Abstract
Objective:
To assess our long-term complications from complete axillary lymph node dissection (AXLND) in patients with breast cancer.
Summary Background Data:
Complete AXLND as part of the surgical therapy for breast cancer has come under increased scrutiny due the use of the sentinel lymph node (SLN) biopsy technique to assess the status of the axillary nodes. As the enthusiasm for the SLN technique has increased, our impression has been that the perceived complication rate from AXLND has increased dramatically while the negative aspects of the SLN technique have been underemphasized.
Methods:
Female patients seen in routine follow-up over a 1-year period were eligible for our retrospective study of the long-term complications from AXLND if they were a minimum of 1 year out from all primary therapy; ie, surgery, radiation, and/or chemotherapy. All patients had previously undergone either a modified radical mastectomy (MRM) or a segmental mastectomy with axillary dissection and postoperative radiation (SegAx/XRT). All patients had a Level I–III dissection. Objective measurements, including upper and lower arm circumferences and body mass index (BMI), were obtained, and a subjective evaluation from the patients was conducted.
Results:
Ninety-four patients were eligible for our study; 44 had undergone MRM, and 50 had undergone SegAx/XRT. The average number of nodes removed was 25.6 (standard deviation, 8). Thirty-three percent of the patients had positive nodal disease, 95% of the patients had an upper arm circumference within 2 cm of the unaffected side, and 93.3% had a lower arm circumference within 2 cm of the unaffected side. Subjectively, 90.4% of the patients had either no or minimal arm swelling, and 96.8% of the patients had “good” or “excellent” overall arm function. The most common long-term symptom was numbness involving the upper, inner aspect of the affected arm (25.5%).
Conclusions:
Our data indicate that a complete AXLND can be performed with minimal long-term morbidity. The lower the morbidity of AXLND, the less acceptable are the unique complications of the SLN technique.
Our data evaluating the long-term complications of a complete axillary lymph node dissection indicate that this operation can be performed with minimal long-term morbidity. The recent literature appears to overestimate the complications of axillary node dissection and underestimate the complications of the sentinel lymph node technique in the understandable enthusiasm surrounding a new methodology. The implications of the sentinel lymph node technique are discussed.
Complete axillary lymph node dissection (AXLND) as part of the surgical therapy for breast cancer has come under increased scrutiny over the last several years, mainly due to the use of the sentinel lymph node (SLN) biopsy technique to assess the status of the axillary nodes. This technique has the advantages of avoiding an AXLND in those patients with pathologically negative nodes and, possibly more importantly, of identifying those nodes at highest risk for disease. This allows the pathologists to focus their efforts more thoroughly and economically on those nodes most likely to be positive. The principal downside of the SLN technique is the possibility of a false-negative result; ie, the sentinel node is negative, but there is disease elsewhere within the axilla that is not identified. Consequently, disease is left behind and the patient's disease status is downstaged with resultant undertreatment.
As with all new surgical procedures, there is an associated learning curve for the SLN biopsy technique. As a result, the false negative rate seems to be inversely proportional to the experience of the surgeon. According to the Louisville group,1 the false negative rate based on a review of the current literature ranges from 0% to 16.7%, with an estimate of approximately 5% for experienced surgeons.
As the number of published papers on the SLN technique for breast cancer has increased, it has been our impression that the perceived complication rate of AXLND has increased dramatically. According to Singletary's recent review of the “Current Status of Axillary Dissection,”2 up to 60% of patients experience long-term side effects as a result of this procedure. The Louisville group stated that the incidence of clinically evident lymphedema that interferes substantially with patients’ quality of life may be as high as 15%.1 This is in contradistinction to earlier data from Morton’s group,3 the originators of the SLN technique, who in the 1970s and 1980s routinely performed prophylactic lymphadenectomies, including AXLND, for malignant melanoma (>0.76 mm), and reported only minimal morbidity associated with the procedures.
Our view has been that the long-term morbidity of AXLND in experienced hands is minimal, that there are multiple factors in addition to the extent of axillary dissection contributing to this morbidity, and that AXLND should remain a reasonable treatment option until long-term recurrence and survival data are available with the SLN technique, particularly for patients with invasive cancers > 1.0 cm.
A review of our long-term complications with complete (Levels I–III) AXLND for breast cancer is presented herein.
METHODS
Female patients treated for breast cancer were seen in routine follow-up over a 1-year period. They were eligible for our retrospective study of the long-term complications from AXLND if they were a minimum of 1 year out from all primary therapy; ie, surgery, radiation, and/or chemotherapy. All patients in the study had previously undergone either a modified radical mastectomy (MRM) or a segmental mastectomy with AXLND and postoperative radiation (SegAx/XRT). All operations were performed by 1 surgeon (AWS), and all axillary dissections included Levels I–III lymph nodes. Surgeries were performed over a 14-year period (1986 to 2000), and 100% of the patients were followed at regular intervals. Objective measurements, including upper and lower arm circumferences and body mass index (BMI), were ascertained, and a subjective evaluation from the patients was conducted by our surgical oncology research nurse (SB). This included the patient's perception of the degree of lymphedema or arm swelling (none, minimal, moderate, severe, incapacitating); the patient's complaints about other symptoms like pain, tingling, and numbness; and the patient's perception of arm function, including range of motion, strength, and overall function. If a patient were seen more than once during the study period, the data from the last follow-up visit were used for our evaluation. Additional information including the number of lymph nodes removed, the presence and number of positive nodes, the adjuvant chemotherapy received, the radiation fields used, the timing (immediate or delayed) and type of breast reconstruction (implant, autologous flap), and the length of postoperative observation were also reviewed at the time of each assessment.
Means for continuous variables as well as frequencies and percentages for categorical variables were calculated. Stepwise regressions were performed to evaluate patient variables that might be predictive of either lymphedema measured clinically or arm swelling perceived by the patient.
RESULTS
Ninety-four female patients who had undergone either a MRM or SegAx/XRT for breast cancer were seen in routine follow-up over a 1-year period. At least 1 year had elapsed since the completion of their primary therapy (surgery, radiation, chemotherapy). Forty-four patients had undergone MRM, and 50 patients had undergone SegAx/XRT. Four patients, 2 in each group, had undergone AXLND bilaterally. Sixty-four percent of the patients were < than 5 years post-therapy; 23% were 5 to 10 years post-therapy; and 11% were > 10 years post-therapy. Six patients died during the study period: 2 from metastatic breast cancer, and 4, who were disease-free from breast cancer, from unrelated illnesses. One patient was alive with metastatic disease. None of the patients who died or were alive with metastatic disease had had a loco-regional recurrence.
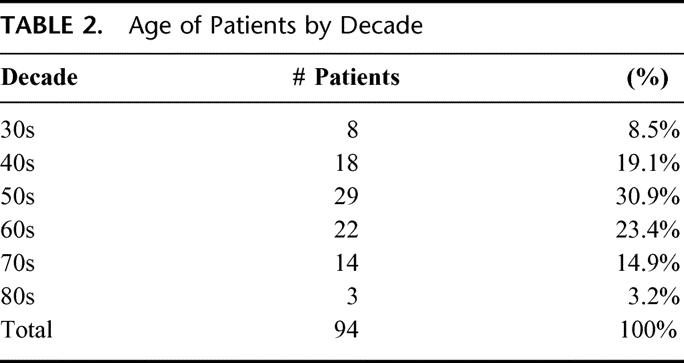
The average number of lymph nodes removed was 25.5, with a median of 25 and a range of 7 to 52. Seventy-six percent of the patients had at least 20 nodes removed. Only 2 patients had fewer than 15 nodes removed. Thirty-one (33%) of 94 patients had positive lymph nodes. Of those with positive nodes, 14 (45%) of 31 patients had at least 4 positive nodes (Table 1). The age of the patients at the time of the AXLND is noted in Table 2. 28% of the patients were < 50 years of age, 54% were aged between 50 and 69 years, and 18% were 70 years or older.
TABLE 1. Surgical Treatment Data
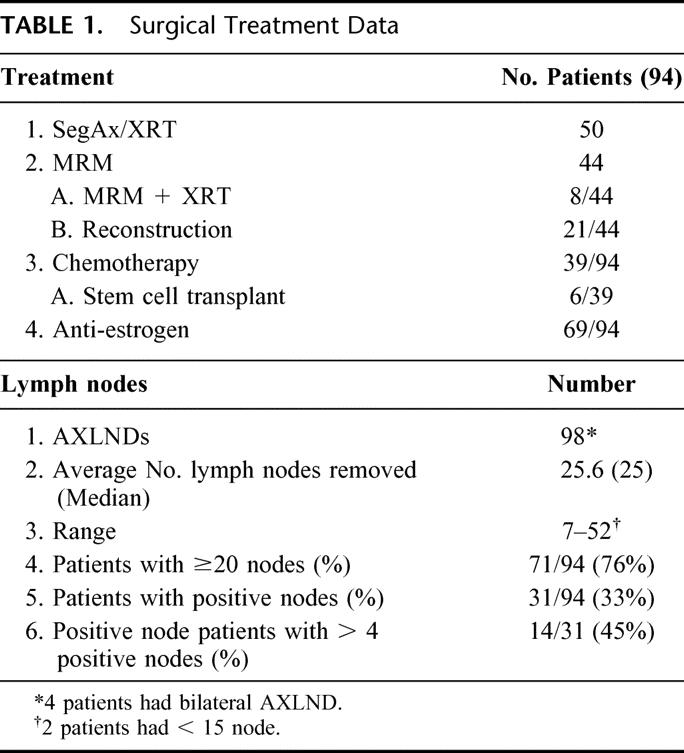
TABLE 2. Age of Patients by Decade
The objective measurements of upper arm and lower arm circumferences are shown in Figure 1. The upper arm circumference was within 1 cm of the unaffected side in 58 of 90 patients (4 patients had bilateral AXLND) and within 2 cm in an additional 27 patients; 5 of 90 patients had a > 2-cm difference. The lower arm circumference was within 1 cm of the unaffected side in 53 of 90 patients; within 2 cm in another 30 patients; and > 2 cm in 7 of 90 patients. Only 1 patient had > 2-cm difference in both upper and lower arm circumference. Thus, 85 (94.4%) of 90 patients had an upper arm circumference within 0 to 2 cm of the unaffected side, and 83 (92.2%) of 90 patients had a lower arm circumference within 0 to 2 cm of the unaffected side. The BMI was normal in 42 (44.7%) of the 94 patients; 30 (31.9%) of 94 had a BMI in the overweight range; and 22 (23.4%) of 94 were in the obese range (Table 3). The subjective perceptions of the patients regarding lymphedema (arm swelling) and other symptoms (pain, tingling, and numbness) are shown in Figure 2. Sixty-five of the 94 patients felt that they had no arm swelling, and 20 patients described their arm swelling as minimal. The objective measurements on 16 of the 20 patients with minimal swelling demonstrated less than a 2-cm difference between the affected and nonaffected sides. Thus, 81 (86.2%) of 94 patients had virtually no arm swelling. Nine patients (10%) considered themselves as having moderate arm swelling. Of the 29 patients who felt they had some degree of arm swelling, 5 of 20 patients with minimal swelling, and 4 of 9 patients with moderate swelling (9 of 29; 31%) had positive nodes. Nineteen of the 29 patients (65.5%) had received either preoperative or postoperative radiation therapy. No patient had severe arm swelling or arm swelling so incapacitating that it affected her lifestyle. Fifteen of the 29 patients with subjective swelling had a BMI > 24.9. The most common long-term symptom was numbness involving mainly the upper, inner aspect of the affected arm. This was mentioned by 24 (25.5%) of 94 patients and had no effect on lifestyle.
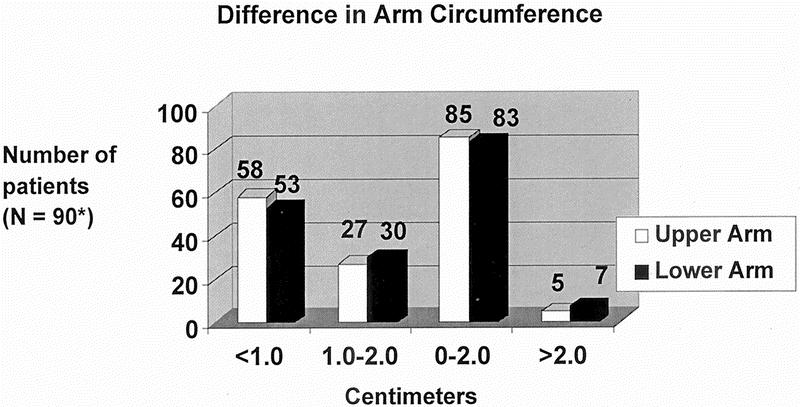
FIGURE 1. Differences in upper and lower arm circumferences: affected versus unaffected side
TABLE 3. Body Mass Index (BMI)
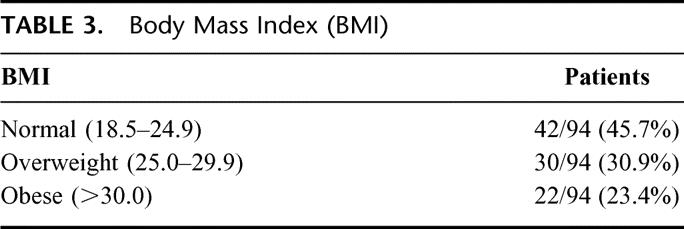
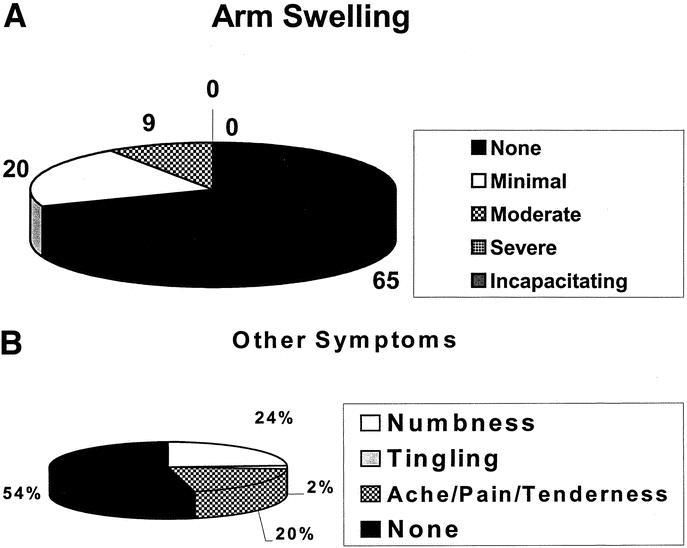
FIGURE 2. Patient's perception of complications following AXLND. (A) Arm swelling; (B) other symptoms
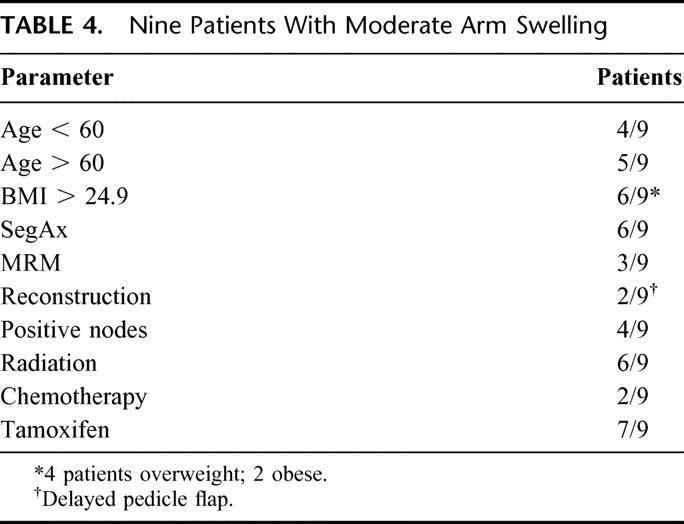
Arm function, including range of motion, strength, and overall function, is described in Figure 3. Over 96% of the patients had either good or excellent arm function, with most in the excellent range. The data in Table 4 focuses on the 9 patients who described themselves as having moderate arm swelling. Stepwise regressions were performed to evaluate patient variables that might be predictive of either arm swelling measured clinically or arm swelling perceived by the patient. The 4 patients with bilateral AXLND were not included in the analyses. All variables including age, BMI, years of follow-up, number of nodes removed, number of nodes positive for metastatic disease, type of operation, and, whether or not the patient received chemotherapy (including stem cell transplantation), preoperative or postoperative radiation, or antiestrogen therapy were analyzed. No patient variables were statistically significant in predicting which patients would develop clinically measurable lymphedema or subjective swelling of the affected arm, although there was a trend demonstrating an association of arm swelling with age, length of follow-up, and obesity.
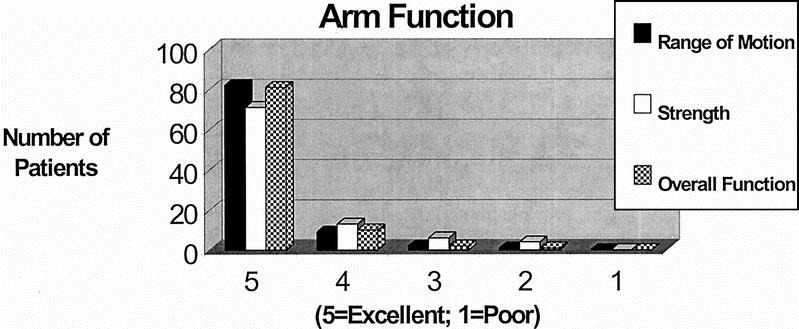
FIGURE 3. Patient's perception of arm function following AXLND
TABLE 4. Nine Patients With Moderate Arm Swelling
DISCUSSION
Our data on the long-term complications of a complete (Levels I–III) AXLND indicate that this operation can be performed with minimal long-term morbidity. Some degree of numbness was the most common symptom and was observed in 25.5% of our patients. It occurred mainly in the upper, inner aspect of the affected arm and was most likely due to the inclusion of the intercostal-brachial nerve(s) with the specimen. It has been our practice to preserve this nerve when possible; however, in the face of suspicious or grossly positive nodal disease, we prefer to sacrifice the nerve and include it with the specimen rather than cut through the specimen and potentially contaminate the operative field.
Twenty of our patients described their arm swelling as minimal, and 9 patients described themselves as having moderate arm swelling. Of the 29 patients with some degree of arm swelling, 31% had positive nodes; thus even if they had undergone a SLN biopsy, 9 of the 29 patients (including 4 of 9 of the patients with moderate swelling) would have had a subsequent AXLND.
Obesity as an important variable in lymphedema after AXLND was described by Petrek et al4 from Memorial Sloan-Kettering Cancer Center. Of 15 potential predictive factors that they analyzed, only 2, arm infection/injury and weight gain (obesity), were statistically associated with lymphedema. In another study from the same institution, Werner et al5 reported that the level of node dissection was not statistically related to the development of arm edema; the only factor that was significantly associated was an elevated BMI. Silberman,6 from the Norris Cancer Center at the University of Southern California, reviewed the problem of lymphedema after AXLND for breast cancer and pointed out that there was little increase in the incidence of lymphedema with inclusion of the Level III nodes: 0 to 2.8% of patients developed lymphedema undergoing axillary sampling procedures; 2.7 to 7.4% of patients developed lymphedema with a Level I–II dissection; and 3.1 to 8.0% developed lymphedema with a Level III dissection. Axillary radiation without surgery was associated with an incidence of lymphedema of 2.1 to 8.3%. The combination of AXLND and radiation was synergistic in their effect on the development of lymphedema. We prefer to use the term “arm swelling” as opposed to lymphedema because there are multiple causes of a swollen arm after AXLND other than lymphedema. For example, any technical error causing encroachment of the axillary vein can lead to arm swelling. A radiation-induced stricture of the innominate, subclavian, or axillary vein can lead to arm swelling. In addition, insertion of a central venous catheter on the side of the AXLND could lead to swelling, simply due to the presence of the catheter, or as a result of a complication induced by the catheter (clot) or the infused chemotherapy (venous sclerosis).
Our results are in agreement with the experience of Morton’s group,3 who performed 126 AXLNDs for melanoma. Their immediate complication rate was described as very low, and no patient developed permanent arm edema. The experience with prophylactic AXLND for melanoma is very useful because postoperative radiation is rarely, if ever, given; hence the melanoma experience with AXLND acts as a “surgery-alone” control and demonstrates that AXLND can be performed with minimal long-term complication. Our experience with prophylactic AXLND for melanoma also demonstrated negligible morbidity.7 In our 29 patients who had some degree of lymphedema, 65.5% (13 of 20 with minimal swelling and 6 of 9 with moderate swelling) received either preoperative or postoperative radiation. As noted above, radiation alone can produce lymphedema, and the combination of radiation and AXLND is synergistic.
We find it disconcerting that long-term complication rates as high as 60% are now being reported for AXLND and that as many as 15% of the patients have sequelae from the operation that are so incapacitating that their lifestyle is affected.1,2 The reasons for these high complication rates are unclear, but, like all operations, must certainly be related to the experience of the surgeon. Of the many papers on the SLN technique that were published in the year 2002, we find with some dismay that all alluded to the serious complications of AXLND, and only 1 discussed the authors’ own experience with AXLND.8 We assume that those publishing their results on the SLN technique are experienced breast cancer surgeons and would have their own large experience with AXLND with which to compare their SLN technique.
We believe that the SLN technique has an important role to play in patients with breast cancer, particularly those with high-grade ductal carcinoma in situ (DCIS) and small T1 invasive cancers. In both these situations, the risk of positive nodal disease is very low; thus the SLN technique obviates the need for AXLND in these low-risk patients. However, with lesions > 1.0 cm, the risk of positive nodal disease becomes substantial, at least 30%, and increases with increasing size of the primary tumor.9 At the same time, the false negative rate of the SLN technique increases. Other complications specific to the SLN technique include the rare allergic reaction to the dye;10,11 and the need for reoperation in a substantial percentage of the patients whose sentinel node is positive, since the diagnosis of positive nodal disease is often not made on frozen section at the time of the original operation. The need for a second operation and a second anesthetic with its attendant risks and cost seems to us to be understated in the current literature. In addition, a second operation will occasionally delay subsequent adjuvant therapy. Consider that approximately 200,000 women in the United States will be diagnosed with invasive breast cancer in the year 2004 (and an additional 54,000 women will be diagnosed with breast carcinoma in situ).12 Most of these patients will present with clinically node-negative disease and will therefore be eligible for the SLN technique. Approximately one third of these patients will have positive nodal disease. With an intraoperative (frozen section) false-negative rate for identifying metastatic disease in the SLN of 45 to 60% for T1 lesions,13 the volume of reoperative axillary surgery that needs to be performed is, to say the least, considerable. Moreover, there have been concerns raised regarding the morbidity of subsequent axillary surgery in those patients with a positive SLN found on permanent pathologic evaluation that underwent an immediate autologous breast reconstruction at the time of mastectomy and SLN biopsy.14
Another understated development as a result of the SLN technique is the inclusion of the axilla in the radiation field in those patients who underwent segmental mastectomy (lumpectomy) and had a negative SLN biopsy.15 This is done by the radiation oncologist in an effort to avoid an axillary recurrence in a patient who may have a false negative SLN biopsy. Weir et al16 from British Columbia recently noted that the regional relapse rate in node-negative patients was significantly increased with smaller numbers of nodes removed. Polednak et al,17 from the Connecticut Tumor Registry, analyzed over 69,000 patients with node-negative breast cancer and found a significantly higher risk of death from breast cancer when less than 10 nodes were removed compared with more than 20. The implication is that positive nodal disease is missed and that the patient is understaged and thus undertreated. This increased relapse rate may be overcome by the use of systemic therapy. The use of radiation and/or chemotherapy in an effort to make up for poorly done surgery does not seem to us to be an effective use of these modalities. These therapies have specific complications of their own and need to be discussed in the context of the SLN technique if they are used as a substitute for lesser surgery or a poorly performed operation. There is no question that the surgical complications of a SLN biopsy are less than that of an AXLND; however, to compare the SLN technique with AXLND, the potential consequences of the SLN technique must also be taken into account.
We believe that the complication rate of AXLND has been overestimated and the negative aspects of the SLN technique underestimated in the understandable enthusiasm surrounding the development of a new technique. Even though we perform a Level I–III dissection, our morbidity has been minimal; thus, a long-term complication rate of 60% for the standard Level I–II AXLND2 is an entirely unacceptable surgical result and simply fuels the fear of our patients. If one routinely extends the radiation field to include the axilla in a patient who has undergone segmental mastectomy and negative SLN biopsy in an effort to cover the possibility of a false-negative result, one needs to factor in that additional morbidity when comparing the SLN technique with AXLND.
Radiation delivered to the axilla in a patient found to have a positive SLN on permanent section in an effort to avoid a second operation has also become increasingly common and similarly understated. Not reoperating on a patient with a positive SLN would leave residual disease behind in a substantial number of patients. In our series of 215 breast cancer patients who underwent either MRM or SegAx/XRT, 41.2% of our patients had at least 1 positive node. Of those with positive nodes, 54.8% had 1 to 3 positive nodes, and 45.2% had > 3 positive nodes.18 Veronesi et al19 pointed out that if the Level I nodes were positive, the statistical chance that the Level II–III nodes would be positive was 41%. This percentage increases with increasing tumor size. Moreover, control of locoregional disease in node-positive patients improves survival. The University of Chicago group noted that in patients with T1 lesions with fewer than 4 positive nodes, the long-term disease-free survival was comparable to that for patients who were node-negative.20 This result was the same even for patients that did not receive systemic chemotherapy, indicating that the surgical therapy (AXLND) was not only diagnostic, but also therapeutic. The recently published radiation trials in high-risk (positive nodes or T3 lesions), premenopausal breast cancer patients also concluded that improved locoregional disease control prolongs survival.21,22 Thus, the axilla must be adequately treated to obtain the best oncologic result.
We believe that the SLN technique is a major advance in the treatment of early-stage breast cancer. However, we also believe that the long-term complication rate of AXLND is sufficiently low that patients at significant risk for positive nodal disease may be better served with an AXLND rather than the SLN technique with its own unique set of complications.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Achilles A. Demetriou, Leon Morgenstern, and Howard Silberman for their constructive criticism of the manuscript and Dr. Janet Elashoff for her assistance with the statistical analyses.
Footnotes
Reprints: Allan W. Silberman, MD, PhD, Cedars-Sinai Comprehensive Cancer Center, 8700 Beverly Blvd. Los Angeles, California 90048. E-mail: AWS222@aol.com.
REFERENCES
- 1.Wong SL, Abell TD, Chao C, et al. Optimal use of sentinel node biopsy versus axillary node dissection in patients with breast carcinoma: a decision analysis. Cancer. 2002;95:478–487. [DOI] [PubMed] [Google Scholar]
- 2.Singletary SE. Current status of axillary node dissection. Contemp Surg. 2002;58:334–340. [Google Scholar]
- 3.Holmes EC, Moseley HS, Morton DL, et al. A rational approach to the surgical management of melanoma. Ann Surg. 1977;186:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrek JA, Senie RT, Peters M, et al. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–1377. [DOI] [PubMed] [Google Scholar]
- 5.Werner RS, McCormick B, Petrek JA, et al. Arm edema in conservatively managed breast cancer: obesity is a major predictive factor. Radiology. 1991;180:177–184. [DOI] [PubMed] [Google Scholar]
- 6.Silberman H. Axillary lymphadenectomy for breast cancer: impact on survival. In: Silberman H, Silberman AW, eds. Surgical Oncology: Multidisciplinary Approach to Difficult Problems. London: Arnold; 2002:369–385. [Google Scholar]
- 7.Silberman AW. Malignant melanoma: practical considerations concerning prophylactic regional lymph node dissection. Ann Surg. 1987;206:206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burak WE, Hollenbeck St, Zervos EE, et al. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Amer J Surg. 2002;183:23–27. [DOI] [PubMed] [Google Scholar]
- 9.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. [DOI] [PubMed] [Google Scholar]
- 10.Kuerer HM, Wayne JD, Ross MI. Anaphylaxis during breast cancer lymphatic mapping. Surgery. 2001;129:119–120. [DOI] [PubMed] [Google Scholar]
- 11.Leong SP, Donegan E, Heffernon W, et al. Adverse reactions to isosulfan blue during selective sentinel lymph node dissection in melanoma. Ann Surg Oncol. 2000;7:361–366. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Tivari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. [DOI] [PubMed] [Google Scholar]
- 13.Weiser MR, Montgomery LL, Susnik B, et al. Is routine intraoperative frozen-section examination of sentinel lymph nodes in breast cancer worthwhile? Ann Surg Oncol. 2000;7:651–655. [DOI] [PubMed] [Google Scholar]
- 14.Kronowitz SJ, Chang DW, Robb GL, et al. Implications of axillary sentinel lymph node biopsy in immediate autologous breast reconstruction. Plast Reconstr Surg. 2002;109:1888–1896. [DOI] [PubMed] [Google Scholar]
- 15.Schlembach PJ, Buchholz TA, Ross MI, et al. Relationship of sentinel and axillary level I-II lymph nodes to tangential fields used in breast irradiation. Int J Rad Oncol Biol Phys. 2001;51:671–678. [DOI] [PubMed] [Google Scholar]
- 16.Weir L, Speers C, D'yachkova Y, et al. Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J Clin Oncol. 2002;20:1793–1799. [DOI] [PubMed] [Google Scholar]
- 17.Polednak AP. Survival of lymph node-negative breast cancer patients in relation to number of lymph nodes examined. Ann Surg. 2003;237:163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silberman AW, Sarna GP, Palmer D. Adjuvant radiation trials for high-risk breast cancer patients: adequacy of lymphadenectomy. Ann Surg Oncol. 2000;7:357–360. [DOI] [PubMed] [Google Scholar]
- 19.Veronesi U, Rilke F, Luini A, et al. Distribution of axillary node metastases by level of invasion. An analysis of 539 cases. Cancer. 1987;59:682–687. [DOI] [PubMed] [Google Scholar]
- 20.Quiet CA, Ferguson DJ, Weichselbaum RR, et al. Natural history of node-positive breast cancer: the curability of small cancers with a limited number of positive nodes. JCO. 1996;14:3105–3111. [DOI] [PubMed] [Google Scholar]
- 21.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949–955. [DOI] [PubMed] [Google Scholar]
- 22.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. [DOI] [PubMed] [Google Scholar]


