Abstract
Mutation of the parkin gene, which encodes an E3 ubiquitin-protein ligase, is the major cause of autosomal recessive juvenile parkinsonism (ARJP). Although various substrates for parkin have been identified, the mechanisms that regulate the ubiquitin ligase activity of parkin are poorly understood. Here we report that 14-3-3η, a chaperone-like protein present abundantly in neurons, could bind to parkin and negatively regulate its ubiquitin ligase activity. Furthermore, 14-3-3η could bind to the linker region of parkin but not parkin with ARJP-causing R42P, K161N, and T240R mutations. Intriguingly, α-synuclein (α-SN), another familial Parkinson's disease (PD) gene product, abrogated the 14-3-3η-induced suppression of parkin activity. α-SN could bind tightly to 14-3-3η and consequently sequester it from the parkin–14-3-3η complex. PD-causing A30P and A53T mutants of α-SN could not bind 14-3-3η, and failed to activate parkin. Our findings indicate that 14-3-3η is a regulator that functionally links parkin and α-SN. The α-SN-positive and 14-3-3η-negative control of parkin activity sheds new light on the pathophysiological roles of parkin.
Keywords: α-synuclein, 14-3-3η, parkin, Parkinson's disease, ubiquitin ligase
Introduction
In the last decade, people working in the field of Parkinson's disease (PD) witnessed a tremendous progress in uncovering the mechanisms of PD, and several familial PD genes were discovered in succession (Vila and Przedborski, 2004). Of these hereditary PD genes, parkin (PARK2), the causative gene of autosomal recessive juvenile parkinsonism (ARJP), is of a special interest because it encodes a ubiquitin ligase, a critical component of the pathway that covalently attaches ubiquitin to specific proteins with a polymerization step to form a degradation signal (Shimura et al, 2000). Indeed, parkin catalyzes the addition of ubiquitin to target proteins prior to their destruction via the proteasome, suggesting that the misregulation of proteasomal degradation of parkin substrate(s) is deleterious to dopaminergic neurons (Dawson and Dawson, 2003; Bossy-Wetzel et al, 2004; Kahle and Haass, 2004). Consequently, impaired protein clearance can induce dopaminergic cell death, supporting the concept that defects in the ubiquitin–proteasome system may underlie nigral degeneration in ARJP and perhaps sporadic forms of PD (McNaught and Olanow, 2003). On the other hand, it was recently reported that parkin also catalyzes the formation of the K63-linked polyubiquitylation chain, independent of proteasomal destruction, in which the K48-linked polyubiquitylation chain is necessary (Doss-Pepe et al, 2005; Lim et al, 2005). Thus, it is plausible that parkin shares two roles as an E3 ligase; that is, one linking to and the other independent of the proteasome.
Among the products of major familial PD genes (Vila and Przedborski, 2004), α-synuclein (α-SN) is a product of familial PD gene (PARK1) identified as a presynaptic protein of unknown function. α-SN is considered in the molecular mechanisms of PD mainly because it is one of the major components of the cytoplasmic Lewy body (LB) inclusion present in the remaining nigral dopaminergic neurons of PD patients, which is the pathological hallmark of sporadic and some familial PDs (Forno, 1996). Although various studies have been conducted on α-SN (Dawson and Dawson, 2003; Bossy-Wetzel et al, 2004; Kahle and Haass, 2004), its pathophysiological role(s) and the interplay between α-SN and parkin are largely unknown.
To date, little is known about the role of parkin as a ubiquitin E3 ligase with respect to the underlying molecular mechanism(s) of ARJP or PD. Here we report for the first time that 14-3-3η, a member of the 14-3-3 family (β/α, γ, ɛ, η, ζ/δ, σ, and τ/θ) (Berg et al, 2003; Bridges and Moorhead, 2004; Mackintosh, 2004) identified in LB (Kawamoto et al, 2002; Ubl et al, 2002), binds primarily to the linker region of parkin and functions as a novel negative regulator of parkin. We also show that α-SN relieves parkin activity suppressed by 14-3-3η, indicating that 14-3-3η is a novel molecule handling both parkin and α-SN, and that functionally links the two familial PD gene products.
Results
Parkin specifically interacts with 14-3-3η but not with other 14-3-3 isoforms
We first examined the physical association of parkin with 14-3-3 isoforms, which are abundantly expressed in the brain (Martin et al, 1994; Baxter et al, 2002). Parkin was immunoprecipitated from mouse brain extracts, and the presence of 14-3-3 was analyzed by Western blotting (Figure 1A). 14-3-3 was clearly detected in the parkin immunoprecipitant, but not in those of control IgG or parkin antibody preabsorbed with recombinant parkin protein (1 μg). Intriguingly, two 14-3-3 signals were evident: a faint band and a strongly stained band, indicating that the 14-3-3 may form homo- and/or hetero-dimers. Subsequently, we determined the type(s) of 14-3-3 species that interacts with parkin in the mouse brain in more detail. In the parkin immunoprecipitant, 14-3-3η, but not other 14-3-3 isoforms examined, that is, β, γ, ɛ, and τ, was detected (Figure 1B). In the next step, we examined whether parkin is coimmunoprecipitated with anti-14-3-3η antibody and found parkin in the 14-3-3η immunoprecipitant (Figure 1C). These reciprocal immunoprecipitation experiments revealed that parkin is associated with 14-3-3η in the mouse brain.
Figure 1.
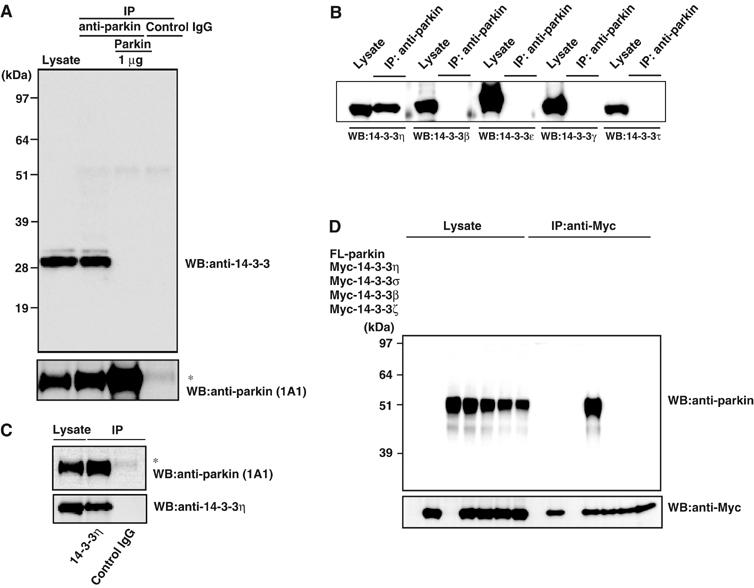
Physical interaction between parkin and 14-3-3η. (A) Immunoprecipitation by anti-parkin antibody in the mouse brain. Mouse brain lysates were prepared and treated with anti-parkin or control IgG as described in Materials and methods. The resulting immunoprecipitates were subjected to SDS–PAGE, followed by Western blotting with anti-14-3-3 and parkin (1A1) antibodies. In all, 1 μg of recombinant parkin was pretreated with anti-parkin prior to immunoprecipitation. Left lane: the brain lysate (1.5% input). Asterisk denotes an IgG heavy chain. (B) Specificity analysis of 14-3-3 species. The immunoprecipitation with anti-parkin and subsequent SDS–PAGE were carried out as in (A). Western blotting was conducted with antibodies against various 14-3-3 isoforms as indicated for lysates and anti-parkin immunoprecipitates. (C) Immunoprecipitation by anti-14-3-3η antibody. After immunoprecipitation with anti-14-3-3η or control IgG of the brain lysate, the immunoprecipitates were analyzed by Western blotting with anti-parkin (1A1) and 14-3-3η antibodies, similar to (A). Left lane: the brain lysate (1.5% input). Asterisk denotes an IgG heavy chain. (D) Interaction between parkin and 14-3-3η in HEK293 cells. FL-parkin (5 μg), Myc-14-3-3η, σ, β, or ζ (2 μg) plasmids were transfected as indicated into HEK293 cells. After 48 h, the cell lysate was prepared and used for immunoprecipitation with anti-Myc antibody. The immunoprecipitates and the lysate (7.5% input) were analyzed by Western blotting with anti-parkin and Myc antibodies, as in (A).
To confirm the specific interaction of parkin with 14-3-3η, Myc-tagged 14-3-3η, σ, β, or ζ was cotransfected with FLAG (FL)-parkin into HEK293 cells, and their interactions were tested. FL-parkin was detected in the immunoprecipitant of Myc-14-3-3η, but not those of Myc-14-3-3σ, β, and ζ (Figure 1D). Taken together with the results of Figure 1B, our data indicate that parkin mainly interacts with 14-3-3η.
Parkin domain interacts with 14-3-3η
We next investigated the region of parkin necessary for interaction with 14-3-3η. Structurally, parkin is characterized by the presence of the N-terminal ubiquitin-like domain (UBL) (which is highly homologous to ubiquitin), the C-terminal RING box, consisting of two RING finger motifs, RING1 and RING2, flanked by one IBR (in between RING finger) motif, and a linker region, which connects these N- and C-terminal regions (Shimura et al, 2000). In these experiments, various deletion mutants of FL-tagged parkin were expressed in HEK293 cells and immunoprecipitated by FL-antibody beads (Figure 2A). FL-parkin or its derivatives on the beads were further incubated with cell lysates that expressed Myc-14-3-3η, and then the amounts of Myc-14-3-3η bound to the beads were determined (Figure 2B). The full-length parkin could bind 14-3-3η. Deletion of either UBL or RING-box domain reduced the binding compared to the full-length parkin, although these deletion mutants retained the ability to bind to 14-3-3η. Furthermore, mutants with combined deletions of the UBL and RING-box domains, that is, the linker region, could also bind 14-3-3η to a lesser extent. Conversely, deletion of the linker region resulted in the loss of ability to bind 14-3-3η. Taken together, it is concluded that the linker region is necessary for the interaction between parkin and 14-3-3η, although the UBL and RING-box domains may enhance the binding affinity.
Figure 2.
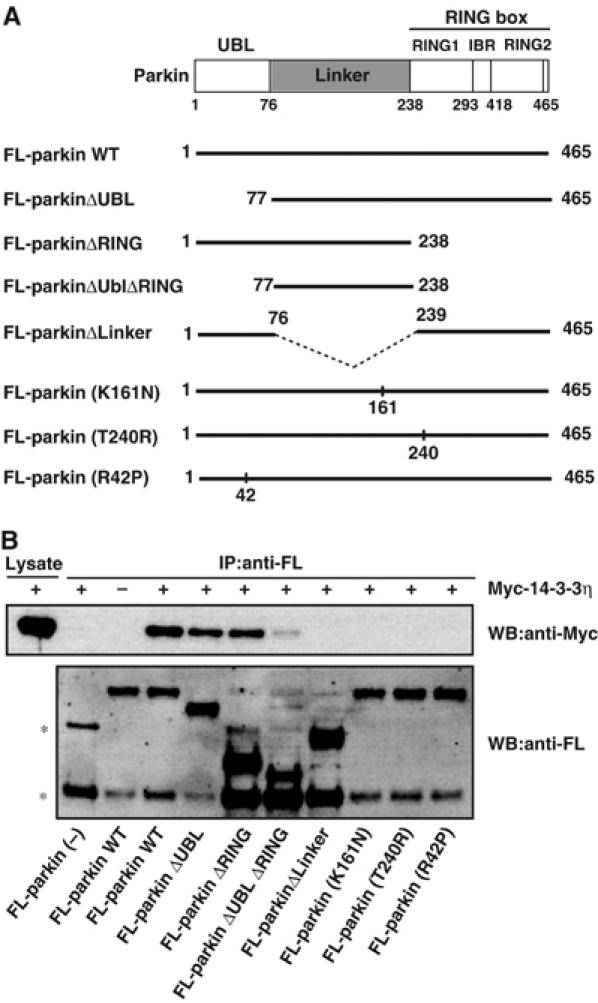
Domain analysis of the parkin region that interacts with 14-3-3η. (A) Schematic representation of WT parkin and its deletion- and disease-related missense mutants. See text for the domain structures of parkin and mutants. The dotted line denotes the deleted region. (B) Interaction between 14-3-3η and parkin mutants. FL-parkin (2 μg) or its mutant (10 μg) plasmids were transfected into HEK293 cells, as described in Figure 1D. The cell lysates (200–600 μl) were immunoprecipitated with anti-FL-antibody beads. Note that various amounts of the lysates were used to adjust roughly the levels of expressed parkin mutants. The resulting immunoprecipitates were mixed with other cell lysates (200 μl) prepared from cells that had been transfected with Myc-14-3-3η plasmid (2 μg) and incubated for 6 h at 4°C. Then, the extensively washed immunoprecipitates and cell lysate (7.5% input) were analyzed by Western blotting with anti-Myc and FL antibodies. Asterisks denote nonspecific bands.
Interestingly, the ARJP disease-causing missense mutation within the linker region, that is, parkin(K161N), in which the Lys residue at position 161 was replaced by Asn residue, showed complete loss of binding to 14-3-3η, confirming the importance of the linker region in the interaction between 14-3-3η and parkin. Unexpectedly, other disease-causing missense mutations of the UBL region, parkin(R42P), and the RING1 region, parkin(T240R), also showed complete loss of interaction with 14-3-3η (Figure 2). Thus, although the UBL and RING-box domains are not primarily required for the binding, both R42P and T240R mutations in the UBL and RING-box domains, respectively, deleteriously affect the neighboring linker domain. Alternatively, since 14-3-3 is known to form a homo- or hetero-dimer, and thus has two binding sites (Aitken et al, 2002), it is plausible that 14-3-3η interacts with two distinct regions of parkin, one major site of which is the linker region.
Effect of suppression of 14-3-3η on parkin E3 activity
We next investigated the role of parkin–14-3-3η binding on parkin activity. At first, we tested its effect on the ubiquitin ligase activity of parkin. We incubated recombinant His-parkin with ubiquitin, E1, and E2 (UbcH7) in vitro. Under this condition, His-parkin appeared as a smear band, which likely reflects self-ubiquitylation (Figure 3A). Addition of recombinant GST-14-3-3η (Figure 3A, left panel) or untagged 14-3-3η (Figure 3A, right panel) to the reaction reduced the smear of His-parkin, and such reduction was proportionate to the added amount of GST-14-3-3η or 14-3-3η and resulted in the recovery of His-parkin of intact size. In addition, we found that 14-3-3η had no effect on the ubiquitylating activity of phosphorylated IκBα by a fully in vitro reconstituted system, containing E1, E2 (Ubc4), and E3 (the SCFβTrCP complex; Kawakami et al, 2001), indicating that 14-3-3η does not interfere with ubiquitylating reactions in general (data not shown). These results strongly suggest that 14-3-3η suppresses the intrinsic self-ubiquitylation activity of parkin.
Figure 3.
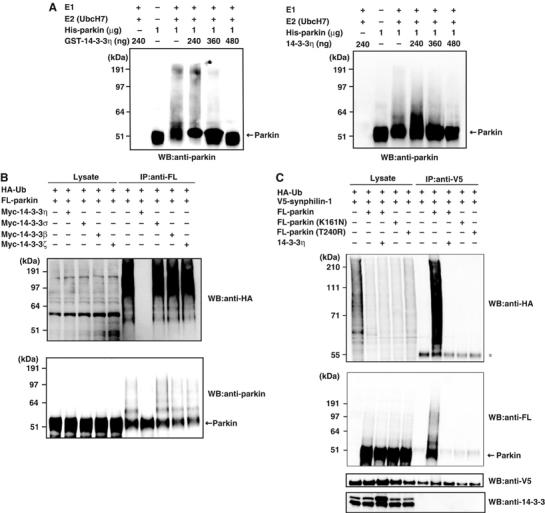
Effects of 14-3-3η on the E3 activity of parkin. (A) In vitro autoubiquitylation. The ubiquitylating assay was conducted as described in Materials and methods with or without various amounts of GST-14-3-3η (left panel) or 14-3-3η (right panel). After incubation, the reaction mixtures were subjected to SDS–PAGE, followed by Western blotting with anti-parkin. Arrow on the right indicates the position of His-parkin. (B) In vivo autoubiquitylation. HA-Ub (3 μg), FL-parkin (3 μg), and Myc-14-3-3η, σ, β, or ζ (6 μg) plasmids were transfected for 48 h into HEK293 cells as indicated. After immunoprecipitation with anti-FL, Western blotting was performed using antibodies against HA and parkin. Western blotting of all lysates was performed to test the expression levels (Lysate). (C) Ubiquitylation of synphilin-1 in HEK293 cells. HA-Ub (2 μg), FL-parkin (3 μg), FL-parkin(K161N) (3 μg), FL-parkin(T240R) (3 μg), Myc-14-3-3η (6 μg), and V5-synphilin-1 (4 μg) plasmids were transfected into HEK293 cells as in (B) at the indicated combinations. After immunoprecipitation with anti-V5 antibody, Western blotting was performed using antibodies against HA, FL, V5, and 14-3-3. Asterisk denotes an IgG heavy chain.
We next tested whether 14-3-3η also affects the ubiquitylation activity of parkin in HEK293 cells. First, we examined the self-ubiquitylation of parkin, whose activity was observed by cotransfections of HA-ubiquitin and FL-parkin. Myc-14-3-3η almost completely suppressed the self-ubiquitylation activity of parkin, while Myc-14-3-3σ, β, and ζ had no inhibitory effect (Figure 3B), indicating the specific role of 14-3-3η for parkin. Second, we examined the effect of 14-3-3η on the ubiquitylation of a model substrate for parkin. When V5-tagged synphilin-1, a known parkin substrate (Chung et al, 2001), was transfected with FL-parkin and HA-ubiquitin in the cells, V5-synphilin-1 was found in ubiquitylated form, as demonstrated by the poly-ubiquitin chain formation (detected by anti-HA antibody) in anti-V5 immunoprecipitant (Figure 3C, top panel). V5-synphilin-1 was not ubiquitylated when FL-parkin was not cotransfected, suggesting that this ubiquitylation is mediated by coexpressed FL-parkin. Indeed, FL-parkin was found to be associated with V5-synphilin-1, further supporting the above notion (Figure 3C, second panel from the top). Note that the polyubiquitylated bands observed as the smear profile were considered to include not only major synphilin-1 bands over 90-kDa size but also self-ubiquitylated bands of parkin over 52-kDa size.
In the next step, we tested the effects of 14-3-3η on the ubiquitylation and binding activities of parkin to V5-synphilin-1. Cotransfection of 14-3-3η resulted in almost complete inhibition of the ubiquitylation of synphilin-1 by parkin and/or self-ubiquitylation of parkin (Figure 3C, top and second panels), as well as inhibition of the interaction between synphilin-1 and parkin (Figure 3C, second panel). 14-3-3η did not interact with synphilin-1 (Figure 3C, bottom panel). Taken together, these results suggest that 14-3-3η does not only inhibit the intrinsic ubiquitylation activity of parkin, but also its binding activity to the substrate and its ubiquitylation.
The ARJP disease-related parkin(K161N) and parkin(T240R) mutants, which cannot bind with 14-3-3η, could not bind and ubiquitylate synphilin-1 and/or self-ubiquitylation of parkin even in the absence of 14-3-3η (Figure 3C, top panel). Hence, the linker and RING-box domains of parkin are essential not only for the negative regulation by 14-3-3η, but also for the substrate recognition and ubiquitin-ligase activity. These results illustrate the importance of these regions of parkin on its positive and negative regulation.
Since parkin is known to associate with E2 (Shimura et al, 2000), we also examined the effect of 14-3-3η on the ability of parkin to recruit E2. For this purpose, we coexpressed HA-parkin with FL-UbcH7 or FL-Ubc7, both of which are known to bind to parkin (Shimura et al 2000; Imai et al, 2001). Almost the same amounts of UbcH7 (Figure 4A) and Ubc7 (Figure 4B) were detected in the anti-parkin immunoprecipitants irrespective of cotransfection with Myc-14-3-3η. These findings indicate that 14-3-3η does not influence the recruitment of E2, that is, UbcH7 or Ubc7, to parkin.
Figure 4.
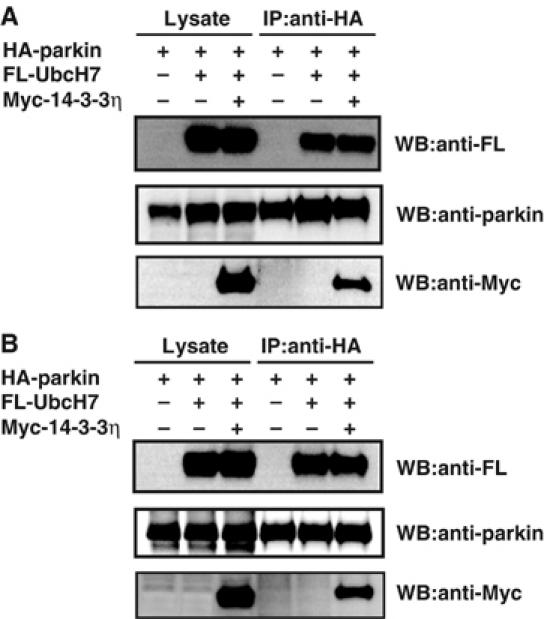
Effect of 14-3-3η on the recruitment of E2 (UbcH7 or Ubc7) to parkin. (A) HA-parkin (3 μg), FL-UbcH7 (3 μg), or Myc-14-3-3η (6 μg) plasmids were transfected for 48 h into HEK293 cells at the indicated combinations. After immunoprecipitation with anti-HA antibody, Western blotting was performed using antibodies against FL, Myc, and parkin. (B) The experiment was conducted as in (A), except that FL-Ubc7 was used instead of FL-UbcH7.
α-Synuclein abrogates 14-3-3η-related parkin inactivation
Based on the above findings, we next examined the mechanism that regulates the 14-3-3η–parkin binding. As α-SN partly has a high homology to 14-3-3 isoforms (Ostrerova et al, 1999), we tested the effects of α-SN on the 14-3-3η-induced suppression of parkin. By cotransfection experiments in HEK293 cells, parkin again ubiquitylated synphilin-1, and 14-3-3η inhibited the parkin-mediated ubiquitylation (Figure 5A). Coexpression of α-SN resulted in the recovery of ubiquitylation of synphilin-1 and the association of synphilin-1 with parkin, suggesting that α-SN abrogates the 14-3-3η-induced suppression of parkin (Figure 5A, top panel). Importantly, the familial PD-related mutants of α-SN(A30P) (Kruger et al, 1998) and α-SN(A53T) (Polymeropoulos et al, 1997) could not abrogate the inhibitory role of 14-3-3η. Similar results were observed by detection of self-ubiquitylation activity of parkin (Figure 5A, second panel).
Figure 5.
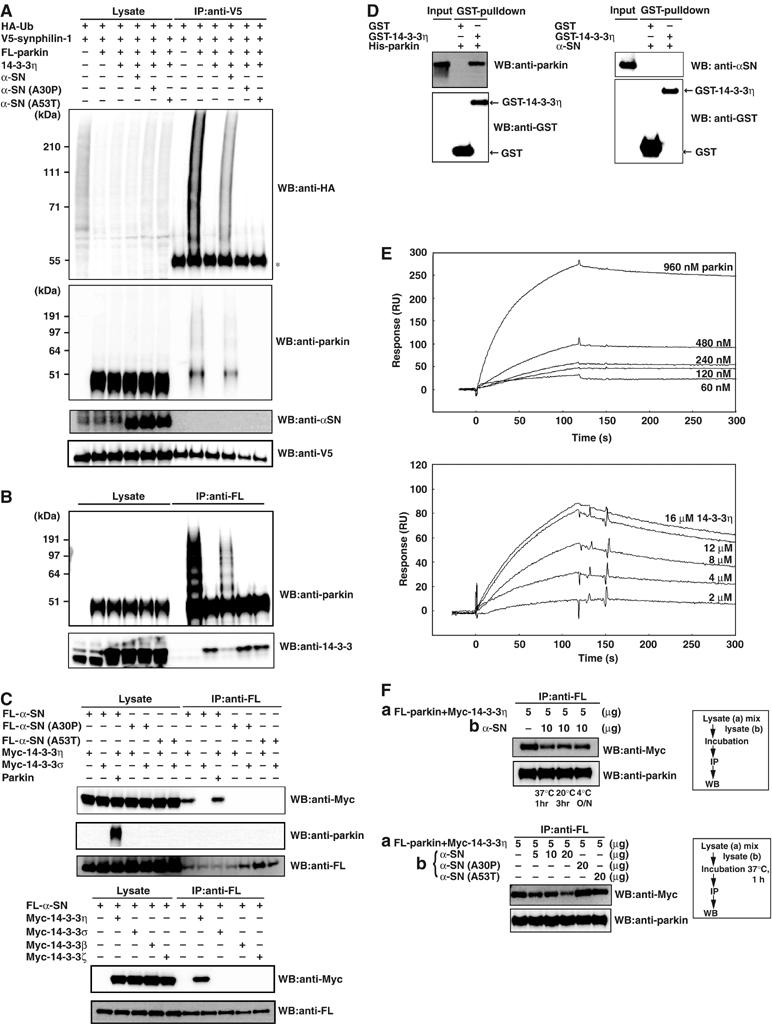
Effects of α-SN on 14-3-3η-induced suppression of parkin E3 activity and interaction between 14-3-3η and α-SN in HEK293 cells. (A) Ubiquitylation of synphilin-1. Transfection was conducted at various combinations, as in Figure 3C, except for cotransfection of 4 μg of α-SN, α-SN(A30P), and α-SN(A53T). After immunoprecipitation with anti-V5 antibody, Western blotting was carried out with antibodies against HA, parkin and α-SN, and V5. Asterisk denotes an IgG heavy chain. (B) Autoubiquitylation of parkin. Transfection was performed as in (A). After immunoprecipitation with anti-FL antibody, Western blotting was carried out with antibodies against parkin and 14-3-3. (C) Interaction of 14-3-3η and α-SN with or without parkin. Various expression vectors at the indicated combinations were transfected. Immunoprecipitation was conducted by anti-FL antibody and the resulting immunoprecipitates were used for Western blotting with antibodies against Myc, parkin, and FL. (D) Physical interaction between 14-3-3η and parkin (left panel) or α-SN (right panel) in recombinant proteins. After recombinant His-tagged parkin produced from baculovirus-infected HiFive insect cells (3 μg) or GST-α-SN expressed in E. coli whose GST moiety was removed by PreScission Protease digestion prior to use (3 μg) was incubated for 1 h at 32°C with 3 μg of GST or GST-tagged 14-3-3η expressed in E. coli, glutathione-Sepharose was added and the incubation vessels were slowly rotated for 3 h at 4°C. The washed Sepharose resin was eluted with 50 μl of 50 mM Tris–HCl (pH 8.0) buffer containing 10 mM reduced glutathione, and aliquots (15 μl) were analyzed by Western blotting with antibodies against parkin (left-top panel), α-SN (right-top panel), and GST (bottom panel). Input: 500 ng of parkin or α-SN. (E) SPR analyses of parkin and α-SN binding to 14-3-3η. Upper: subtracted sensorgrams of interaction between a subset of parkin concentrations and immobilized 14-3-3η. Lower: subtracted sensorgrams of interaction between a subset of 14-3-3η concentrations and immobilized α-SN. (F) Sequestration of 14-3-3η by α-SN from the parkin–14-3-3η complex. Various expression vectors were transfected as indicated. Upper panel: 5 μg of the lysate-(a) from cells co-expressing FL-parkin and Myc-14-3-3η were mixed with 10 μg of cellular lysate-(b) expressing α-SN. The mixtures were incubated under various conditions; that is, 37°C for 1 h, 20°C for 3 h, or overnight at 4°C (O/N), then immunoprecipitation by anti-FL antibody was conducted, followed by Western blotting with antibodies against Myc (14-3-3η) and parkin. Lower panel: the experiments were conducted as for the top panel, except that incubation was carried out at 37°C for 1 h using α-SN-, α-SN(A30P)-, or α-SN (A53T)-expressing lysates as indicated. The experimental protocol is shown in the flow charts on the right.
We then tested whether α-SN can release the binding of Myc-14-3-3η from parkin in cotransfection experiment. As shown in Figure 5B (top panel), FL-parkin was self-ubiquitylated in the absence of 14-3-3η. Coexpression of 14-3-3η inhibited the self-ubiquitylation of parkin, and this was accompanied by the binding of 14-3-3η to FL-parkin. Coexpression of α-SN abrogated the binding of 14-3-3η to parkin and resulted in the recovery of self-ubiquitylation of parkin. These effects were not seen by coexpression of α-SN(A30P) and α-SN(A53T) (Figure 5B, top panel). In addition, while the 14-3-3η–parkin interaction was considerably reduced by α-SN, it was not reduced by α-SN(A30P) or α-SN(A53T) (Figure 5B, bottom panel). Taken together, α-SN, but not α-SN(A30P) or α-SN(A53T), binds strongly to 14-3-3η and thereby releases parkin from the parkin–14-3-3η complex.
We also tested the interaction of Myc-14-3-3η with FL-α-SN, FL-α-SN(A30P), and FL-α-SN(A53T). Myc-14-3-3η interacted only with FL-α-SN, but not α-SN(A30P) nor α-SN(A53T) (Figure 5C, upper-top panel), suggesting that α-SN relieves parkin activity from binding to 14-3-3η. The 14-3-3η/α-SN interaction was not affected by parkin (Figure 5C, upper-top panel), and parkin was not associated with α-SN (Figure 5C, upper-second panel). Interestingly, FL-α-SN did not interact with Myc-14-3-3σ, β, and ζ in the same experiment (Figure 5C, lower panel). These results further strengthen the notion that α-SN specifically activates parkin through binding 14-3-3η.
We then investigated whether the interaction of 14-3-3η and parkin is direct or indirect by using purified recombinant His-parkin and GST-14-3-3η. GST or GST-14-3-3η was mixed with His-parkin, and pulled down by glutathione beads. His-parkin bound to GST-14-3-3η, but not GST (Figure 5D, left panel), indicating that parkin directly interacts with 14-3-3η. On the other hand, a similar in vitro binding assay showed that GST-14-3-3η did not interact with recombinant α-SN (Figure 5D, right panel), suggesting that certain modification(s) of α-SN may be required for the interaction of 14-3-3η.
Subsequently, we measured the binding affinities of parkin and α-SN for 14-3-3η by the surface plasmon resonance (SPR) method. As shown in Figure 5E, parkin bound 14-3-3η with a considerably strong affinity (Kd=4.2 nM, upper), whereas the affinity of α-SN for 14-3-3η was much lower than that of parkin (Kd=1.1 μM, lower). These results are consistent with those of the immunoprecipitation/Western analysis using recombinant proteins (Figure 5D).
Finally, we examined whether 14-3-3η bound to parkin can be released by α-SN. To test this, we first mixed the lysates coexpressing FL-parkin and Myc-14-3-3η of HEK293 cells with those expressing α-SN. Then the mixtures were incubated under three different conditions, as indicated in the upper panel of Figure 5F. Next, the lysates were immunoprecipitated with anti-FL antibody, and followed by Western blotting with anti-Myc and anti-parkin antibodies. As shown in Figure 5F (upper panel), the amount of 14-3-3η bound to parkin was significantly lower in all incubation conditions, when the cell lysates that simultaneously expressed both parkin and 14-3-3η were incubated with α-SN-expressing lysates. Incubation for 1 h at 37°C reduced the amount of 14-3-3η bound to parkin in proportion to the added amount of α-SN-expressing cell lysate (Figure 5F, lower panel). Intriguingly, the α-SN(A30P) and α-SN(A53T) mutants had no effect on the release of 14-3-3η, unlike wild-type (WT) α-SN (Figure 5F, lower panel). These observations strongly indicate that α-SN, but not α-SN(A30P) or α-SN(A53T), can capture and release 14-3-3η from the parkin–14-3-3η complex, which supports our notion that the negative regulation of parkin activity by 14-3-3η is relieved by α-SN (Figure 5A and B).
Parkin, 14-3-3η, and α-SN levels in the substantia nigra of PD
Finally, we analyzed the levels of parkin, 14-3-3η, and α-SN in the substantia nigra of the midbrain from patients with sporadic PD. Western blotting revealed no significant differences in parkin, 14-3-3η, and actin in the substantia nigra between control (patients without PD) and PD patients, whereas α-SN was significantly increased in the substantia nigra of PD patients (Figure 6A, upper panel). As parkin did not interact physically with α-SN in our immunoprecipitation analysis (Figure 5C; data not shown), we then examined the interactions of 14-3-3η with parkin or α-SN by measuring these proteins in the anti-14-3-3η immunoprecipitant. Whereas the levels of parkin associated with 14-3-3η from PD appeared to be decreased relative to the control, the levels of α-SN that interacted with 14-3-3η were clearly increased in patients with PD (Figure 6A, lower panel). Thus, it is suggested that the elevated levels of α-SN are associated with its interaction with 14-3-3η and the activity of parkin may be aberrantly regulated in the substantia nigra of sporadic PD.
Figure 6.
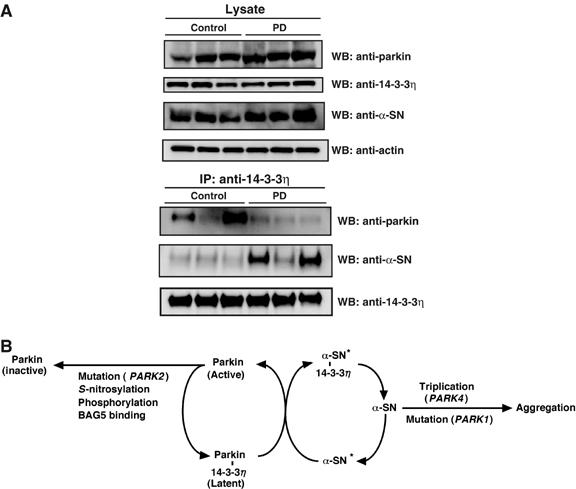
(A) Levels of parkin, 14-3-3η, and α-SN in the substantia nigra of PD. Brain of a representative patient with PD (upper panel). Samples (30 μg) of the crude extract of the brains (substantia nigra) of control (patients without PD) and PD patients were subjected to SDS–PAGE, following Western blotting against antibodies against parkin, 14-3-3η, α-SN, and actin. Physical interaction between 14-3-3η and parkin or α-SN (lower panel). After the same samples used in the upper panel were immunoprecipitated with anti-14-3-3η, Western blotting was carried out using antibodies against parkin, α-SN, and 14-3-3η. (B) A schematic diagram showing the pathways involved in the regulation of parkin activity by 14-3-3η and α-SN. α-SN, α-synuclein; α-SN*, modified form of α-SN. Note that whether parkin is phosphorylated to bind to 14-3-3η remains unknown at present. See text for details.
Discussion
The major finding of the present study was the identification of 14-3-3η as a novel regulator of parkin. First, parkin was in a complex with 14-3-3η, but not β, γ, ɛ, or τ isoforms, in the mouse brain (Figure 1). 14-3-3η could bind primarily to the linker region of parkin, but not with the ARJP-causing missense mutant parkin (K161N), which has a mutation in the linker region (Figure 2). Second, the binding of 14-3-3η to parkin was associated with suppression of the ubiquitin-ligase activity, suggesting that certain parkin bound to 14-3-3η is present at a latent status in the brain (Figure 3). Third, overexpression of α-SN abrogated the 14-3-3η-induced suppression of parkin activity, indicating that α-SN relieves the negative regulation of parkin by 14-3-3η (Figure 5A and B). Intriguingly, PD-causing A30P and A53T mutations of α-SN could not bind 14-3-3η and failed to activate parkin. These results indicate that 14-3-3η is a regulator that functionally links parkin and α-SN, as illustrated in Figure 6B.
It is of particular note that we report unusual isoform specificity for 14-3-3η to interact with parkin among all 14-3-3 species examined. However, the possibility that the other species are also involved in the interaction by forming a heterodimer with 14-3-3η cannot be excluded in vivo, because 14-3-3 bands immunoprecipitated by anti-parkin antibody from the brain extracts showed doublet with one weak signal for Western blotting (Figure 1A). Nevertheless, herein we address that recombinant parkin could directly bind to 14-3-3η (Figure 5D, left panel), with considerably high affinity (Kd=approximately 4 nM) (Figure 5E) and that the 14-3-3η homodimer is a negative factor for autoubiquitylating activity of parkin in vitro (Figure 3A).
It is known that the 14-3-3 family proteins interact with the majority, but not all, proteins after their phosphorylation (Aitken et al, 2002; Bridges and Moorhead, 2004; Mackintosh, 2004). Indeed, parkin contains the RKDSPP sequence in the linker region that resembles the typical binding motifs with a potential phosphorylation residue for 14-3-3 proteins (Yaffe et al, 1997; Mackintosh, 2004). It is also known that parkin has several possible phosphorylation sites, and recent studies showed that parkin is phosphorylated in vitro (Yamamoto et al, 2004), although there is no direct evidence demonstrating phosphorylation of parkin in vivo to date. However, it remains elusive whether or not phosphorylation of parkin is responsible for its specific binding to 14-3-3η, because known potential phosphorylation motifs are capable of associating with many 14-3-3 species in general. The specificities of 14-3-3: client–protein interactions do not result from different specificities for the phosphopeptide-binding motifs, but probably arises from contacts made on the variable surface of 14-3-3 outside the binding cleft, as discussed previously by Yaffe et al (1997). In this regard, some reports showed functional specificities of 14-3-3 isoforms (Aitken, 2002; Aitken et al, 2002; Roberts and de Bruxelles, 2002), and indeed several enzymes retain several nonphosphorylated binding motifs for 14-3-3s (Hallberg, 2002; Sribar et al, 2003), though parkin lacks such 14-3-3-interacting sequences. Thus, parkin, in particular its linker region, may have a new binding motif(s) for 14-3-3η, but the interacting motif(s) remains to be identified. If 14-3-3η binds to parkin through two sites as a dimmer, it is plausible that the phosphorylation of parkin is involved in their interactions at least in part.
With regard to the mechanistic action of 14-3-3η, it may suppress parkin activity by preventing access of the substrate, because the binding of synphilin-1 (used here as a model substrate to parkin) was inhibited by 14-3-3η (Figure 3C). Accumulating evidence suggests that parkin can bind various targets by the UBL domain or the RING box, in particular the RING 1 domain (Dawson and Dawson, 2003). Accordingly, 14-3-3η may have function(s) other than suppressing the access of the substrate to parkin. Indeed, 14-3-3η strongly inhibits substrate-independent self-ubiquitylation of parkin, indicating blockage of the intrinsic E3 activity. It was also anticipated that 14-3-3η hinders the recruitment of E2 to parkin. However, this was not the case, because 14-3-3η had no effect on the binding of UbcH7 and Ubc7 to parkin (Figure 4). Thus, while the mechanism of 14-3-3η-induced suppression of parkin activity remains to be identified, it is possible that it involves preventing the positioning of the ubiquitin-charged E2 toward the target Lys residue by steric hindrance due to the association of 14-3-3η to parkin.
It is worth noting that parkin does not interact with α-SN directly, because we could not demonstrate the physical binding of parkin to α-SN in vivo and in vitro (data not shown; see also Dawson and Dawson, 2003). Nevertheless, we found that the negative regulation of parkin by 14-3-3η was relieved by α-SN, which could bind tightly to 14-3-3η in vivo (Figure 5A–C). In this regard, it is of note that the amounts of 14-3-3η bound to parkin were decreased when the lysates of cells coexpressing parkin and 14-3-3η were incubated with those expressing WT α-SN, but not PD-related α-SN(A30P) or α-SN(A53T) mutants in vitro (Figure 5F). These results clearly indicate that 14-3-3η bound to parkin is sequestered by α-SN, but not competition by α-SN toward the binding of 14-3-3η to parkin. Unlike the association between parkin and 14-3-3η, there is little or no interaction between α-SN and 14-3-3η in vitro, as recombinant α-SN did not bind to 14-3-3η (Figure 5D, right panel, and E). Thus, it is plausible that certain modification(s) of α-SN is required for its association to 14-3-3η in mammalian cells (see our model in Figure 6B). Judging from the characteristic properties of 14-3-3 family proteins capable of binding many phosphorylated proteins (Yaffe et al, 1997), certain phosphorylation(s) of α-SN seems quite possible for the interaction with 14-3-3η. Indeed, there are several reports regarding phosphorylation of α-SN (Fujiwara et al, 2002; Hirai et al, 2004). Although previous studies clearly demonstrated that α-SN deposited in synucleinopathy brains is extensively phosphorylated at Ser-129 (Fujiwara et al, 2002; Hirai et al, 2004), this is probably not the case in our study, because the chemically synthesized peptide phosphorylated at Ser-129 of α-SN did not bind to the 14-3-3η (our unpublished results). However, the possibility that α-SN is phosphorylated at other site(s) cannot be exclusively ruled out. Alternatively, one cannot exclude a possible, though yet unknown, modification(s) of α-SN other than phosphorylation as a mechanism responsible for the increased affinity toward 14-3-3η. In this regard, α-SN is structurally related to 14-3-3 family proteins (Ostrerova et al, 1999), but it is unknown whether the homologous region is involved in the physical interaction with α-SN. Further studies are required to clarify the mode of α-SN modification.
In the present study, we found reciprocal regulation of parkin activity by α-SN and 14-3-3η, whose tripartite control could enhance our understanding of the pathogenesis of PD. As illustrated in Figure 6B, to date there are several reports on the post-translational modification of parkin. Recent findings indicate that the ubiquitin E3 ligase activity of parkin is modified by nitric oxide (NO). Namely, parkin is S-nitrosylated in PD patients and an in vivo mouse model of PD, and S-nitrosylation shows inhibition of the E3 activity of parkin (Chung et al, 2004; Kahle and Haass, 2004; Yao et al, 2004), which could contribute to the degenerative process in PD by impairing the ubiquitylation of parkin substrates. Moreover, Kalia et al (2004) showed that the bcl-2-associated athanogene 5 (BAG5) enhanced the death of dopaminergic neurons in an in vivo model of PD by inhibiting the E3 ligase activity of parkin. In addition, recent studies reported that phosphorylation of parkin causes a small but significant reduction of parkin auto-ubiquitylating activity (Yamamoto et al, 2004). More recently, it was reported that Nrdp1/FLRF RING-finger E3 ligase binds and ubiquitylates parkin, resulting in reduction of parkin activity, implying its involvement in the pathogenesis of PD (Zhong et al, 2005). Considered together, these results indicate that the apparent loss of parkin E3 ubiquitin ligase activity associated with the pathogenesis of PD (see the model displayed in Figure 6B) is in agreement with the ARJP-linked mutations that lead to loss of function of parkin, and that the functional loss of parkin activity is linked to the death of dopaminergic neurons. In addition, we reported herein the imbalance of tripartite interactions among parkin, 14-3-3η, and α-SN levels in the substantia nigra of sporadic PD, but it is still not clear how these alterations influence parkin activity in neural cells. Thus, parkin is an E3 ubiquitin ligase involved in the ubiquitylation of proteins, irrespective of its involvement of K48- or K63-linked ubiquitylation (Doss-Pepe et al, 2005; Lim et al, 2005), that are important in the survival of dopaminergic neurons in PD.
In the present study, we found that parkin E3 activity is regulated positively and negatively by α-SN and 14-3-3η, respectively, suggesting that derangements of this regulation may be responsible for ARJP. For instance, the activated parkin free from 14-3-3η may be labile, and thus sensitive to other stresses, such as S-nitrosylation, and inactivated secondarily in PD. This situation resembles the effect of S-nitrosylation, in which nitrosative stress leads to S-nitrosylation of WT parkin, which leads initially to a marked increase followed by a decrease in the E3 ligase–ubiquitin–proteasome degradative pathway (Yao et al, 2004). The initial increase in the activity of parkin's E3 ubiquitin ligase leads to autoubiquitylation of parkin and subsequent inhibition of its activity, which would impair ubiquitylation and clearance of parkin substrates. In turn, 14-3-3η may protect against impairment of parkin induced by various environmental stresses, including S-nitrosylation. It is also noteworthy that, although 14-3-3η acts as a negative regulator of parkin, it may play a positive role in maintaining a large pool of parkin by preventing its self-ubiquitylation in the brain. Finally, we assume that gradual reduction of parkin activity may be associated with the development of ARJP as well as sporadic PD.
Current evidence suggests that α-SN increases in response to various stresses (Sherer et al, 2002; Gomez-Santos et al, 2003). This finding is compatible with the results of recent studies that dopamine-dependent neurotoxicity (Tabrizi et al, 2000; Zhou et al, 2000; Junn and Mouradian, 2002) is mediated by the formation of protein complexes that contain α-SN and 14-3-3, which are selectively increased in the substantia nigra in PD (Xu et al, 2002). Further studies are needed to determine the levels of parkin, 14-3-3η, α-SN, and parkin and α-SN-14-3-3η complexes in the substantia nigra of the midbrain of patients with sporadic PD.
Here we suggest that α-SN and parkin function through the same pathway. Indeed, both proteins, if not all, are associated with presynaptic vesicles (Dawson and Dawson, 2003). So far, however, the physiological role of α-SN is largely unknown, though various roles including its involvement in synaptic plasticity have been suggested (Liu et al, 2004). We here provided the first evidence that α-SN acts as a positive regulator of parkin E3 activity. It is worth noting that disease-causing mutations of α-SN(A30P) and α-SN(A53T) could not activate the latent parkin–14-3-3η complex, and thus, these mutations may accelerate the development of PD by failing to activate parkin. Our results identified a functional link between these two familial PD-gene products, thus highlighting the existence of a novel regulatory mechanism that could help us further understand the pathogenesis of ARJP as well as sporadic PD. However, it must be stressed here that α-SN is the causative gene product of familial PD. It is noteworthy that α-SN is an aggregation-prone protein due to its natively unfolded protein nature. It is of note that the locus of PARK4 is triplication of the α-SN gene (PARK1) (Singleton et al, 2003), indicating that overexpression of α-SN itself is toxic and induces dopaminergic neuronal death. Indeed, α-SN tends to self-aggregate, and this tendency, which is augmented in the α-SN(A30P) and α-SN(A53T) mutants (Conway et al, 2000) (see our model in Figure 6B), causes autosomal dominant PD (Narhi et al, 1999). Both WT and mutant α-SN form amyloid fibrils akin to those seen in LBs, as well as nonfibrillary oligomers termed protofibrils (Dawson and Dawson, 2003; Bossy-Wetzel et al, 2004). However, whether aggregation and fibrillary formation of α-SN- and PD-linked mutants play a role in neuronal dysfunction and death of neurons in PD are a matter of fierce debate. At this point of view, we emphasize that the feature of α-SN as an aggregation-prone protein is probably not linked directly to its role as a potent activator of parkin E3 in the pathogenesis of PD. Even if these two unique properties of α-SN account for the development of PD independently or synergistically, however, it is clear that their mechanistic actions differ as illustrated in Figure 6B.
Materials and methods
Immunological analysis
For immunoprecipitation analysis of endogenous proteins in the brains of adult mouse and human, these brains were homogenized in three volumes of ice-cold lysis buffer (20 mM HEPES (pH 7.9) buffer containing 0.2% NP-40, 1 mM dithiothreitol (DTT) and protease inhibitor cocktail (Sigma, Chemical Co., St Louis, MO)). The tissue homogenate was centrifuged at 20 000 g at 4°C for 20 min. The supernatant (2 mg protein) was used for immunoprecipitation with one of the following antibodies: anti-polyclonal parkin (Cell Signaling Technology, Beverly, MA) and anti-14-3-3η antibodies (Immuno-Biological Lab. Co., Gunma) or control IgG (700 ng). The resulting immunoprecipitates were resolved in 30 μl of the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer, and one-third of the samples (10 μl) were subjected to SDS–PAGE, followed by Western blotting with anti-14-3-3 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-14-3-3β, 14-3-3γ, 14-3-3ɛ, 14-3-3η, and 14-3-3τ (Immuno-Biological Lab. Co., Ltd, Japan) and anti-monoclonal parkin (1A1) antibodies (Shimura et al, 1999). In all, 10 μg of the supernatant (lysate) was used as input (1.5%).
For immunoprecipitation analysis of the cell culture system, HEK293 cells were transfected with the respective plasmids. After 48 h, the cells were washed with ice-cold PBS (in mM, 10 Na2PO4, 2 KH2PO4, 137 NaCl, and 2.7 KCl), pH 7.4, and harvested in the lysis buffer (600 μl). The lysate was then rotated at 4°C for 1 h, followed by centrifugation at 20 000 g for 10 min. The supernatant (200 μl) was then combined with 50 μl protein G-Sepharose (Amersham Life Science, Buckinghamshire, UK), pre-incubated with anti-Myc (Santa Cruz Biotechnology, Santa Cruz), V5 (Invitrogen), and HA (Santa Cruz Biotechnology) antibodies or anti-FL antibody beads (Sigma) for 3 h. The protein G-Sepharose or FL-beads were precipitated and the pellets were extensively washed using the lysis buffer containing 500 mM NaCl. The precipitates were used for Western blot analysis using anti-parkin, Myc, FL, HA, V5, 14-3-3, and α-SN (BD Transduction Lab.) antibodies, as mentioned above. A volume of 5 μl of the supernatant was used as input (7.5%).
In vitro autoubiquitylation assay
Recombinant GST-14-3-3η was produced in Escherichia coli. Untagged 14-3-3η was produced from GST-14-3-3η by digestion with PreScission Protease (Amersham Bioscience). Recombinant His-parkin and E1 were produced from baculovirus-infected HiFive insect cells. Reactions were performed for 3 h at 37°C in 50 μl of assay mixture containing 40 mM Tris–HCl buffer (pH 7.5), 5 mM MgCl2, 2 mM ATP, 2 mM DTT, 15 μg ubiquitin (Sigma), 200 ng of E1, and 600 ng of E2 (UbcH7) (Affiniti-Research, Exeter, Devon, UK) in the presence or absence of GST-14-3-3η or 14-3-3η. After incubation, the reaction was terminated by the addition of the sample buffer for SDS–PAGE (17 μl), and aliquots (15 μl) were subjected to SDS–PAGE followed by Western blotting with anti-parkin antibody.
In vivo ubiquitylation assay
HEK293 cells were transfected for 48 h with pcDNA3.1 expression plasmids, in which FL-tagged parkin or FL-parkin mutants, α-SN or α-SN mutants, 14-3-3η, V5-synphilin-1, and HA-ubiquitin cDNAs were ligated. MG132 (50 μM) was added for 20 min, prior to harvesting of the cells. Then, the cells were washed with cold PBS and lysed by 50 mM Tris–HCl buffer (pH 8.0), containing 150 mM NaCl, 1% Nonidet-P40, 1% deoxycolate, 0.1% SDS, 5 mM ethylenediaminetetraacetic acid, and protease inhibitor cocktail. Preparation of the cell lysate, immunoprecipitation, and Western blot analyses were essentially the same for the immunological analysis as described above. In all experiments, the cell lysates (10 μg, 7.5% input) were used for Western blotting as controls to check the expression levels.
For the method sections of ‘Cell culture and transfection', ‘Plasmids', and ‘Surface plasmon resonance (SPR) analysis', see Supplementary data.
Supplementary Material
Supplementary Material
Acknowledgments
We thank Y Goto for providing the 14-3-3σ, β, and ζ expression plasmids, and T Ichimura for the valuable discussion. This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Aitken A (2002) Functional specificity in 14-3-3 isoform interactions through dimer formation and phosphorylation. Chromosome location of mammalian isoforms and variants. Plant Mol Biol 50: 993–1010 [DOI] [PubMed] [Google Scholar]
- Aitken A, Baxter H, Dubois T, Clokie S, Mackie S, Mitchell K, Peden A, Zemlickova E (2002) Specificity of 14-3-3 isoform dimer interactions and phosphorylation. Biochem Soc Trans 30: 351–360 [DOI] [PubMed] [Google Scholar]
- Baxter HC, Liu WG, Forster JL, Aitken A, Fraser JR (2002) Immunolocalisation of 14-3-3 isoforms in normal and scrapie-infected murine brain. Neuroscience 109: 5–14 [DOI] [PubMed] [Google Scholar]
- Berg D, Holzmann C, Riess O (2003) 14-3-3 proteins in the nervous system. Nat Rev Neurosci 4: 752–762 [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA (2004) Molecular pathways to neurodegeneration. Nat Med 10 (Suppl S2): S2–S9 [DOI] [PubMed] [Google Scholar]
- Bridges D, Moorhead GB (2004) 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE 2004: re10. [DOI] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM (2004) S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304: 1328–1331 [DOI] [PubMed] [Google Scholar]
- Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM (2001) Parkin ubiquitinates the α-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med 7: 1144–1150 [DOI] [PubMed] [Google Scholar]
- Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT Jr (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA 97: 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson's disease. Science 302: 819–822 [DOI] [PubMed] [Google Scholar]
- Doss-Pepe EW, Chen L, Madura K (2005) α-Synuclein and parkin contribute to the assembly of ubiquitin lysine 63-linked multiubiquitin chains. J Biol Chem 280: 16619–16624 [DOI] [PubMed] [Google Scholar]
- Forno LS (1996) Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol 55: 259–272 [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4: 160–164 [DOI] [PubMed] [Google Scholar]
- Gomez-Santos C, Ferrer I, Santidrian AF, Barrachina M, Gil J, Ambrosio S (2003) Dopamine induces autophagic cell death and α-synuclein increase in human neuroblastoma SH-SY5Y cells. J Neurosci Res 73: 341–350 [DOI] [PubMed] [Google Scholar]
- Hallberg B (2002) Exoenzyme S binds its cofactor 14-3-3 through a non-phosphorylated motif. Biochem Soc Trans 30: 401–405 [DOI] [PubMed] [Google Scholar]
- Hirai Y, Fujita SC, Iwatsubo T, Hasegawa M (2004) Phosphorylated α-synuclein in normal mouse brain. FEBS Lett 572: 227–232 [DOI] [PubMed] [Google Scholar]
- Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R (2001) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105: 891–902 [DOI] [PubMed] [Google Scholar]
- Junn E, Mouradian MM (2002) Human α-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci Lett 320: 146–150 [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Haass C (2004) How does parkin ligate ubiquitin to Parkinson's disease? EMBO Rep 5: 681–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia SK, Lee S, Smith PD, Liu L, Crocker SJ, Thorarinsdottir TE, Glover JR, Fon EA, Park DS, Lozano AM (2004) BAG5 inhibits parkin and enhances dopaminergic neuron degeneration. Neuron 44: 931–945 [DOI] [PubMed] [Google Scholar]
- Kawakami T, Chiba T, Suzuki T, Iwai K, Yamanaka K, Minato N, Suzuki H, Shimbara N, Hidaka Y, Osaka F, Omata M, Tanaka K (2001) NEDD8 recruits E2-ubiquitin to SCF E3 ligase. EMBO J 20: 4003–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto Y, Akiguchi I, Nakamura S, Honjyo Y, Shibasaki H, Budka H (2002) 14-3-3 proteins in Lewy bodies in Parkinson disease and diffuse Lewy body disease brains. J Neuropathol Exp Neurol 61: 245–253 [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O (1998) A30P mutation in the gene encoding α-synuclein in Parkinson's disease. Nat Genet 18: 106–108 [DOI] [PubMed] [Google Scholar]
- Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y, Tanaka Y, Smith W, Engelender S, Ross CA, Dawson VL, Dawson TM (2005) Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci 25: 2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, Narasanna A, Kolodilov N, Dauer W, Hawkins RD, Arancio O (2004) Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J 23: 4506–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh C (2004) Dynamic interactions between 14-3-3 proteins and phosphoproteins regulate diverse cellular processes. Biochem J 381: 329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H, Rostas J, Patel Y, Aitken A (1994) Subcellular localisation of 14-3-3 isoforms in rat brain using specific antibodies. J Neurochem 63: 2259–2265 [DOI] [PubMed] [Google Scholar]
- McNaught KS, Olanow CW (2003) Proteolytic stress: a unifying concept for the etiopathogenesis of Parkinson's disease. Ann Neurol 53 (Suppl 3): S73–S84 [DOI] [PubMed] [Google Scholar]
- Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. (1999) Both familial Parkinson's disease mutations accelerate α-synuclein aggregation. J Biol Chem 274: 9843–9846 [DOI] [PubMed] [Google Scholar]
- Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B (1999) α-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci 19: 5782–5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047 [DOI] [PubMed] [Google Scholar]
- Roberts MR, de Bruxelles GL (2002) Plant 14-3-3 protein families: evidence for isoform-specific functions? Biochem Soc Trans 30: 373–378 [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT (2002) An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered α-synuclein metabolism and oxidative damage. J Neurosci 22: 7006–7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin–protein ligase. Nat Genet 25: 302–305 [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Yoshikawa M, Kitada T, Matsumine H, Asakawa S, Minoshima S, Yamamura Y, Shimizu N, Mizuno Y (1999) Immunohistochemical and subcellular localization of Parkin protein: absence of protein in autosomal recessive juvenile parkinsonism patients. Ann Neurol 45: 668–672 [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K (2003) α-Synuclein locus triplication causes Parkinson's disease. Science 302: 841. [DOI] [PubMed] [Google Scholar]
- Sribar J, Sherman NE, Prijatelj P, Faure G, Gubensek F, Fox JW, Aitken A, Pungercar J, Krizaj I (2003) The neurotoxic phospholipase A2 associates, through a non-phosphorylated binding motif, with 14-3-3 protein γ and ɛ isoforms. Biochem Biophys Res Commun 302: 691–696 [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Orth M, Wilkinson JM, Taanman JW, Warner TT, Cooper JM, Schapira AH (2000) Expression of mutant α-synuclein causes increased susceptibility to dopamine toxicity. Hum Mol Genet 9: 2683–2689 [DOI] [PubMed] [Google Scholar]
- Ubl A, Berg D, Holzmann C, Kruger R, Berger K, Arzberger T, Bornemann A, Riess O (2002) 14-3-3 protein is a component of Lewy bodies in Parkinson's disease-mutation analysis and association studies of 14-3-3η. Brain Res Mol Brain Res 108: 33–39 [DOI] [PubMed] [Google Scholar]
- Vila M, Przedborski S (2004) Genetic clues to the pathogenesis of Parkinson's disease. Nat Med 10 (Suppl): S58–S62 [DOI] [PubMed] [Google Scholar]
- Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA (2002) Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med 8: 600–606 [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91: 961–971 [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Friedlein A, Imai Y, Takahashi R, Kahle PJ, Haass C (2004) Parkin phosphorylation and modulation of its E3 ubiquitin ligase activity. J Biol Chem 280: 3390–3399 [DOI] [PubMed] [Google Scholar]
- Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, Uehara T, Lipton SA (2004) Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA 101: 10810–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Tan Y, Zhou A, Yu Q, Zhou J (2005) RING finger ubiquitin-protein isopeptide ligase Nrdp1/FLRF regulates parkin stability and activity. J Biol Chem 280: 9425–9430 [DOI] [PubMed] [Google Scholar]
- Zhou W, Hurlbert MS, Schaack J, Prasad KN, Freed CR (2000) Overexpression of human α-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res 866: 33–43 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


