (compare Fig. 1A and Fig. 8B). These results suggest that ERBIN is not necessary for the selective targeting of ERBB2 to the basolateral membranes of MDCK cells. More recently, Jaulin-Bastard et al. (17) demonstrated that the last 15 C-terminal amino acids of ERBB2 (containing the ERBIN binding site) were sufficient to redirect a heterologous protein from the apical to the basolateral membrane. Given the contradictory results described above, we created a similar chimeric receptor consisting of the extracellular and transmembrane domains of P75NTR fused to the last 15 C-terminal amino acids of ERBB2 [P75B2(C15)]. As can be seen in Fig. 8B, the last 15 C-terminal amino acids of ERBB2 were sufficient to redirect a heterologous apically localized protein to the basolateral membrane, in agreement with the published data. However, the last 15 C-terminal amino acids of ERBB2 (PTAENPEYLGLDVPV) appear to contain two motifs that conform to the consensus sequence of tyrosine-based basolateral targeting signals (underlined), N-P-X-Y and Y-X-X-Φ, where Φ represents a hydrophobic residue. To determine whether this sequence in a juxtamembrane context might act as a targeting motif, we mutated the critical tyrosine residue to alanine [P75B2(C15)YA]. Since the tyrosine is also involved in ERBIN binding (17), we generated a second mutation that would affect ERBIN binding but is outside the putative basolateral targeting motif [P75B2(C15)VA]. The results show that the mutation of tyrosine causes a significant redirection of P75NTR to the apical membrane, whereas the mutation of the C-terminal valine gives a predominantly basolateral localization similar to that of the unmutated chimeric receptor (Fig. 8C and D). This indicates that the basolateral targeting activity of the isolated ERBIN binding site is due to a cryptic tyrosine-based basolateral targeting motif.
FIG. 8.
Mutation analysis of the C-terminal ERBIN binding domain of ERBB2. (A) Schematic depiction of the ERBB2 protein and the C-terminal mutations within the ERBIN binding domain. (B) MDCK cell clones containing inducible mutated ERBB2 expression vectors as shown in panel A were grown on transwell filters to confluence before induction with tetracycline (1 μg/ml). Induced cells were processed and stained as described in the legend to Fig. 1. (C) Schematic depiction of a chimera composed of the extracellular and transmembrane domains of P75NTR fused in frame to the ERBIN binding domain of ERBB2 with or without alanine substitution mutations as shown. (D) Localization of the chimeras diagrammed in panel C after transfection into MDCK cells. Bars, 5 μm.
DISCUSSION
Extensive mutation analyses have demonstrated the presence of a basolateral sorting signal in the cytoplasmic juxtamembrane region of ERBB2, between amino acids 692 and 701. Alanine substitution mutations revealed a bipartite motif composed of the amino acids QETE and (EP)LT. Since substitution of AA for EP in the second part of the motif had no effect on sorting, it is possible that LT are the more important residues. However, the fact that single alanine substitutions throughout the whole region had no significant effect on targeting suggests a degree of plasticity in the sorting sequence that might be reflected in the EP-to-AA mutation. The requirement for both elements of the consensus is supported by the results of internal deletions (Fig. 3). For example, a receptor protein missing LQETE but retaining LVEPTL was essentially apical, as was a receptor protein retaining LQETE but missing LVEPTL (Fig. 3B). A number of membrane receptors have been shown to contain a dihydrophobic amino acid pair that is necessary for basolateral membrane sorting. These include E-cadherin, CD44, and the Fc receptor, and substitution of alanine for one or more of the hydrophobic residues abolishes basolateral targeting. The ERBB2 receptor contains two dihydrophobic residue pairs (LLQETELVEPLT) within the region containing the basolateral sorting motif, but mutation of either or both residue pairs did not affect basolateral targeting. Furthermore, basolateral proteins which rely on a dihydrophobic sorting signal are correctly sorted in both MDCK and LLC-PK1 cells. In the experiments described here, ERBB2 was sorted to the basolateral membrane in MDCK cells but mainly to the apical membrane in LLC-PK1 cells. This observation is consistent with ERBB2 not using a dihydrophobic sorting motif.
LLC-PK1 cells missort proteins such as the transferrin receptor and the LDL receptor that utilize a tyrosine-containing basolateral sorting signal (10). While the ERBB2 receptor is also aberrantly directed to the apical membrane in LLC-PK1 cells, interestingly, it does not contain a tyrosine in the identified sorting signal. The defect in the basolateral targeting of receptor proteins with tyrosine-containing sorting signals in LLC-PK1 cells appears to reside in the loss of the μ1B subunit of the AP1 adaptor complex, since reintroduction of a cDNA encoding the μ1B subunit rescues basolateral sorting of these receptors (10, 11, 31). Basolateral localization of ERBB2 and the transferrin receptor can be rescued by cotransfection with μ1B, although in our hands the rescue appears to be less complete than previously demonstrated for the transferrin receptor (10). Hence, the missorting of ERBB2 in LLC-PK1 cells suggests that AP1B, at least in part, may be instrumental in sorting receptors with non-tyrosine containing motifs, although this would need to be firmly established through binding studies using the adaptin heteromeric complex proteins.
Bipartite basolateral sorting motifs are not unusual. For example, the LDL receptor and the aquaporin 4 (AQP4) water channel each have a tyrosine-containing element as well as one encompassing a cluster of acidic residues (25, 27). However, while the ERBB2 sorting signal contains two or three acidic residues, they are dispersed and do not really qualify as a true acidic cluster. For example, in the LDL receptor and AQP4 water channel, 3 out of 4 key amino acid residues are acidic (25, 27). Moreover, AQP4 also has a dihydrophobic pair of amino acids adjacent to the acidic cluster that is needed for efficient basolateral sorting. A similar dihydrophobic pair of amino acids (LV) follows the two acidic residues in ERBB2 but did not appear to contribute to basolateral sorting. Furin endopeptidase also appears to contain a bipartite basolateral sorting sequence. In this case, a separate cluster of 4 acidic residues is followed by a separate phenylalanine-isoleucine pair (34). The FI pair may be equivalent to the dihydrophobic signal found in many other receptors. Therefore, it appears that the ERBB2 sorting sequence is similar to, but distinct from, those previously described.
A region of 23 amino acids in the cytoplasmic juxtamembrane of the epidermal growth factor receptor has been shown to encompass a basolateral sorting signal (14). In this study it was suggested that these amino acids might form a pair of amphipathic helices. However, the sequence we define in ERBB2 is no more than 10 amino acids long and locates on the basis of amino acid similarity only to the distal helix and the region between the two proposed helical domains of the human epidermal growth factor receptor. Nevertheless, this study, together with the results reported here, provides strong evidence for a basolateral sorting sequence in the juxtamembrane region in this family of receptors.
The extreme C-terminal region of ERBB2 contains a binding domain for the PDZ protein ERBIN, which colocalizes with ERBB2 at basolateral membranes (3). The studies of Borg et al. found that ERBIN was necessary for the basolateral localization of ERBB2, since mutations that abolished ERBIN binding also led to the mislocalization of ERBB2 to apical membranes. These findings are not consistent with the results of our experiments, since we did not find that the ERBIN binding site was necessary for basolateral targeting of the ERBB2 receptor in MDCK cells (Fig. 8). Indeed, mutation of the ERBIN binding site did not cause full-length ERBB2 to mislocalize. However, the carboxy-terminal residues of ERBB2 have a capacity to act as a basolateral targeting motif, since they were able to direct a heterologous protein to the basolateral membrane (Fig. 8).
It is not clear why our results differ from those of Borg et al., although we consider that the most likely explanation lies in their use of a chimeric receptor containing the extracellular domain of epidermal growth factor receptor fused to the cytoplasmic domain of ERBB2. It is possible that the juxtamembrane targeting signal is in some way compromised in the chimera, causing a greater emphasis to be placed on the PDZ domain. However, the internal deletion analysis presented here would not support this notion. It is also possible that basolateral sorting of these receptors could be influenced by their interaction with other proteins in the secretory pathway, such that they may provide a chaperone function. Alternatively, the persistence of ERBB2 at the basolateral membrane might be dependent on the PDZ domain acting as a retention determinant, as seen in the GABA transporter (32). Interestingly, the ability of the isolated ERBIN binding site to direct P75NTR to the basolateral membrane may rely on the presence of a cryptic sorting signal that functions when placed in a juxtamembrane position. This is strongly supported by the mutation analysis of the ERBIN binding site shown in Fig. 8. Moreover, similar tyrosine-based motifs have previously been demonstrated to act as basolateral targeting signals in the LDL receptor (NPVY) and the human immunodeficiency virus type 1 envelope glycoprotein (YSPL) (24, 27).
We conclude that the PDZ binding domain is not likely to direct basolateral sorting of ERBB2 in MDCK cells; rather, sorting is dependent on a novel bipartite juxtamembrane signal. This signal appears to be autonomous and may utilize, directly or indirectly, the AP1B adaptor complex as one means of trafficking to the cell surface.
Acknowledgments
We thank Senthil Muthuswamy (Cold Spring Harbor Laboratories, Plainview, N.Y.) for providing the P75NTR cDNA, Colin Hopkins (Imperial College) for the transferrin receptor expression vector and antibody, and Sharon Tooze (Cancer Research UK) for critical reading of the manuscript.
REFERENCES
- 1.Alroy, I., and Y. Yarden. 1997. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 410:83-86. [DOI] [PubMed] [Google Scholar]
- 2.Bonifacino, J. S., and E. C. Dell'Angelica. 1999. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borg, J. P., S. Marchetto, A. Le Bivic, V. Ollendorff, F. Jaulin-Bastard, H. Saito, E. Fournier, J. Adelaide, B. Margolis, and D. Birnbaum. 2000. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat. Cell Biol. 2:407-414. [DOI] [PubMed] [Google Scholar]
- 4.Breuza, L., M. Garcia, M. H. Delgrossi, and A. Le Bivic. 2002. Role of the membrane-proximal O-glycosylation site in sorting of the human receptor for neurotrophins to the apical membrane of MDCK cells. Exp. Cell Res. 273:178-186. [DOI] [PubMed] [Google Scholar]
- 5.Carraway, K. L., III, and C. Sweeney. 2001. Localization and modulation of ErbB receptor tyrosine kinases. Curr. Opin. Cell Biol. 13:125-130. [DOI] [PubMed] [Google Scholar]
- 6.Coussens, L., T. L. Yang-Feng, Y. C. Liao, E. Chen, A. Gray, J. McGrath, P. H. Seeburg, T. A. Libermann, J. Schlessinger, U. Francke, et al. 1985. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230:1132-1139. [DOI] [PubMed] [Google Scholar]
- 7.Darcy, K. M., D. Zangani, A. L. Wohlhueter, R. Y. Huang, M. M. Vaughan, J. A. Russell, and M. M. Ip. 2000. Changes in ErbB2 (her-2/neu), ErbB3, and ErbB4 during growth, differentiation, and apoptosis of normal rat mammary epithelial cells. J. Histochem. Cytochem. 48:63-80. [DOI] [PubMed] [Google Scholar]
- 8.Di Fiore, P. P., J. H. Pierce, M. H. Kraus, O. Segatto, C. R. King, and S. A. Aaronson. 1987. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science 237:178-182. [DOI] [PubMed] [Google Scholar]
- 9.Fanning, A. S., and J. M. Anderson. 1999. Protein modules as organizers of membrane structure. Curr. Opin. Cell Biol. 11:432-439. [DOI] [PubMed] [Google Scholar]
- 10.Folsch, H., H. Ohno, J. S. Bonifacino, and I. Mellman. 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99:189-198. [DOI] [PubMed] [Google Scholar]
- 11.Folsch, H., M. Pypaert, P. Schu, and I. Mellman. 2001. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 152:595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graus-Porta, D., R. R. Beerli, J. M. Daly, and N. E. Hynes. 1997. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 16:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobert, M., and C. Carlin. 1995. Cytoplasmic juxtamembrane domain of the human EGF receptor is required for basolateral localization in MDCK cells. J. Cell. Physiol. 162:434-446. [DOI] [PubMed] [Google Scholar]
- 14.Hobert, M. E., S. J. Kil, M. E. Medof, and C. R. Carlin. 1997. The cytoplasmic juxtamembrane domain of the epidermal growth factor receptor contains a novel autonomous basolateral sorting determinant. J. Biol. Chem. 272:32901-32909. [DOI] [PubMed] [Google Scholar]
- 15.Hogan, B. L. 1999. Morphogenesis. Cell 96:225-233. [DOI] [PubMed] [Google Scholar]
- 16.Hunziker, W., and C. Fumey. 1994. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 13:2963-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaulin-Bastard, F., H. Saito, A. Le Bivic, V. Ollendorff, S. Marchetto, D. Birnbaum, and J. P. Borg. 2001. The ERBB2/HER2 receptor differentially interacts with ERBIN and PICK1 PSD-95/DLG/ZO-1 domain proteins. J. Biol. Chem. 276:15256-15263. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, D., A. Lanahan, C. R. Buck, A. Sehgal, C. Morgan, E. Mercer, M. Bothwell, and M. Chao. 1986. Expression and structure of the human NGF receptor. Cell 47:545-554. [DOI] [PubMed] [Google Scholar]
- 19.Kim, S. K. 1997. Polarized signaling: basolateral receptor localization in epithelial cells by PDZ-containing proteins. Curr. Opin. Cell Biol. 9:853-859. [DOI] [PubMed] [Google Scholar]
- 20.Klapper, L. N., S. Glathe, N. Vaisman, N. E. Hynes, G. C. Andrews, M. Sela, and Y. Yarden. 1999. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc. Natl. Acad. Sci. USA 96:4995-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knust, E. 2000. Control of epithelial cell shape and polarity. Curr. Opin. Genet. Dev. 10:471-475. [DOI] [PubMed] [Google Scholar]
- 22.Le Bivic, A., Y. Sambuy, A. Patzak, N. Patil, M. Chao, and E. Rodriguez-Boulan. 1991. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J. Cell Biol. 115:607-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Gall, A. H., S. K. Powell, C. A. Yeaman, and E. Rodriguez-Boulan. 1997. The neural cell adhesion molecule expresses a tyrosine-independent basolateral sorting signal. J. Biol. Chem. 272:4559-4567. [DOI] [PubMed] [Google Scholar]
- 24.Lodge, R., J. P. Lalonde, G. Lemay, and E. A. Cohen. 1997. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 16:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madrid, R., S. Le Maout, M. B. Barrault, K. Janvier, S. Benichou, and J. Merot. 2001. Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. EMBO J. 20:7008-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matter, K. 2000. Epithelial polarity: sorting out the sorters. Curr. Biol. 10:R39-R42. [DOI] [PubMed] [Google Scholar]
- 27.Matter, K., W. Hunziker, and I. Mellman. 1992. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell 71:741-753. [DOI] [PubMed] [Google Scholar]
- 28.Mikaelian, I., and A. Sergeant. 1992. A general and fast method to generate multiple site directed mutations. Nucleic Acids Res. 20:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miranda, K. C., T. Khromykh, P. Christy, T. L. Le, C. J. Gottardi, A. S. Yap, J. L. Stow, and R. D. Teasdale. 2001. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J. Biol. Chem. 276:22565-22572. [DOI] [PubMed] [Google Scholar]
- 30.Mostov, K. E., M. Verges, and Y. Altschuler. 2000. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 12:483-490. [DOI] [PubMed] [Google Scholar]
- 31.Ohka, S., H. Ohno, K. Tohyama, and A. Nomoto. 2001. Basolateral sorting of human poliovirus receptor alpha involves an interaction with the μ1B subunit of the clathrin adaptor complex in polarized epithelial cells. Biochem. Biophys. Res. Commun. 287:941-948. [DOI] [PubMed] [Google Scholar]
- 32.Perego, C., C. Vanoni, A. Villa, R. Longhi, S. M. Kaech, E. Frohli, A. Hajnal, S. K. Kim, and G. Pietrini. 1999. PDZ-mediated interactions retain the epithelial GABA transporter on the basolateral surface of polarized epithelial cells. EMBO J. 18:2384-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmen, T., S. Honing, A. Icking, R. Tikkanen, and W. Hunziker. 2002. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 4:154-159. [DOI] [PubMed] [Google Scholar]
- 34.Simmen, T., M. Nobile, J. S. Bonifacino, and W. Hunziker. 1999. Basolateral sorting of furin in MDCK cells requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster. Mol. Cell. Biol. 19:3136-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas, D. C., C. B. Brewer, and M. G. Roth. 1993. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J. Biol. Chem. 268:3313-3320. [PubMed] [Google Scholar]
- 36.Yamamoto, T., S. Ikawa, T. Akiyama, K. Semba, N. Nomura, N. Miyajima, T. Saito, and K. Toyoshima. 1986. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature 319:230-234. [DOI] [PubMed] [Google Scholar]
- 37.Yeaman, C., A. H. Le Gall, A. N. Baldwin, L. Monlauzeur, A. Le Bivic, and E. Rodriguez-Boulan. 1997. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J. Cell Biol. 139:929-940. [DOI] [PMC free article] [PubMed] [Google Scholar]