Abstract
The core complex of Coat Protein I (COPI), known as coatomer, is sufficient to induce coated vesicular-like structures from liposomal membrane. In the context of biological Golgi membrane, both palmitoyl-coenzyme A (p-coA) and ARFGAP1, a GTPase-activating protein (GAP) for ADP-Ribosylation Factor 1, also participate in vesicle formation, but how their roles may be linked remains unknown. Moreover, whether COPI vesicle formation from Golgi membrane requires additional factors also remains unclear. We now show that Brefeldin-A ADP-Ribosylated Substrate (BARS) plays a critical role in the fission step of COPI vesicle formation from Golgi membrane. This role of BARS requires its interaction with ARFGAP1, which is in turn regulated oppositely by p-coA and nicotinamide adenine dinucleotide, which act as cofactors of BARS. Our findings not only identify a new factor needed for COPI vesicle formation from Golgi membrane but also reveal a surprising mechanism by which the roles of p-coA and GAP are linked in this process.
Keywords: ARFGAP1, BARS, COPI, membrane fission, vesicle formation
Introduction
Coat proteins play a key role in intracellular transport by coupling the deformation compartmental membrane for vesicle formation with cargo sorting that involves coats binding to specific sequences in the cytoplasmic domain of cargo proteins for their proper packaging into nascent vesicles. Coat Protein I (COPI) is one of the best characterized coat complexes (Bonifacino and Lippincott-Schwartz, 2003). Upon activation of the small GTPase ADP-Ribosylation Factor 1 (ARF1), coatomer (the core complex of COPI) becomes recruited onto Golgi membrane (Donaldson et al, 1992). Incubation of an artificial liposomal membrane with purified coatomer and ARF1 has been shown to induce coated vesicular-like structures from a larger liposomal membrane (Spang et al, 1998; Bremser et al, 1999), providing evidence that coatomer represents the core machinery in COPI vesicle formation.
To identify the additional factors critical for COPI vesicle formation from the biological Golgi membrane, reconstitutions have been performed by incubating isolated Golgi membrane with purified coatomer and ARF1. When this membrane was washed to release peripheral membrane factors, palmitoyl coenzyme-A (p-CoA) was revealed to play a role in COPI vesicle formation at the fission step (Ostermann et al, 1993). How p-coA accomplishes this role remains unclear, although one mechanism has been suggested to involve the transfer of the palmitoyl chain to target proteins and/or lipids (Pfanner et al, 1989). In this respect, an intriguing potential connection has been the recent finding that phospholipid acyltransferase activity participates in the fission of compartmental membranes. Endophilin has been identified to possess such an activity to mediate the fission of clathrin-coated vesicles (Schmidt et al, 1999). Moreover, Brefeldin-A (BFA)-induced ADP-Ribosylation Substrate (BARS) has been shown to possess such an activity, and also induce the fission of Golgi tubules (Weigert et al, 1999). This latter finding has been surprising, as BARS is a splice variant of C-Terminal Binding Protein 1 (CtBP1), which has been well characterized as a transcription corepressor (Chinnadurai, 2002). Nevertheless, recent studies have begun to validate the fission activity of BARS, as it is critical for Golgi fragmentation during mitosis (Hidalgo Carcedo et al, 2004), and participates in the formation of post-Golgi transport carriers (Bonazzi et al, 2005).
In the context of COPI transport, ARFGAP1, a GTPase-activating protein (GAP) for ARF1, can substitute for p-coA in reconstituting the formation of COPI vesicles from Golgi membrane (Yang et al, 2002). Besides its known role as a negative regulator of ARF1, GAP was shown to possess a novel function as an ARF1 effector by acting as a component of the COPI coat complex (Yang et al, 2002; Lee et al, 2005). In the current study, we elucidate a role for BARS in the fission step of COPI vesicle formation, and also reveal a mechanism by which the roles of GAP and p-coA are linked in this process.
Results
BARS is critical for reconstituting COPI vesicles from Golgi membrane
Using an established two-stage incubation system to reconstitute COPI vesicles (Ostermann et al, 1993), we previously showed that the addition of GAP at the second-stage incubation was sufficient for vesicle formation from Golgi membrane washed with 0.5 M KCl (Yang et al, 2002). However, when Golgi membrane was washed more stringently with 3 M KCl, we found that the addition of GAP at the second stage was no longer sufficient (Figure 1A). Analyzing the wash fraction by gel filtration, we found that one fraction restored vesicle formation (Figure 1B). As this fraction comigrated with the 43 kDa marker, and BARS is a 50 kDa protein that has been shown to have fission activity on Golgi membrane (Weigert et al, 1999), we next immunoblotted the different fractions for BARS, and found that only the active fraction contained a significant level of BARS (Figure 1B). Consistent with this result, BARS was detectable on unwashed Golgi membrane and on membranes washed with 0.5 M KCl, but the level became significantly reduced on Golgi membrane washed with 3 M KCl (Figure 1C). Confirming that BARS was critical in reconstituting COPI vesicles from Golgi membrane washed with 3 M KCl, we found that vesicle formation was restored, when recombinant BARS was incubated in conjunction with GAP during the second-stage incubation. Similar results were obtained using Golgi membrane isolated from CHO cells (Figure 1D) or from rat liver (Supplementary Figure 1A).
Figure 1.
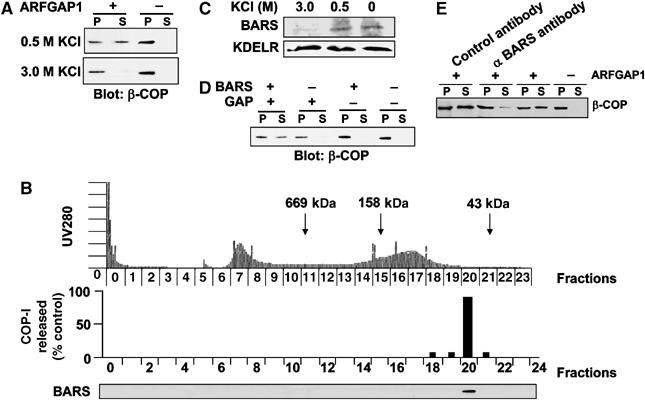
BARS is a peripheral Golgi membrane protein critical for reconstituting COPI vesicles. (A) GAP is no longer sufficient to complete the second stage of the vesicle reconstitution system when Golgi membrane is washed with 3 M KCl. CHO Golgi membrane was subjected to salt washes as indicated and then used for the two-stage incubation system, followed by immunoblotting of pellet (P) and supernatant (S) for β-COP after the second-stage incubation. (B) The critical activity lost upon washing Golgi membrane with 3 M KCl contains BARS. The 3 M KCl wash fraction was analyzed by gel filtration with eluted fractions monitored for total proteins (top panel), assayed for activity in restoring COPI vesicle formation (middle panel), and immunoblotted for BARS (bottom panel). (C) Endogenous BARS on Golgi membrane is markedly reduced by 3 M KCl wash. CHO Golgi membrane is subjected to salt washes as indicated, followed by immunoblotting for proteins as indicated. The level of the transmembrane KDELR is used to assess the levels of Golgi membrane in each condition. (D) The addition of BARS restores the ability of GAP to complete the second-stage incubation using Golgi membrane washed with 3 M KCl. CHO Golgi membrane washed with 3 M KCl was used for the two-stage incubation system, followed by immunoblotting of pellet (P) and supernatant (S) for β-COP after the second-stage incubation. (E) Antibody against endogenous BARS on Golgi membrane inhibits the reconstitution of COPI vesicles. CHO Golgi membrane washed with 0.5 M KCl was incubated with an anti-BARS antibody or an anti-mannosidase I antibody (as control), and then subjected to the two-stage incubation system, followed by immunoblotting of pellet (P) and supernatant (S) for β-COP after the second-stage incubation.
As confirmation that BARS was critical for the active fraction of the salt wash, we found that this activity was lost upon the immunodepletion of BARS (Supplementary Figure 1B). The addition of recombinant BARS to the depleted fraction restored vesicle formation (Supplementary Figure 1C), thus excluding the possibility that a copurified protein other than BARS was the critical factor in the active fraction. We also confirmed that endogenous BARS was critical for COPI vesicle formation, as the addition of an anti-BARS antibody blocked the reconstitution of COPI vesicles from Golgi membrane washed with 0.5 M KCl, which would have retained endogenous BARS (Figure 1E).
We next characterized COPI vesicles reconstituted using the new method of washing Golgi membrane with 3 M KCl. We had shown previously by a rapid, high-speed ultracentrifugation procedure that the redistribution of coatomer from Golgi membrane washed with 0.5 M KCl during the second-stage incubation represented mostly COPI vesicles (Lee et al, 2005). Using the same approach, we found that the release of coatomer from the second-stage incubation using Golgi membrane washed with 3 M KCl also represented mostly COPI vesicles. First, coatomer from the supernatant fraction of this second-stage incubation was detected virtually all in the pelleted fraction upon high-speed centrifugation, while purified soluble coatomer, either alone (Figure 2A) or in combination with GAP and BARS (Supplementary Figure 1D), remained virtually all in the supernatant. Second, examination of the pelleted coatomer fraction by electron microscopy (EM) revealed mostly vesicles of approximately 80 nm diameter, which were positive for subunits of coatomer (Figure 2B).
Figure 2.
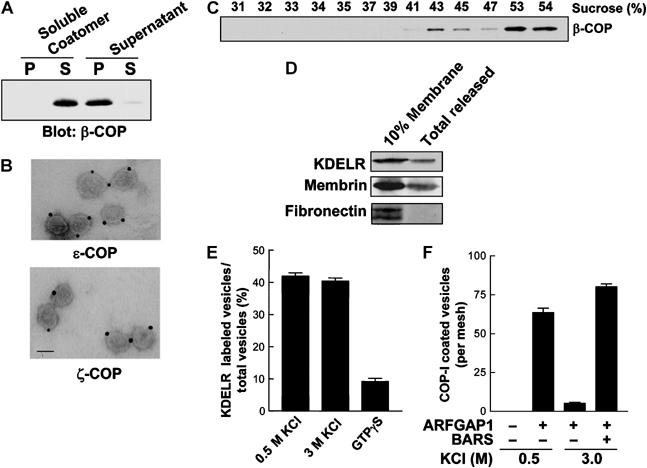
Characterization of vesicle reconstitution using Golgi membrane washed with 3 M KCl. (A) Coatomer released from Golgi membrane during the second-stage incubation is mainly membrane-bound. CHO Golgi membrane was washed with 3 M KCl, and then subjected to the two-stage incubation system. The supernatant from the second-stage incubation, or soluble coatomer as control, was subjected to ultracentrifugation at 200 000 g for 1 h to obtain pellet (P) and supernatant (S), followed by immunoblotting for β-COP. (B) The pellet fraction after ultracentrifugation of the supernatant derived from the second-stage incubation contains mostly COPI vesicles. The pellet fraction in (A) was analyzed by whole-mount EM with immunogold labeling using antibodies directed against subunits of COPI as indicated; bar, 50 nm. (C) Vesicles reconstituted from Golgi membrane washed with 3 M KCl have density characteristic of COPI vesicles. CHO Golgi membrane washed with 3 M KCl was used in the two-stage incubation system, and then the supernatant from the second stage was subjected to equilibrium centrifugation, followed by fractionation and immunoblotting for β-COP. (D) Golgi membrane washed with 3 M KCl selectively releases cargo proteins during the second-stage incubation. Golgi membrane was isolated from CHO cells stably transfected with myc-tagged KDELR, washed with 3 M KCl, and then used for the two-stage incubation system. The supernatant after the second-stage incubation was then immunoblotted for proteins as indicated, and, as comparison, 10% of Golgi membrane used for the reconstitution was also immunoblotted. (E) Vesicles derived from Golgi membrane washed with 3 M KCl have similar levels of KDELR as those derived from Golgi membrane washed with 0.5 M KCl. Golgi membrane was isolated from CHO cells stably transfected with myc-tagged KDELR, and then washed as indicated for use in the two-stage incubation system. The level of myc-tagged KDELR on vesicles was quantified by EM. The mean derived from three independent experiments is shown with standard error. As a reference point for cargo sorting, GTPγS is used for the two-stage incubation system using Golgi membrane washed with 0.5 M KCl, as has been shown previously to inhibit cargo sorting. (F) Golgi membrane washed with 3 M KCl reconstitutes a similar level of COPI vesicles as those washed with 0.5 M KCl. CHO Golgi membrane was subjected to different washes as indicated and then used for the two-stage incubation system with components added at the second stage as indicated. The incubation was then examined by EM to quantify the level of COPI-positive vesicles using antibodies directed against COPI subunits. The mean derived from three independent experiments is shown with standard error.
The level of immunogold labeling on the reconstituted vesicles was also similar to that previously observed for COPI vesicles reconstituted by the more traditional methods of using either GTPγS or p-coA (Yang et al, 2002), or for COPI vesicles in vivo (Orci et al, 1997). Nevertheless, because the degree of immunogold labeling could vary due to multiple technical factors, we confirmed the coating density of the reconstituted vesicles by showing that they floated to 43% sucrose upon equilibrium centrifugation (Figure 2C), which was similar to COPI vesicles previously reconstituted using less stringently washed Golgi membrane (Yang et al, 2002). Moreover, as observed previously using GTP in the reconstitution (Yang et al, 2002), the new method of reconstituting COPI vesicles also allowed their subsequent uncoating, as indicated by coatomer at the bottom of the flotation gradient (>53% sucrose in Figure 2C). As an additional insight, we noted that, even though GAP and BARS were needed at similar levels to complete the second-stage incubation of the reconstitution system (Supplementary Figure 1E), gradient analysis showed that the distribution of BARS did not exhibit a similar peak that marked COPI vesicles as seen for coatomer and GAP (Supplementary Figure 1F). Thus, we concluded that BARS was unlikely to function as a structural coat component on COPI vesicles, as previously concluded for GAP (Yang et al, 2002).
Assessing cargo sorting, we detected both the KDEL receptor (a cargo protein specific for COPI retrograde transport (Girod et al, 1999; White et al, 1999)) and membrin (a Golgi SNARE that interacts with ARF1 (Honda et al, 2005)), to be released from Golgi membrane during the second-stage incubation (Figure 2D). In contrast, fibronectin, a luminal secretory protein that also has a significant Golgi distribution (Ledger et al, 1980), remained with the Golgi membrane (Figure 2D). Confirming this suggested selectivity in cargo sorting into reconstituted COPI vesicles, we found by quantitative immunogold EM that KDELR was detected to a similar extent in vesicles reconstituted either by the new method (using Golgi membrane washed with 3 M KCl) or the previous method (using Golgi membrane washed with 0.5 M KCl) (Figure 2E).
We also ruled out more conclusively that the 3 M KCl wash was too harsh to allow Golgi membrane to reconstitute COPI vesicles through physiologic mechanisms. First, examining the morphology of the Golgi membrane, we found that it was not significantly altered by the 3 M KCl wash (Supplementary Figure 2A). Second, this wash did not result in the luminal fibronectin being released into the wash fraction (Supplementary Figure 2B). Third, tethering proteins, such as p115, GRASP55, and GRASP65, were also mostly retained on Golgi membrane after washing with 3 M KCl (Supplementary Figure 2C). Fourth, the effect of washing Golgi membrane with 3 M KCl could be reproduced by washing Golgi membrane with 2 M urea (Supplementary Figure 2D). Notably, 2.5 M urea had been used previously to wash ER membrane in the reconstitution of COPII vesicles (Spang and Schekman, 1998), providing precedence for highly stringent washing still enabling the physiologic mechanisms of vesicle formation. Fifth, as control, BARS alone (Figure 1D) or incubation of ARFGAP1 in conjunction with the Golgi tethering complex COG as control (Supplementary Figure 2E) did not allow the completion of the second-stage incubation. Sixth, compared to reconstitution using Golgi membrane washed with 0.5 M KCl, we found by quantitative immunogold EM that the new reconstitution using Golgi membrane washed with 3 M KCl produced a similar level of COPI-positive vesicles (Figure 2F). Thus, we concluded that Golgi membrane washed with 3 M KCl was able to support the physiologic mechanisms of COPI vesicle formation.
COPI vesicle formation does not require the acyltransferase activity of BARS
To gain insight into how BARS functioned in COPI vesicle formation, we noted that the crystal structure of BARS had revealed that it contained at least two functional domains (Nardini et al, 2003), a central domain that bound nicotinamide adenine dinucleotide (NAD), referred as the NAD-binding domain (NBD), and the rest of the protein that comes together to form one domain, referred as the substrate-binding domain (SBD). In addition to these domains, we also generated other domain constructs: N-terminal portion (NTP, consisting of the N-terminal subdomain of SBD and NBD), C-terminal portion (CTP, consisting of the C-terminal subdomain of SBD and NBD), and C-terminal domain (CTD, representing the extreme C-terminal subdomain) (Figure 3A).
Figure 3.
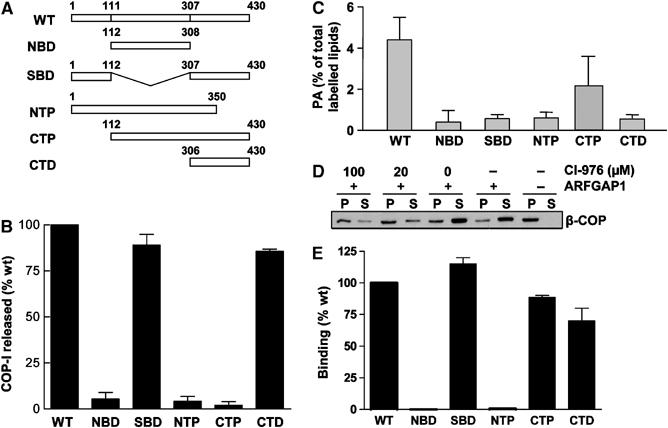
COPI vesicle formation does not require the acyltransferase activity of BARS. (A) Schematic representation of the different domain mutants of BARS. (B) COPI vesicle formation in the presence of different BARS mutants. CHO Golgi membrane washed with 3 M KCl was used for the two-stage incubation system, with the second-stage incubation containing GAP and different forms of BARS as indicated. The level of β-COP released into the supernatant after this stage was quantified, and then normalized to that derived from using the wild-type BARS for incubation. The mean of this normalized value derived from three independent experiments is shown with standard error. (C) Acyltransferase activity of different mutant BARS. The level of phosphatidic acid (PA) generated by the transfer of fatty acyl-CoA onto lysophosphatidic acid, catalyzed by the different forms of BARS, was measured. The mean derived from three independent experiments is shown with standard error. (D) COPI vesicle formation requires an acyltransferase activity. CHO Golgi membrane washed with 0.5 M KCl was used for the two-stage incubation, with the second stage using components as indicated, followed by immunoblotting of pellet (P) and supernatant (S) for β-COP after the second-stage incubation. (E) Binding to GAP by different mutant BARS. GAP fused to GST that had been gathered onto glutathione beads was incubated with different forms of BARS in a pulldown assay. The level of different BARS on beads was quantified, normalized to the level of GAP, and then compared to that of the wild-type BARS. The mean derived from three independent experiments is shown with standard error.
These domain mutants were initially examined for their ability to support vesicle formation from Golgi membrane washed with 3 M KCl. Quantifying this result, we found that NBD, NTP, and CTP were markedly impaired in vesicle formation as compared to the full-length BARS, while SBD and CTD still retained significant ability to support vesicle formation (Figure 3B). We ruled out that the CTD construct resulted in a misfolded hydrophobic protein that induced vesicle formation by a nonspecific process, as deliberate denaturation of the CTD mutant by boiling eliminated its activity in vesicle formation (Supplementary Figure 3A). We then assessed whether the different BARS mutants still possessed acyltransferase activity (Figure 3C), which was shown previously to be associated with the ability of BARS to induce fission of Golgi tubules (Weigert et al, 1999). Surprisingly, the SBD and CTD mutants, which still possessed significant ability to support vesicle formation, had markedly impaired acyltransferase activity (compare Figure 3B and C). Thus, we concluded that the acyltransferase activity of BARS was not the only mechanism by which it participated in COPI vesicle formation.
However, we also noted that COPI vesicle formation had been shown previously to require an acyltransferase activity on Golgi membrane (Pfanner et al, 1989). To sort out an explanation, we found that an inhibitor of phospholipid acyltransferase activity on Golgi membrane, CI-976 (Drecktrah et al, 2003), also inhibited vesicle formation in our reconstitution system (Figure 3D). Thus, as this acyltransferase activity is known to be possessed by multiple proteins other than BARS, we concluded that, while such an activity was required to reconstitute COPI vesicles from Golgi membrane, this activity possessed specifically by BARS was not essential.
As an insight into an alternate mechanism to explain how BARS participated in COPI vesicle formation, we explored whether BARS interacted with GAP in a pulldown assay (Supplementary Figure 3B). Quantifying the interactions, we found that SBD, CTP, and CTD bound to GAP similarly as the full-length protein, while NBD and NTP showed marked impairment (Figure 3E). The noted interactions were likely specific, as wild-type BARS did not exhibit significant binding to other ARF GAPs, such as ACAP1 and PAP (Supplementary Figure 3C). We also noted that the recruitment of BARS to Golgi membrane was not affected by the presence of GAP (Supplementary Figure 3D). Moreover, the CTD domain could interact directly with membrane lipids, as indicated by its direct binding to liposomal membrane (Supplementary Figure 3E). Thus, we concluded that the interaction between BARS and GAP likely occurred after BARS became membrane-bound.
The CTP mutant accumulates COPI-positive buds on Golgi membrane by affecting the fission step
To gain further insight into the role of the interaction between GAP and BARS, we initially tested whether this interaction affected the GAP catalytic activity. However, we observed no significant effect (Supplementary Figure 4A). Thus, as a clue to an alternate mechanism, we noted that, while the binding results of the different forms of BARS largely correlated with their ability to support vesicle formation, a notable exception was the CTP mutant, because it retained significant ability to bind GAP, but did not support vesicle formation. As this mutant BARS contained the CTD fragment, which was fully able to support vesicle formation, an intriguing possibility was that the additional sequences in CTP would render this mutant acting dominant negatively in suppressing vesicle formation.
We first confirmed this possibility, as incubation with an equal molar ratio of wild type and CTP led to significant inhibition in vesicle formation, with this inhibition being nearly complete when the CTP mutant was added at 10-fold excess (Supplementary Figure 4B). This inhibition was reversible, as adding the wild-type protein in excess of the CTP mutant reversed the effect (Supplementary Figure 4C). Consistent with these findings, the addition of CTP to the vesicle reconstitution system using Golgi membrane washed with 0.5 M KCl, which retained endogenous BARS, also inhibited vesicle formation (Figure 4A). Thus, we examined the Golgi membrane after this incubation to gain insight into how the CTP mutant inhibited vesicle formation. Strikingly, we detected an accumulation of COPI-positive buds (Figure 4B). Quantifying the immunogold labeling for both COPI and BARS, we found that the CTP mutant was localized mainly at the neck of the buds, while COPI was more randomly distributed throughout the arrested buds (Figure 4C). Quantitative EM also revealed that more COPI-positive buds were detected on Golgi membrane in the presence of the CTP mutant as compared to the control condition without CTP (Figure 4D). Parallel to this finding, significantly fewer COPI vesicles were formed in the presence of the CTP mutant as compared to the control condition, when only GAP was added during the second-stage incubation (Figure 4E). Thus, we concluded that BARS played a critical role at the fission step during COPI vesicle formation.
Figure 4.
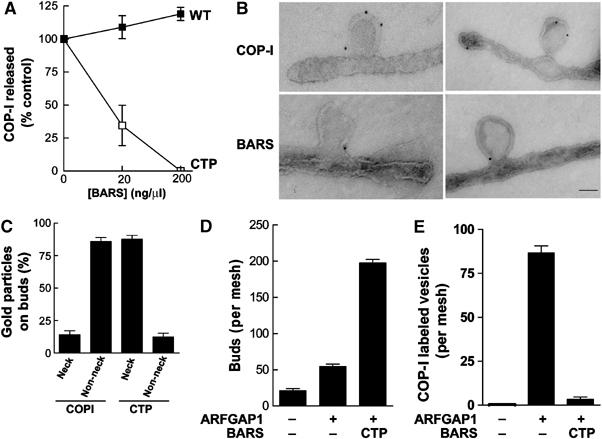
The CTP mutant induces the accumulation of COPI-positive buds on Golgi membrane. (A) The CTP mutant inhibits the reconstitution of COPI vesicles using Golgi membrane washed with 0.5 M KCl. CHO Golgi membrane washed with 0.5 M KCl was used for the two-stage incubation system, with the second-stage incubation containing GAP and varying levels of different BARS as indicated. The level of β-COP released into the supernatant was quantified and then normalized to control, which is derived from the condition that used only GAP in the incubation. The mean of this normalized value derived from three independent experiments is shown with standard error. (B) The CTP mutant induces the accumulation of COPI-positive buds on Golgi membrane. CHO Golgi membrane washed with 0.5 M KCl was used for the two-stage incubation system, with GAP and CTP added at the second stage, followed by EM examination using anti-COPI subunit antibodies (upper panels) and anti-BARS antibody (lower panels); bar, 50 nm. (C) CTP localizes mostly to the neck of buds on Golgi membrane. Ten fields from the EM that resulted (B) were randomly selected for quantitation of the level of gold particles on either the neck region of the bud or the rest of the bud, and then expressed as fractional levels. The mean derived from three independent experiments is shown with standard error. (D) Quantitation of COPI-positive buds on Golgi membrane induced by the CTP mutant. The two-stage incubation system was performed using Golgi membrane washed with 0.5 M KCl, followed by EM examination with labeling using anti-ɛ-COP and anti-ζ-COP antibodies. For each condition, five meshes were randomly selected for counting. The mean derived from three independent experiments is shown with standard error. (E) CTP inhibits COPI vesicle formation. The same quantitation as described above (D) was carried out assessing for vesicles rather than buds.
BARS is critical for COPI transport in vivo
We next sought confirmatory in vivo evidence by examining the effect of knocking down endogenous BARS. Using a small interfering RNA (siRNA) directed against a common nucleotide sequence to both human and monkey BARS, we initially confirmed efficient reduction in the endogenous level of BARS (Supplementary Figure 5A). To assess whether this knockdown affected COPI transport in vivo, we examined the retrograde transport of a KDELR chimera (Cole et al, 1998). This chimera consists of the luminal domain of a temperature-sensitive form of vesicular stomatitis virus G protein (VSVG-ts045) appended to the amino-terminus of the KDELR. Upon shifting from the permissive (32°C) to the nonpermissive (40°C) temperature, the chimera was shown to redistribute from the Golgi to the ER, because the misfolded VSVG domain at the nonpermissive temperature prevented its subsequent anterograde transport from the ER. In cells treated with siRNA against BARS, we found that the KDELR chimera was inhibited in redistributing to the ER at nonpermissive temperature (Figure 5A).
Figure 5.
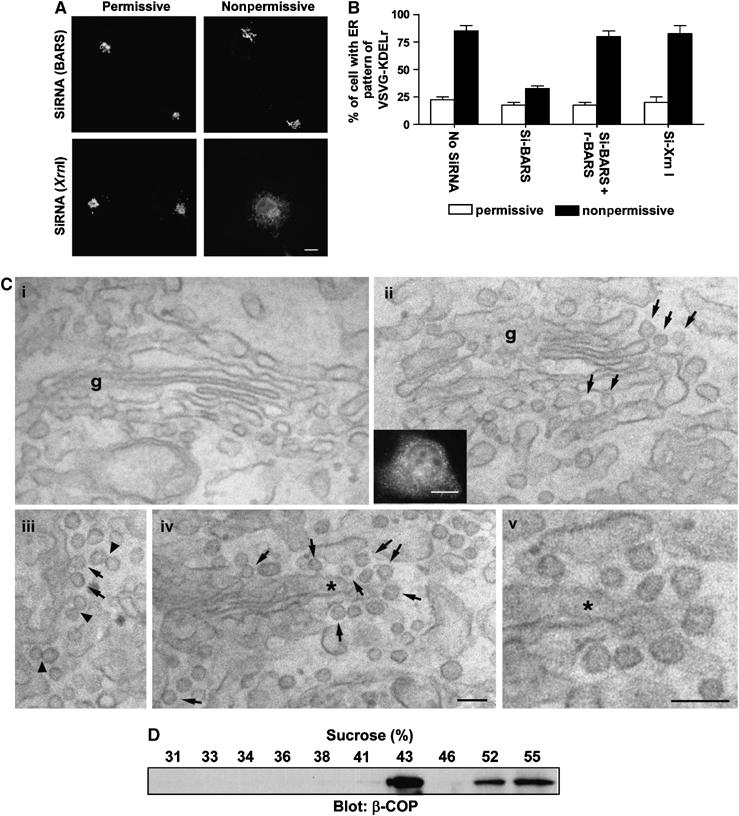
In vivo confirmation that BARS is critical for fission of COPI vesicles. (A) Knocking down BARS inhibits the retrograde transport of a chimeric KDELR. COS cells were transfected with VSVG-ts045-KDELR-myc, and siRNA against either BARS or Xrn1 (an exoribonuclease as control). Cells were then examined for the distribution of the chimeric KDELR by immunofluorescence microscopy upon shift to the nonpermissive temperature for 1 h. Representative images are shown (bar, 10 μm). (B) Ectopic expression of rat BARS restores retrograde transport of a chimeric KDELR that had been inhibited by siRNA directed against monkey BARS. The same experiment in (A) was performed, except for the additional condition of ectopically expressing rat BARS in COS cells. The mean with standard error was obtained from three independent experiments. (C) The CTP mutant induces increased coated buds in Golgi areas of transfected cells. COS cells were either mock transfected (i) or transfected with the CTP mutant (ii–v). Correlative light-EM technique was then used. A representative transfected cell is examined at both the light level (inset in ii) and at the EM level (ii–v). Note the presence of many 50–80 nm buds (arrows) with visible coating and narrow ‘necks' in CTP-transfected cells, suggesting that they are coated buds. EM tomography (not shown) was also performed on 200 nm sections to confirm that most of these round profiles were buds. Arrowheads indicate double vesicles/buds with an electron-dense connection, g (Golgi complex). The asterisk in (iv) indicates the region enlarged and shown in (v); bar, 100 nm for EM images and 10 μm for light microscopy image. (D) The CTP-induced buds have similar buoyant density as reconstituted COPI vesicles. CHO Golgi membrane washed with 0.5 M KCl was used for the two-stage incubation system, with GAP and CTP added at the second stage. After incubation, the reaction was subjected to high-salt treatment and pipette-induced shearing, followed by centrifugation to release buds into the supernatant fraction. This fraction was then analyzed by equilibrium centrifugation, followed by immunoblotting of gradient fractions with β-COP.
Two lines of evidence indicated that this inhibition was specific. First, a rescue experiment was performed by taking advantage of the observation that the siRNA designed against the human and monkey nucleotide sequence of BARS contained significant mismatches to the rat counterpart. Upon the ectopic expression of rat BARS in COS cells, the inhibition in the ER redistribution of the KDELR chimera was reversed (Figure 5B). Second, anterograde transport from the ER to the Golgi, by tracking VSVG-ts045 upon shifting from nonpermissive to permissive temperature (Cole et al, 1998), was not affected by the siRNA (Supplementary Figure 5B). This latter finding was consistent with the recent appreciation that COPI is not responsible for all retrograde transport (Girod et al, 1999; White et al, 1999). Thus, inhibition of COPI retrograde transport would not necessarily block all anterograde transport from the ER (Gaynor and Emr, 1997).
Using an alternate way of inhibiting BARS activity in vivo, we found that the ectopic expression of the CTP mutant also inhibited the ER redistribution of the KDELR chimera at the nonpermissive temperature (Supplementary Figure 5C). Correlative light-EM (Polishchuk et al, 2000) confirmed that CTP-expressing cells had increased level of coated buds on Golgi cisternae (Figure 5C and Supplementary Figure 5D). Moreover, deliberately inducing the release of the CTP-arrested buds from Golgi membrane, using high salt and pipette-induced shearing as done previously to form COPI vesicles in the presence of GTPγS (Serafini and Rothman, 1992), we found by equilibrium centrifugation that the sheared buds accumulated near 43% sucrose density (Figure 5D). Taken together, these findings further confirmed that BARS was critical for the fission step of COPI vesicle formation.
Differential regulation of BARS by p-coA and NADH acting as cofactors
As p-coA had been shown previously to be critical for the fission step also (Ostermann et al, 1993), we next explored a potential mechanistic connection between BARS and p-coA. Previously, the addition of p-coA at the second-stage incubation using Golgi membrane washed with 0.25 M KCl was able to induce COPI vesicle formation (Ostermann et al, 1993). However, when Golgi membrane was washed with 3 M KCl, we found that vesicle formation, as reflected by the release of COPI from Golgi membrane during the second-stage incubation, was inhibited (Figure 6A). Thus, this observation suggested that the effect of p-coA also required at least one peripheral membrane protein that was released by the stringent washing of Golgi membrane. Indeed, when BARS was added in conjunction with p-coA during the second-stage incubation, we observed that vesicle formation was restored (Figure 6A), as further confirmed by the evaluation of the released fraction by immunogold EM, which revealed COPI vesicles (Figure 6B). Thus, we concluded that BARS was at least one peripheral membrane protein that mediated the ability of p-coA to promote vesicle fission.
Figure 6.
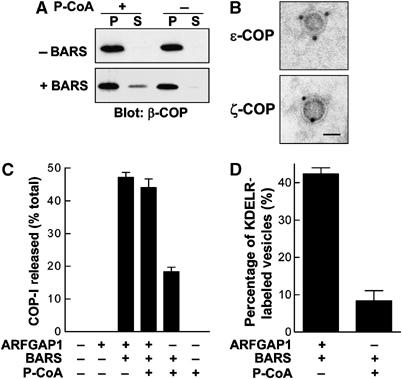
BARS mediates a mechanism by which p-coA induces COPI vesicle formation. (A) The ability of p-coA to induce the release of COPI from Golgi membrane at the second-stage incubation is lost when the membrane is washed with 3 M KCl and is restored by the addition of BARS. CHO Golgi membrane washed with 3 M KCl was used for the two-stage incubation, with the second stage using components as indicated, followed by immunoblotting of pellet (P) and supernatant (S) for β-COP after the second-stage incubation. (B) The release of coatomer from the more stringently washed (3 M KCl) Golgi membrane using the combination of BARS and p-coA at the second-stage incubation represents mostly COPI vesicles. The supernatant as described above in (A), derived from the coincubation of p-coA and BARS, was labeled with antibodies directed to subunits of COPI as indicated followed by immunogold EM. Bar, 50 nm. (C) The relative efficiency of COPI release from Golgi membrane induced by adding p-coA and BARS versus that by adding GAP and BARS at the second-stage incubation. The two-stage incubation system was performed using Golgi membrane washed with 3 M KCl. After the second-stage incubation that used components as indicated, the level of β-COP released into the supernatant is quantified, and then expressed as a percentage of total (represented by β-COP level in both the pellet and supernatant). The mean of this value derived from three independent experiments is shown with standard error. (D) Cargo sorting is impaired using the combination of p-coA and BARS rather than the combination of GAP and BARS. Golgi membrane was prepared from a CHO stable cell line that expresses myc-tagged KDELR and then washed with 3 M KCl for the two-stage incubation system. At the second stage, different components as indicated were added for incubation, followed by examination by EM for the level of vesicles positive for the tagged KDELR. The mean derived from three independent experiments is shown with standard error.
However, we also noticed that vesicle reconstitution using the combination of p-coA and BARS seemed less robust than that seen using the combination of GAP and BARS (Figure 6C). Moreover, the addition of p-coA did not significantly further enhance vesicle formation that was already induced by the combination of GAP and BARS (Figure 6C). As GAP has been shown recently to function as a component of the COPI coat complex (Yang et al, 2002; Lee et al, 2005), we examined the efficiency of cargo sorting using different combinations of purified components. The level of KDELR in COPI vesicles was reduced when the combination of BARS and p-coA was used as compared to the combination of BARS and GAP (Figure 6D). Thus, we concluded that the more efficient reconstitution of vesicle formation in the presence of GAP was likely due to its requirement for the COPI coat complex to be fully functional.
To elucidate further how BARS mediated the ability of p-coA to induce fission of COPI vesicles, we were led by a previous prediction that that binding to p-coA by BARS would be antagonized by NAD/NADH that acted competitively (Nardini et al, 2003). We first confirmed that p-coA could bind directly to BARS (Figure 7A), and then found that the presence of p-coA promoted the interaction between BARS and GAP, while the presence of NADH inhibited this interaction (Figure 7B). These findings also suggested that a causal relationship between the interaction of GAP and BARS and induction of COPI vesicle formation could be more firmly established by examining whether disrupting this interaction would lead to reduced vesicle formation. Cotitrating in different levels of NADH and p-coA, we found that an increase in the relative level of NADH inhibited vesicle formation, and this inhibition was reversed by increasing the relative level of p-coA (Figure 7C).
Figure 7.
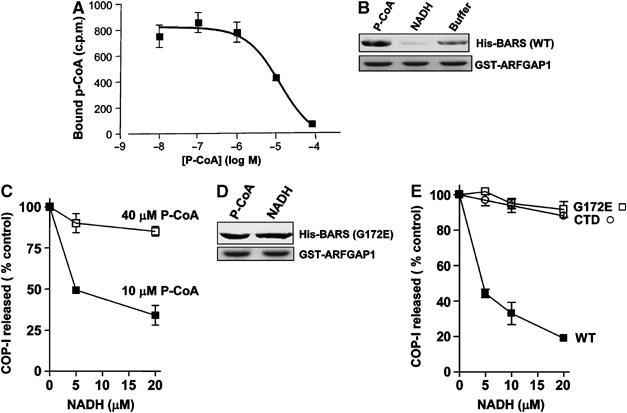
The interaction of BARS and GAP is oppositely regulated by p-coA and NADH, which act as cofactors to affect vesicle formation. (A) Binding to p-coA by BARS. BARS was incubated with labeled p-coA and increasing concentrations of unlabeled p-coA as indicated. The amount of radiolabeled p-coA that remained bound was then quantified. The mean derived from three independent experiments is shown with standard error. (B) The interaction between BARS and GAP is enhanced in the presence of p-coA and reduced in the presence of NADH. Soluble BARS was preincubated with either p-coA or NADH, followed by incubation with GAP on beads as GST fusion protein for a pulldown assay. Beads were then immunoblotted for BARS (upper panel) and Coomassie-stained for GST-GAP (lower panel). (C) NADH inhibits the release of COPI from Golgi membrane in the vesicle reconstitution assay, and this inhibition is reversed by titrating in increasing levels of p-coA. The two-stage incubation system was performed using Golgi membrane washed with 3 M KCl. After the second-stage incubation that used GAP and BARS and levels of p-coA and/or NADH as indicated, the level of β-COP released into the supernatant was quantified and then normalized to control, which is derived from the condition that used only GAP and BARS without adding either p-coA or NADH in the incubation. The mean of this normalized value derived from three independent experiments is shown with standard error. (D) NADH does not inhibit binding to GAP by the G172E point mutant of BARS. Soluble G172E mutant BARS was preincubated with either p-coA or NADH, followed by incubation with GAP on beads as GST fusion protein for a pulldown assay. Beads were then immunoblotted for BARS (upper panel) and Coomassie-stained for GST-GAP (lower panel). (E) NADH does not inhibit the release of coatomer from Golgi membrane during the second-stage incubation using either the G172E point mutant or the CTD mutant of BARS. The same assay was used as described in (C), with only increasing level of NADH present as indicated.
To show that these effects of p-coA and NADH on vesicle formation were through BARS, we were led by another previous observation that binding to NADH by BARS could be abrogated by mutating glycine at position 172 to glutamate (G172E) (Nardini et al, 2003). First, we confirmed that the interaction of this mutant BARS with GAP was not inhibited by NADH (Figure 7D), and then found that vesicle formation was no longer inhibited by NADH when this mutant rather than wild-type BARS was used in the reconstitution system (Figure 7E). Consistent with this result, the CTD mutant that did not possess the G172 residue was also resistant to the effect of NADH in inhibiting vesicle formation (Figure 7E). Thus, these findings on how NADH affected vesicle formation indicated that it specifically targeted BARS rather than other potential targets on Golgi membrane. Moreover, the observed effects of p-coA and NADH altogether revealed that they acted as cofactors of BARS in modulating the interaction between BARS and GAP to regulate COPI vesicle formation.
Discussion
We show that BARS plays a critical role in the fission step of COPI vesicle formation by multiple approaches. This role is initially suggested by modifying a well-characterized vesicle reconstitution system to use more stringently washed (3 M KCl) Golgi membrane, and then confirmed by additional approaches that did not involve this modification, including the use of a neutralizing antibody and a dominant-negative CTP mutant in the context of Golgi membrane washed under less stringency as done previously (Ostermann et al, 1993; Yang et al, 2002). The results were further confirmed by in vivo evidence derived by inhibiting BARS, including siRNA and the ectopic expression of the CTP mutant that acts as a dominant negative.
Coatomer has been shown to be sufficient to reconstitute coated vesicles from liposomal membrane (Spang et al, 1998; Bremser et al, 1999). However, the initial hint that vesicle formation from biological Golgi membrane may require more than just coatomer comes from the finding that p-coA is needed for the fission step (Ostermann et al, 1993). This observation has led to a long search for an underlying mechanistic explanation, which has been elusive thus far. In well-known examples, p-coA has been shown to participate in the palmitoylation of proteins and lipids by acyltransferases using p-coA as the source of palmitoyl fatty acids. However, we have found that the acyltransferase activity of BARS is not critical for its role in COPI vesicle formation, a finding that is similar to that shown for BARS in Golgi fission during mitosis (Hidalgo Carcedo et al, 2004), and in the formation of post-Golgi carriers (Bonazzi et al, 2005). Elucidating an alternate mechanism, we show that p-coA is needed as a cofactor of BARS in regulating its interaction with ARFGAP1. Thus, we have revealed an unexpected mechanism by which p-coA participates in COPI vesicle formation.
However, an outstanding mechanistic question is how its interaction with GAP mediates the role of BARS in vesicle fission. Although the GAP catalytic activity has been shown recently to be critical for COPI vesicle formation (Lee et al, 2005), we have found that this activity is not affected by the presence of BARS. In considering an alternate explanation, we are intrigued by a possibility suggested by studies on endophilin. Although its acyltransferase activity has been suggested to participate in the fission of clathrin-coated vesicles (Schmidt et al, 1999), another mechanism involves the ability of endophilin to induce membrane curvature independent of its acyltransferase activity (Farsad et al, 2001). Moreover, just as dynamin regulates the recruitment of endophilin to mediate the fission step of clathrin vesicle formation, our current data suggest a mechanistic parallel whereby ARFGAP1 regulates BARS at a similar step in COPI vesicle formation. Thus, it is tempting to speculate that BARS also has an intrinsic ability to induce membrane curvature, which is regulated by its interaction with GAP. However, as we have detected more COPI-positive buds in the presence of the CTP mutant than the control conditions when no form of BARS is added (see Figure 4D), this observation also suggests that BARS is not only critical for the fission step but may also promote the prior process of bud formation. Clarification of these issues in the future will likely provide important additional insights into mechanisms of COPI vesicle formation.
Another intriguing current finding is that an inhibitor of phospholipid acyltransferase activity on Golgi membrane inhibits COPI vesicle formation, which is consistent with previous work that implicates a role for the transfer of p-coA to proteins and/or lipids during COPI vesicle formation (Pfanner et al, 1989). Thus, as multiple proteins other than BARS are known to possess this acyltransferase activity, a likely explanation is that one or more of these other proteins will also participate in COPI vesicle formation. This possibility also helps to explain a puzzling observation that the double knockout of CtBP1 and CtBP2, which would also lack BARS because it is a splice variant of CtBP1, allows the generation of embryonic fibroblasts from day 9 of developing mouse embryos (Hildebrand and Soriano, 2002). Such a finding seemingly contradicts our current conclusion that BARS is critical for COPI vesicle formation, as COPI transport is thought to be essential for cell viability. In considering a reconciliatory explanation, we are led by studies on the Src family of tyrosine kinases. Although these family members possess a similar kinase activity, the individual members participate in distinct cellular roles that can be demonstrated by their acute loss. Yet, due to developmental compensation by the related family members, a chronic loss of one member in the context of a genetic deletion still allows the generation of viable embryonic cells (Lowell and Soriano, 1996). A similar explanation already applies to the endocytic pathway regulated by BARS, as examination of embryonic cells that lack all CtBP members shows that these cells compensate for the loss of BARS by using a dynamin-dependent mechanism (Bonazzi et al, 2005).
Finally, a more precise understanding of how p-coA and NADH modulate the BARS activity during the fission of COPI vesicles will also be insightful. As these cofactors of BARS are well known to participate in many metabolic events, an intriguing possibility is that their concentration would vary depending on the metabolic state of the cell. In this respect, one would also have to wonder why the CtBP members (in which BARS is a family member as CtBP3) that have been well characterized in transcription regulation should have this activity also modulated by its binding to NADH (Chinnadurai, 2002). When taken altogether, we are tempted to speculate that the binding of cofactors by BARS underlies a complex mechanism by which multiple cellular events that include transcription, transport, and metabolism are coordinated. Thus, future work to address this exciting prospect may reveal the interacting networks among diverse cellular events that have not been well appreciated.
Materials and methods
Chemicals, proteins, and cells
GTP, GTPγS, bovine serum albumin (BSA), NADH, and p-coA (Sigma-Aldrich, St Louis, MO), as well as CI-976 (William Brown, Cornell University, Ithaca, NY), were obtained. Cytosol, coatomer, full-length N-myristoylated ARF1, full-length ARFGAP1, and Golgi membrane were prepared as described previously (Yang et al, 2002). Recombinant ACAP1 and PAP expressed using the baculovirus system were purified as described previously for ARFGAP1 (Yang et al, 2002). COS-7 cells were grown as described previously (Aoe et al, 1997).
Plasmids, siRNA, and antibodies
Details are provided as Supplementary data.
Vesicle reconstitution
The two-stage incubation system has been described (Yang et al, 2002). Details are provided as Supplementary data.
Electron microscopy
EM examination using the whole-mount technique has been described (Yang et al, 2002). Details are provided as Supplementary data.
Gel filtration
The wash fraction was dialyzed against a buffer that contained 25 mM Hepes–KOH, pH 7.2, 50 mM KCl, 2.5 mM Mg(OAc)2, and then loaded onto a Superose6 (Amersham Biosciences, Piscataway, NJ) gel filtration column, at a flow rate of 0.1 ml/min. The eluted fractions were monitored for total proteins as detected by UV at 280 nm, and also for BARS by immunoblotting.
In vivo transport
Retrograde transport of VSVG(ts045)-KDELR and anterograde transport of VSVG(ts045) were performed as described previously (Cole et al, 1998). Details are provided as Supplementary data.
In vitro assays
Assays previously described include: acyltransferase activity of BARS (Weigert et al, 1999) and catalytic activity of GAP (Randazzo et al, 2001). Details of pulldown assays are provided as Supplementary data.
Binding of CTD mutant to liposomal membrane
Liposomal membrane was generated as described previously, to mimic the composition of Golgi membrane (Spang et al, 1998), and then incubated with soluble CTD (3 μg/ml) for 10 min at 37°C, followed by floatation gradient centrifugation at 200 000 g for 16 h.
Binding of p-coA by BARS
BARS (100 pmol) were spotted onto nitrocellulose and incubated with 6 × 104 c.p.m. of [14C]p-coA and different concentrations of unlabeled p-coA as indicated in binding buffer (20 mM Hepes, pH 7.4 and 0.01% Triton X-100). After extensive washing, the amount of labeled p-coA on the nitrocellulose was quantified by Instant Imager.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
SUPPLEMENTARY MATERIALS AND METHODS
Acknowledgments
We thank Jian Li for advice and discussions, Huiya Gilbert for technical assistance, and Francis Barr, William Brown, Dan Cassel, and Wanjin Hong for providing reagents. This work is supported in part by grants to VWH from the NIH, and to AL and DC from AIRC (Milan, Italy) and Telethon Italy.
References
- Aoe T, Cukierman E, Lee A, Cassel D, Peters PJ, Hsu VW (1997) The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J 16: 7305–7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi M, Spano S, Turacchio G, Cericola C, Valente C, Colanzi A, Kweon HS, Hsu VW, Polishchuck EV, Polishchuck RS, Sallese M, Pulvirenti T, Corda D, Luini A (2005) CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat Cell Biol 7: 570–580 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Lippincott-Schwartz J (2003) Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol 4: 409–414 [DOI] [PubMed] [Google Scholar]
- Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes CA, Sollner TH, Rothman JE, Wieland FT (1999) Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell 96: 495–506 [DOI] [PubMed] [Google Scholar]
- Chinnadurai G (2002) CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol Cell 9: 213–224 [DOI] [PubMed] [Google Scholar]
- Cole NB, Ellenberg J, Song J, DiEuliis D, Lippincott-Schwartz J (1998) Retrograde transport of Golgi-localized proteins to the ER. J Cell Biol 140: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Cassel D, Kahn RA, Klausner RD (1992) ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci USA 89: 6408–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D, Chambers K, Racoosin EL, Cluett EB, Gucwa A, Jackson B, Brown WJ (2003) Inhibition of a Golgi complex lysophospholipid acyltransferase induces membrane tubule formation and retrograde trafficking. Mol Biol Cell 14: 3459–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P (2001) Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol 155: 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, Emr SD (1997) COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol 136: 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod A, Storrie B, Simpson JC, Johannes L, Goud B, Roberts LM, Lord JM, Nilsson T, Pepperkok R (1999) Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat Cell Biol 1: 423–430 [DOI] [PubMed] [Google Scholar]
- Hidalgo Carcedo C, Bonazzi M, Spano S, Turacchio G, Colanzi A, Luini A, Corda D (2004) Mitotic Golgi partitioning is driven by the membrane-fissioning protein CtBP3/BARS. Science 305: 93–96 [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P (2002) Overlapping and unique roles for C-terminal binding protein 1 (CtBP1) and CtBP2 during mouse development. Mol Cell Biol 22: 5296–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A, Al-Awar OS, Hay JC, Donaldson JG (2005) Targeting of Arf-1 to the early Golgi by membrin, an ER-Golgi SNARE. J Cell Biol 168: 1039–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledger PW, Uchida N, Tanzer ML (1980) Immunocytochemical localization of procollagen and fibronectin in human fibroblasts: effects of the monovalent ionophore, monensin. J Cell Biol 87: 663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Yang JS, Hong W, Premont RT, Hsu VW (2005) ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J Cell Biol 168: 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell CA, Soriano P (1996) Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev 10: 1845–1857 [DOI] [PubMed] [Google Scholar]
- Nardini M, Spano S, Cericola C, Pesce A, Massaro A, Millo E, Luini A, Corda D, Bolognesi M (2003) CtBP/BARS: a dual-function protein involved in transcription co-repression and Golgi membrane fission. EMBO J 22: 3122–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Sollner TH, Rothman JE (1997) Bidirectional transport by distinct populations of COPI-coated vesicles. Cell 90: 335–349 [DOI] [PubMed] [Google Scholar]
- Ostermann J, Orci L, Tani K, Amherdt M, Ravazzola M, Elazar Z, Rothman JE (1993) Stepwise assembly of functionally active transport vesicles. Cell 75: 1015–1025 [DOI] [PubMed] [Google Scholar]
- Pfanner N, Orci L, Glick BS, Amherdt M, Arden SR, Malhotra V, Rothman JE (1989) Fatty acyl-coenzyme A is required for budding of transport vesicles from Golgi cisternae. Cell 59: 95–102 [DOI] [PubMed] [Google Scholar]
- Polishchuk RS, Polishchuk EV, Marra P, Alberti S, Buccione R, Luini A, Mironov AA (2000) Correlative light-electron microscopy reveals the tubular–saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J Cell Biol 148: 45–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo PA, Miura K, Jackson TR (2001) Assay and purification of phosphoinositide-dependent ADP-ribosylation factor (ARF) GTPase activating proteins. Methods Enzymol 329: 343–354 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Wolde M, Thiele C, Fest W, Kratzin H, Podtelejnikov AV, Witke W, Huttner WB, Soling HD (1999) Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature 401: 133–141 [DOI] [PubMed] [Google Scholar]
- Serafini T, Rothman JE (1992) Purification of Golgi cisternae-derived non-clathrin-coated vesicles. Methods Enzymol 219: 286–299 [DOI] [PubMed] [Google Scholar]
- Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L (1998) Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci USA 95: 11199–11204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Schekman R (1998) Reconstitution of retrograde transport from the Golgi to the ER in vitro. J Cell Biol 143: 589–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert R, Silletta MG, Spano S, Turacchio G, Cericola C, Colanzi A, Senatore S, Mancini R, Polishchuk EV, Salmona M, Facchiano F, Burger KN, Mironov A, Luini A, Corda D (1999) CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 402: 429–433 [DOI] [PubMed] [Google Scholar]
- White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer EH (1999) Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol 147: 743–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Lee SY, Gao M, Bourgoin S, Randazzo PA, Premont RT, Hsu VW (2002) ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol 159: 69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
SUPPLEMENTARY MATERIALS AND METHODS


