Abstract
Objective:
We evaluated the ability of neuromonitoring to predict postoperative outcome in patients undergoing thyroid surgery for different indications.
Summary Background Data:
Neuromonitoring has been advocated to reduce the risk of vocal cord palsy and to predict postoperative vocal cord function.
Methods:
Three hundred twenty-eight patients (502 nerves at risk) were studied prospectively at a single center. Neuromonitoring was performed with the Neurosign 100® device by transligamental placement of the recording electrode into the vocalis muscles. Cumulative distribution of stimulation thresholds was determined by stepwise decreases in current (1 mA to 0.05 mA) for both the vagus and the recurrent nerve. Patients were grouped according to surgical risk (benign and malignant disease, reoperation for benign and for malignant disease).
Results:
If the electrophysiological response was correlated to postoperative vocal cord function, the sensitivity of neuromonitoring was modest (86% in surgery for benign disease) to low (25% in reoperation for malignant disease); the positive predictive value was modest (overall rate 62%) but acceptable (87%) if corrected for technical problems. Specificity and negative predictive values were high (ie, overall >95%). Stimulation thresholds were not augmented in 11 patients, in whom postoperative palsy developed despite normal intraoperative recordings. Similarly, an electrical field response was elicited in 14 of 21 patients with preoperative vocal cord palsy. Electromyographic recordings did not reveal an abnormal amplitude or a decline in nerve conduction velocity.
Conclusions:
Neuromonitoring is useful for identifying the recurrent laryngeal nerve, in particular if the anatomic situation is complicated by prior surgery, large tissue masses, aberrant nerve course. However, neuromonitoring does not reliably predict postoperative outcome.
In a prospective study on 502 nerves at risk, we show that neuromonitoring had a high specificity and negative predictive value for postoperative vocal cord function; sensitivity and positive predictive values were acceptable but only in low-risk patient groups. Electrophysiological parameters that purportedly predict vocal cord palsy were found to be useless. However, neuromonitoring is useful for the reliable identification of the recurrent laryngeal nerve.
Thyroid surgery has 2 dreaded complications that may be of long-term consequence: laryngeal nerve palsy and hypocalcemia caused by the loss of the parathyroid glands. The continuous refinement of the surgical techniques has lowered the incidence of these complications. Nevertheless, it remains a challenge, in particular to the neophyte, to reliably identify these 2 structures and to preserve their functional integrity. Neuromonitoring was proposed some 30 to 40 years ago as a means to verify the functional integrity of the recurrent laryngeal nerve.1–5 Recently, several instrumental setups have become commercially available, and neuromonitoring has gained some popularity in thyroid surgery.6–12 In the present study, we have investigated whether intraoperative neuromonitoring (using the Neurosign 100 instrument) allows one to find and identify the nerve. The underlying working hypothesis assumed neuromonitoring has an adequate sensitivity and specificity to predict postoperative vocal cord function, ie, to identify patients that are prone to develop vocal cord dysfunction and palsy. Stimulation thresholds and related parameters have been proposed as useful for predicting outcome.10 We therefore also attempted to verify these claims and test for the reliability of electrophysiological parameters that are easily accessible under surgical conditions. Our results show that neuromonitoring reliably identifies the recurrent nerve. However, the standard parameters of neuromonitoring (eg, recording the stimulation threshold prior to wound closure) cannot be used to reliably predict the postoperative outcome.
PATIENTS AND METHODS
Patients
The study population comprised 328 patients, 174 and 154 of which were subjected to a bilateral and unilateral resection, respectively. Patients were recruited on a consecutive basis in a single center (8 surgeons, 2 surgical trainees). The analysis of the data did not show any obvious benefit of routine neuromonitoring in patients undergoing primary surgery for benign disease. Thus, we changed the inclusion criteria and subsequently selected patients with relapses or malignant disease to obtain reasonably comparable numbers and to test the performance of neuromonitoring in challenging cases.
The prospective study protocol aimed at a systematic investigation of the Neurosign 100® (Indomed GmbH, Teningen, Germany) and, in particular, in identifying parameters that were useful in predicting postoperative outcome. Thus, after identification of the cricoid and thyroid cartilage, the ipsilateral vocalis muscle was impaled with the bipolar recording electrode through the cricothyroid ligament. The neutral electrode was placed in the sternocleidomastoid muscle. Before any manipulation of the thyroid gland, the vagus nerve was dissected over a short (∼2 cm) stretch to allow for the atraumatic placement of a vessel loop, a procedure that took about 1 to 5 minutes and that did not result in any complication (eg, injury of the jugular vein and veins branching thereof). For our prospective study, it was considered mandatory, but it is obviously an optional step. Thereafter, a first stimulation was performed by placing the (bipolar, concentric) stimulating probe onto the vagus nerve; the current amplitude was 1 mA (rectangular 3 Hz pulse for 1–2 seconds). The electrical field response can be documented by recording the potentials or by converting the electric field response of the muscle to an acoustic signal. The detailed electrophysiological and pharmacological validation of the procedure is published elsewhere.13 For obvious practical purposes, the acoustic response is preferable; it was therefore used on a routine basis. Subsequently, the stimulation threshold was determined by lowering the current in preset steps (0.5, 0.2, 0.1, 0.08) to 0.05 mA. In rare instances, 1 mA did not suffice; in these cases, the amplitude was increased up to 3 mA. Thereafter, the thyroid lobe was mobilized, and the branches of the upper thyroid pole were ligated and severed. An attempt was made to identify the recurrent nerve by using the electrode rather than by palpation and surgical dissection.
The routine procedure called for dissection of the entire recurrent laryngeal nerve; however, surgeons were left the option to adapt the dissection procedure according to the extent of resection and to the anatomic situation. The resection of the thyroid lobe was conducted under visual control of the nerve and under repetitive neuromonitoring. After the removal of the thyroid and prior to wound closure, the stimulating electrode was placed onto the recurrent laryngeal nerve and the vagus nerve; the stimulation threshold was determined as outlined above. This final stimulation threshold was used to compute the distribution of sensitivities shown in Figure 1. The protocol also included the documentation (if possible) of the ramifications of the recurrent laryngeal nerve and the selective stimulation of individual branches.

FIGURE 1. Cumulative distribution of stimulation thresholds after stimulation of the vagus nerve and of the recurrent laryngeal nerve. A, The recurrent laryngeal nerves were stimulated with 3-Hz pulses at currents ranging from 3 mA to 0.01 mA before (triangles) and after dissection but prior to wound closure (circles). The stimulation threshold of the vagus nerve was also recorded prior to wound closure (squares). The cumulative distribution was analyzed separately for patients undergoing surgery for benign disease (closed symbols) and for patients who were reoperated for benign disease (open symbols). B, The stimulation threshold was determined before wound closure in patients undergoing surgery for malignant disease (▴, recurrent nerve = recurrent-mal.; ▵, vagus nerve = vagus-mal.) and reoperation for malignant disease (◆, recurrent nerve = recurrent-mal. reop.; ⋄, vagus nerve = vagus-mal. reop.) The control curves for the recurrent laryngeal (•, recurrent-benign) and the vagus nerve (□, vagus-benign) correspond to patients undergoing surgery for benign disease and are the same as those in A. To normalize for the difference in nerves at risk in each group, the data are expressed as percent of total; the distributions were fitted to the logistic equation N=100* [IP/(IP + I0.5P)], where N is the number of nerves in % of total, I and I0.5 are the individual threshold current and the median current in mA, respectively, and P is the slope factor.
Perioperative Management and Follow-up
The perioperative management was performed as described earlier14: in brief, the diagnosis was performed by standard procedures; vocal cord function was determined in all patients before surgery and on day 3 or 4 after surgery by laryngoscopy. Postoperative nerve injuries were followed up at regular intervals (on day 14, after 2–3, 6 and 9 months) by indirect laryngoscopy with a mirror and stroboscopy using a flexible endoscope (carried out by an ear-nose-throat specialist). In case of postoperative nerve injury, follow-up examinations were on day 14, after 2–3, 6 and 12 months.
Data Analysis
To account for the different types of operations (ie, unilateral and bilateral procedures), we present the data based on the number of nerves at risk. Cumulative distributions of stimulation thresholds were fitted to a 3-parameter logistic equation using a Marquart–Levenberg algorithm. Sensitivity, specificity, positive, and negative predictive values for individual patient groups were calculated from the appropriate 2 × 2 contingency tables.
RESULTS
Study Population
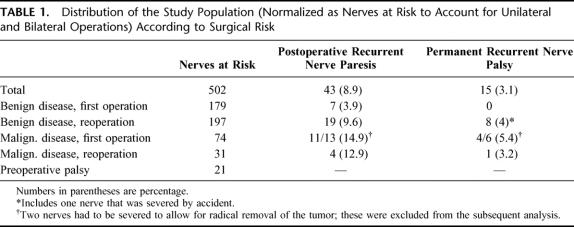
We have used a prospective study to address the usefulness of neuromonitoring in thyroid surgery. The details of the study protocol are summarized above (see Patients and Methods). Table 1 gives an overview of the study population; for the sake of comparison, nerves at risk that were investigated by neuromonitoring are listed rather than patients. Because of the different risks associated with surgery, we distinguish between benign and malignant disease as well as reoperation. It is evident that the first operation in benign disease carries a low risk (as reflected by the absence of permanent palsy). In contrast, the reoperation, as well as surgery in malignant disease, is known to carry a significant risk of permanent palsy, and this is also evident from the data in Table 1. Obviously, the number of patients (and nerves) that were reoperated in malignant disease was too low to draw any conclusions on the risk.
TABLE 1. Distribution of the Study Population (Normalized as Nerves at Risk to Account for Unilateral and Bilateral Operations) According to Surgical Risk
Responsiveness to Vagus and Recurrent Nerve Stimulation in Different Patient Groups
Neuromonitoring is being advocated for (1) the rapid and reliable identification of the recurrent laryngeal nerve and for (2) predicting the postoperative vocal cord mobility.1–12 The study protocol therefore called for identification of the recurrent nerve by using the electrode rather than by palpation and surgical dissection. This obviously required electrical stimulation of structures that looked like a nerve. It is worth noting that under these conditions a substantially higher current (Fig. 1, triangles) was required than for eliciting a contraction via the vagus nerve (Fig. 1, squares) or after surgical exposure of the nerve and hence removal of insulating tissue (Fig. 1, circles). It is also worth pointing out that the recurrent laryngeal nerve was successfully identified by this approach, ie, by moving the electrode through the retrothyroidal space, in 85% (and this values was set 100% in Fig. 1 to allow for comparisons). The cumulative distributions shown in Figure 1A also demonstrate that the vagus nerve was most sensitive to electrical stimulation (50% of the patients reacted at I50=0.159 ± 0.004 mA) whereas substantially higher currents were required to elicit a response via the recurrent nerve (I50=0.357 ± 0.008 mA). We are at loss to explain this unexpected finding. However, we stress that the I50 derived from the cumulative distribution (I50=0.159 ± 0.004) is in excellent agreement with that determined from stimulus-response curves generated by recording the electrical field response in individual patients (I50 = 0.17 ± 0.04; see 13). Furthermore Figure 1A also documents that similar cumulative distributions were reproducibly obtained in 2 different populations, ie, patients subjected to surgery for benign disease (closed symbols) and reoperated patients (open symbols) regardless of whether the sensitivity of the vagus nerve, of the dissected or of the unexposed recurrent nerve was investigated. This indicated that the method was reasonably reliable and that the sample size was large enough to obtain representative data.
This can also be observed in Figure 1B, where the response of the unexposed nerve has been omitted for the sake of clarity. The responsiveness of the vagus nerve in patients suffering from malignant disease (open triangles and diamonds) was comparable to that of the patients in benign disease. This was also true for the distributions of the responsiveness of the recurrent nerve (cf. patients with benign disease = closed circles vs. patients with malignant disease = closed triangles and reoperated patients with malignant disease = closed diamonds). There was only one outlier, which is the stimulation of the vagus nerve in reoperated patients with malignant disease (open diamonds in Fig. 1B), and we attribute this to either a recording error or to the small sample size. Based on these observations, we conclude that there is an individual variability in the responsiveness to electrical stimulation. The distribution of this variability is steeper than predicted from the Gaussian normal distribution; in other words, most patients respond over a narrow range of currents. Nevertheless, the variation in individual responsiveness must be taken into account to the interpretation because it can be readily detected by neuromonitoring.
Predicting Postoperative Outcome by Neuromonitoring
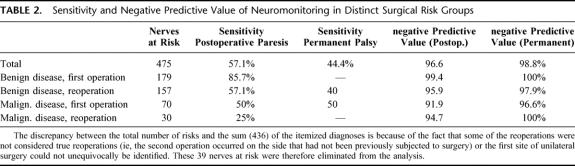
As already mentioned above, neuromonitoring may be of particular relevance if it allows to predict which patients are at a particular risk of developing postoperative and permanent palsy. In principle, it appears reasonable to assume that the intraoperative responsiveness to electrical stimulation is associated with normal postoperative vocal cord function and that the reverse is also true. The data in Table 2 suggest that, in general, neuromonitoring has a low sensitivity, ie, its ability is low to recognize an abnormal response that is associated with paresis. It is, in particular, low in those situations, where a sensitive detection is most needed, namely in complicated thyroid surgical procedures such as malignant disease and reoperations (Table 2, second and third column). In contrast, the sensitivity in surgery for benign disease is acceptable (but dispensable).
TABLE 2. Sensitivity and Negative Predictive Value of Neuromonitoring in Distinct Surgical Risk Groups
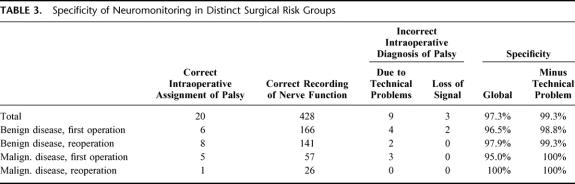
By comparison, the negative predictive values for both postoperative paresis and permanent palsy are excellent (Table 2); these indicate that the probability is high for correctly predicting an intact postoperative nerve function by neuromonitoring. However, this is somewhat misleading because, as noted in Table 1, the incidence of postoperative palsy is generally low. Accordingly, the specificity (which accounts for the false positives) is acceptable. As can be seen from Table 3 (second column from right), the patients were erroneously suspected to develop a postoperative palsy based on the neuromonitoring diagnosis only in 2.7% of all cases. In most of the cases, this was accounted for by intraoperative technical problems (Table 3, right hand column).
TABLE 3. Specificity of Neuromonitoring in Distinct Surgical Risk Groups
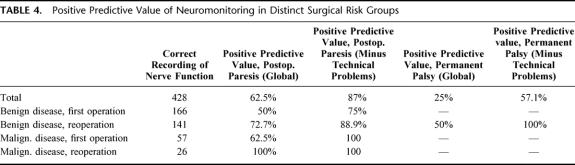
Hence, it is more important to examine the frequency of incorrect intraoperative diagnosis of palsy (listed in Table 3) and to derive the positive predictive values (Table 4) from a comparison of the correct and incorrect intraoperative diagnosis. Technical problems significantly affected the positive predictive values. These technical problems typically comprised the following situations: dislocation of the electrode in the vocal muscle; defects in the electrode and the cables; or an excess of neuromuscular blockage by relaxant (in particular when combined with halogenated ethers). In general, for postoperative paresis, the positive predictive value is acceptable (overall 87%), if the technical problems are accounted for (Table 4, third column). However, it is difficult to judge the positive predictive value for permanent palsy because the incidence of this complication was low.
TABLE 4. Positive Predictive Value of Neuromonitoring in Distinct Surgical Risk Groups
Threshold of Stimulation in Patients Who Developed Postoperative Palsy
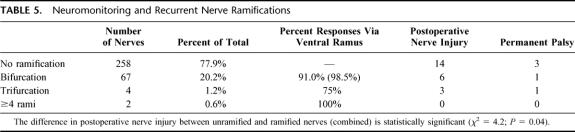
It is worth noting that the positive predictive values listed in Table 4 did in several cases not reach 100% even if the technical problems were accounted for. In other words, patients responded to electrical stimulation, although their vocal cord function was subsequently compromised. It was therefore of interest to evaluate the stimulation threshold in these patients because an impaired conduction may require higher currents for eliciting a response. However, the cumulative distribution plotted in Figure 2A does not suggest that there was a major shift in the responsiveness. The responsiveness of the vagus nerve was somewhat blunted (Fig. 2A symbols): the median current required for triggering the response in 50% of the patients (I50) via vagal stimulation was 0.226 ± 0.010 mA (versus I50=0.159 ± 0.004 mA in the control group). However, the curve that describes the threshold for recurrent nerve stimulation (Fig. 2A, symbols) was shifted to the left (0.231 ± 0.016 mA) when compared with the control curve (I50= 0.359 ± 0.008 mA in the control group). We do not understand the basis for these shifts; they may reflect subtle changes in excitability and/or conductance, but–from a practical perspective–the changes are too small to be useful for a clinical diagnosis or for deriving any prospectively useful information.
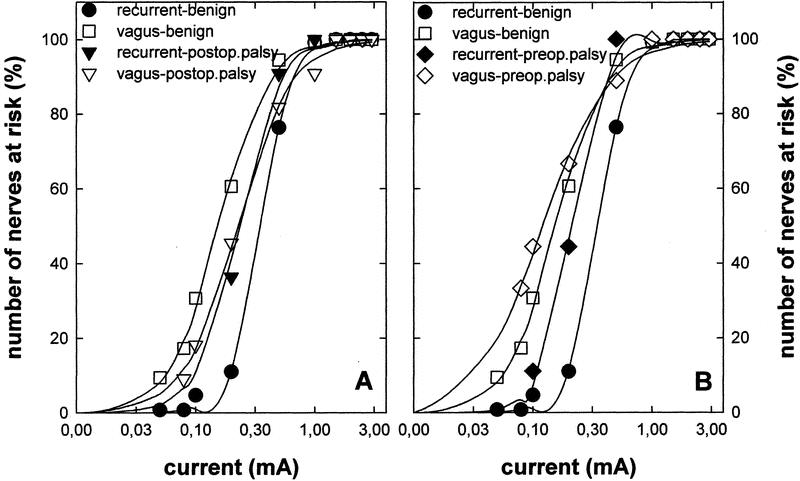
FIGURE 2. Cumulative distribution of stimulation thresholds in patients who developed postoperative palsy (A) and in patients who presented with preoperative palsy (B). The stimulation threshold was determined before wound closure as outlined in B. Shown are the cumulative distributions of those patients who developed postoperative palsy (▴ and ▵, recurrent and vagus nerve abbreviated as recurrent-postoperative. palsy and vagus-postoperative. palsy, respectively) and those patients who presented with preoperative palsy (◆ and ⋄, recurrent and vagus nerve abbreviated as recurrent-postoperative. palsy and vagus-postoperative. palsy, respectively) and in whom an electrical field response was nevertheless recorded. Control patients (same data as in A, •, recurrent nerve = recurrent-benign; □, vagus nerve = vagus-benign) are included for comparison.
Threshold of Stimulation in Patients Presenting With Preoperative Palsy
As can be seen from Table 1, there were 21 patients who presented with preoperative palsy. In 14 of these, intraoperative neuromonitoring demonstrated that an electrical field response could still be recorded via the electrode placed in the vocal muscle. We stress the fact that these nerves were meticulously dissected over their entire cervical course to detect any macroscopical damage. In all instances, the nerves were anatomically intact. Intuitively, we surmised that the threshold required for triggering a response was shifted to higher currents. However, the cumulative distribution curve did not reveal any major change in the I50 for vagal stimulation (Fig. 2B, open diamonds; I50= 0.121 ± 0.005 mA). Similarly, in these patients, the curve for stimulation via the recurrent nerve (Fig. 2B, closed diamond) was not shifted to the right but rather to the left (I50= 0.209 ± 0.007 mA) of the control curve. Again, we are not able to provide an explanation for this somewhat surprising observation.
Acoustic monitoring is ideal from a practical perspective. However, important electrophysiological information is lost, which may allow an understanding of the mechanistic basis for vocal cord palsy. We therefore also recorded the electrical field response after vagal and recurrent nerve stimulation in patients with preoperative palsy; original data obtained with a representative patient are shown in Figure 3. The field response generated by vagal stimulation (Fig. 3A) or by recurrent laryngeal nerve stimulation (Fig. 3C) did not provide any indication for a deviation from recordings made in patients with normal vocal cord function (Fig. 3D). As expected and as described elsewhere in detail,13 the amplitude declined after injection of the muscle relaxant atracurium (Fig. 3B). Nerve conduction velocity can be estimated by comparing the lag between stimulus (=0 time point in Fig. 3) and response, which is about 4 milliseconds after vagus nerve stimulation (Fig. 3A, B) and 2 milliseconds after recurrent nerve stimulation (Fig. 3C). The difference of 2 milliseconds is accounted for by the distance between the 2 stimulation points: the vagus nerve was stimulated at the level of the cricoid cartilage; for recurrent nerve stimulation, the electrode was placed about 3 cm before entry of the nerve into the larynx. Assuming that the nerve loop is about 15 cm long, we calculate a nerve conduction of 75 m/s. This is within the normal range of Aα-fibers and reasonably similar nerve conduction velocities were also estimated for individuals with normal pre- and postoperative vocal cord function (see eg, ∼81 m/s estimated from the recordings shown in Fig. 3D). Thus, the electrical field response did not provide any additional information and failed to resolve the enigma.
FIGURE 3. Electrical field responses in a patient presenting with preoperative vocal cord palsy (A–C) and a patient with normal pre- and postoperative vocal cord function (D). A and B, Original trace recorded after stimulation of the right vagus nerve with 0.5 mA (A) and 1 mA (B). The interval between the 2 recordings is about 30 minutes. Despite the higher current used in B, the amplitude of the electrical field response is lower than in A because the patient was administered a skeletal muscle relaxant in between (10 mg atracurium). C, Electrical field response to stimulation of the right recurrent laryngeal nerve. Note that the lag between stimulation (time point = 0 milliseconds) and electrical field response is shorter than in B (or A): after vagal stimulation, the delay is about 2 milliseconds longer, and this difference can be used to estimate the nerve conduction velocity (∼15 cm/2 milliseconds ≅ 75 m/s). D, Electrical field responses were elicited by stimulating the right laryngeal nerve of a patient with normal pre- and postoperative vocal cord function. Consecutive original traces are superimposed; the upper 3 traces are 3 consecutive recordings obtained by stimulation at a site above the crossing point of the inferior thyroid artery. The electrode was moved about 3 cm (ie, below the inferior thyroid artery) and 4 consecutive traces were recorded; the 2 vertical lines were drawn to illustrate the difference in time-to-peak of the electrical field responses. This difference (= 0.37 milliseconds) can be used to estimate the nerve conduction velocity (∼3 cm/0.37 milliseconds ≅ 81 m/s).
Responsiveness to Disconnection and Ligated Nerve
By contrast with these unexplained findings, we did observe the anticipated response in a case where the recurrent nerve had to be severed for the curative resection of a carcinoma. In this case, the recurrent nerve and the vagal nerve both triggered a muscle response at the threshold value of 0.1 mA; after the recurrent nerve had been seized with a forceps, the stimulation threshold in the vagal nerve increased to 0.5 mA. In contrast, the stimulation threshold in the distal portion of the recurrent nerve (ie, the part adjacent to the larynx) was not affected. Clamping and, obviously, resection abolished the response to vagal nerve stimulation. However, throughout these manipulations the threshold in the distal portion (and subsequently stump) of the recurrent nerve remained constant at 0.1 mA. In a second case a misplaced ligation was fortuitously discovered in a patient (who had a postoperative vocal cord dysfunction and) who had to undergo a reoperation, because the final histopathological diagnosis of cancer was delayed by 3 days. In this case, the distal portion of the recurrent nerve had a stimulation threshold of 0.5 mA whereas vagal nerve stimulation failed to elicit any response.
Ramification of the Recurrent Laryngeal Nerve
It is well known that there are anatomic variations of the recurrent nerve. Neuromonitoring has also been advocated because it may be useful in establishing the anatomic situation (eg, extralaryngeal divisions, nonrecurrent variants). We have tested if the selective stimulation of the individual nerves allows to identify extralaryngeal ramifications by prospectively investigating 331 nerves (summarized in Table 5). Neuromonitoring identified extralaryngeal ramifications in 1 of 5 patients; in most thereof, stimulation of the most ventral ramus triggered the muscular response. The distribution between the patients with normal and variant anatomy matches those found in large studies15 and in intraoperative recordings of vocal cord function by laryngoscopy.12 The surgical risk may increase with the number of ramifications. Because the number of ramification exceeding 2 was too low for a meaningful statistical analysis, we compared postoperative nerve injury in nerves without ramifications to the outcome in ramified nerves. There was a statistically significant increase in postoperative palsy, if the recurrent nerve was ramified (χ2= 4.2; P = 0.04).
TABLE 5. Neuromonitoring and Recurrent Nerve Ramifications
DISCUSSION
In the present study, we used a neuromonitoring approach that is reasonably pragmatic; ie, it can be conducted without major inconvenience to the patient, without interfering with the standard anesthetic procedure (ie, intubation and inhalational anesthetics), and which does not require specialized skills. Furthermore, the surgical approach is only minimally impeded by the electrical cables. There are 3 arguments in favor of neuromonitoring. First, neuromonitoring increases the ability of the surgeon to reliably identify the recurrent nerve, and this is presumably one of the reasons for its increasing popularity. The second reason is to verify the functional integrity of the recurrent laryngeal nerve prior to ending the surgical procedure. The loss of response may influence the decision-making process: assuming that neuromonitoring made reliable predictions about postoperative vocal cord function, the intraoperative loss on one side would, for instance, probably prompt most surgeons to abstain from resecting the second side in recurrent endemic goiter. Third, neuromonitoring may provide guidance for the surgeon in difficult situations; these include anatomic variants, reoperations and surgery for malignant disease.
Authors of a large multicenter study have argued that neuromonitoring reduces the incidence of postoperative paresis.11 However, it is worth noting that in surgery for benign disease, the rates of permanent palsy approaches nil if the recurrent nerve is correctly identified and exposed and these achievements have been made without neuromonitoring. In a large study involving more than 27,000 nerves at risk, we found that the single most important factor in defining outcome was the individual performance of the surgeon.14 Thus, we believe that it is difficult to disentangle these effects and to demonstrate the benefit of neuromonitoring by objective criteria in surgery for benign disease (which comprises most of operations). Finally, an experienced surgeon identifies the nerve with success rates that are comparable or superior to those achieved by simply pointing the electrode to candidate structures.13,14 Obviously, in the training of neophytes, the neuromonitoring device cannot substitute for assistance and supervision by an experienced colleague.
Intuitively, one would anticipate that a normal electrical field response recorded prior to wound closure predicts normal postoperative vocal cord mobility. This is indeed the case and, hence, specificities as high as ≥99% can be achieved (see Table 3). However, we find—surprisingly—that the presence of a response does not guarantee normal postoperative vocal cord function. Accordingly, the positive predictive value is rather disappointing (see Table 4) even if technical problems are being accounted for. Consistent with this inadequate positive predictive value, an electrophysiological response may still be triggered when the patients suffer from preexisting vocal cord palsy (due to a preceding operation or other reasons). Finally and most importantly, we do not find evidence for a threshold that allows the discrimination between lesioned nerves (which are hence prone to palsy) and nerves that support normal postoperative vocal cord mobility (see Fig. 2A). This is in marked contrast to the finding of Brennan et al,10 who proposed that nerves that required ≥0.5 mA as a threshold current were prone to develop postoperative palsy.
There are no systematic reports that investigated the response of the recurrent nerve in patients with documented preexisting vocal cord palsy. Hence, it is difficult to compare the observed frequencies with other studies. Nevertheless, it is quite remarkable that in two thirds (14 of 21) of the patients electrical stimulation of the recurrent (and the vagus) nerve still elicited a measurable depolarization of the vocalis muscle. It has to be stressed, however, that in all these cases the nerve was always anatomically intact. It is even more remarkable that, in these patients, the cumulative distribution curve was not shifted to the right (Fig. 2B). A rightward shift would be indicative of an increased average threshold and thus of an impaired nerve function. This was clearly not seen. Eisele16 also provided anecdotal evidence for a normal stimulation threshold (0.1 mA) in a patient with preoperative palsy (which did not recover during the next 3 years of follow-up). We also examined the electromyographic response in patients with preoperative palsy. It is evident that the electrical field response was not affected and that the estimated nerve conduction velocity was normal within the inherent limitations of a calculation that relies on the estimated length of the nerve loop.
The sensitivity and specificity of neuromonitoring has also been examined in other reports.17–20 To the best of our knowledge, an analysis has, however, not been done where patients were grouped according to surgical risk. We find that this analysis is useful because it highlights the limitations of neuromonitoring. Subgrouping identifies the patients who are at risk because of the low sensitivity; in reoperations the sensitivity drops to less than 60% even in benign disease. We stress that this cannot be accounted for by a difference in sample size; there were 157 nerves at risk in the reoperated group versus 179 in the benign disease/first operation group (see Table 2). The negative predictive value of 95.9 to 91.9% in the high-risk groups imparts a false sense of security. In fact, because permanent palsy occurs only with a low incidence (overall 3.1% and 0% in 1rst operations for benign disease, see Table 1), the high number of—correctly diagnosed—uneventful outcomes detracts from the real problem. In fact, a negative predictive value of 96.6% (Table 2) is a major problem in view of the 5.4% incidence of permanent palsy in patients with malignant disease (Table 1).
In our opinion, the main benefit of neuromonitoring is its ability to guide the surgeon in situations where the anatomic situation diverges from the normal situs. Thus, we find that extralaryngeal ramifications occur frequently enough to impose a significant risk. Our data predict that the inadvertent ligation of the most ventral branch will impair vocal cord function in most cases, for the ventral ramus controls, in most of the cases, the function of the vocalis muscle. Similarly, neuromonitoring is useful in confirming the presence of nonrecurrent nerves (which, however, is a rare incidence) and allows the confirmation of the anatomic assignment in reoperations and in surgery for malignant disease where anatomic structures tend to be dislocated. Thus, it can also be useful in primary surgery. In Austria (and other landlocked European countries with a high rate of endemic goiter), thyroid surgery also is performed in smaller departments where volume is modest and the surgical procedure is less a matter of routine. It is to be expected that the quality of surgery can be improved by neuromonitoring precisely in this situation. Taken together, our observations show that intraoperative neuromonitoring of vocalis muscle function is useful but that its importance must not be overstated. It is specifically dangerous when it creates a false sense of security: recurrent nerves that respond to electrical stimulation do not guarantee a normal postoperative vocal cord function. Similarly, neuromonitoring cannot be used as a training device to replace guidance by experienced colleagues.
Footnotes
Reprints: M. Freissmuth, Institute of Pharmacology, University of Vienna, Währinger Strasse 13a, A-1090 Vienna, Austria. E-mail: michael.freissmuth@univie.ac.at.
REFERENCES
- 1.Faaborg-Andersen. Electromyographic investigation of intrinsic laryngeal muscles in humans. Acta Physiol Scand. 1957;41(Suppl):140. [Google Scholar]
- 2.Peytz F, Rasmussen H, Buchthal F. Conduction time and velocity in human recurrent laryngeal nerve. Dan Med Bull. 1965;12:125. [PubMed] [Google Scholar]
- 3.Flisberg K, Lindholm T. Electrical stimulation of the human recurrent laryngeal nerve during thyroid operation. Acta Otolaryngol (Suppl). 1969;263:63–67. [DOI] [PubMed] [Google Scholar]
- 4.Shedd DP, Burget GC. Identification of the recurrent laryngeal nerve. Arch Surg. 1966;92:861–864. [DOI] [PubMed] [Google Scholar]
- 5.Depisch D. Intraoperative mobility recording of the vocal cord after electrostimulation of the recurrent nerves. Acta Chir Austriaca. 1975;(Suppl 14):1–14.
- 6.Tschopp K, Probst R. New Aspects in surgery of the thyroid gland with intraoperative monitoring of the recurrent laryngeal nerve. Laryngo Rhino Otol. 1994;73:568–572. [DOI] [PubMed] [Google Scholar]
- 7.Djohan RS, Rodriguez HE, Connolly MM, et al. Intraoperative monitoring of recurrent laryngeal nerve function. Am Surg. 2000;66:595–597. [PubMed] [Google Scholar]
- 8.Echeverri A, Flexon PB. Electrophysiologic nerve stimulation for identifying the recurrent laryngeal nerve in thyroid surgery: review of 70 consecutive thyroid surgeries. Am Surg. 1998;64:328–333. [PubMed] [Google Scholar]
- 9.Lamadé W, Meyding-Lamade U, Buchhold C, et al. First continuous nerve monitoring in thyroid gland surgery. Chirurg. 2000;71:551–557. [DOI] [PubMed] [Google Scholar]
- 10.Brennan J, Moore EJ, Shuler KJ. Prospective analysis of the efficacy of continuous intraoperative nerve monitoring during thyroidectomy, parathyroidectomy, and parotidectomy. Otolaryngol Head Neck Surg. 2001;124:537–543. [DOI] [PubMed] [Google Scholar]
- 11.Thomusch O, Sekulla C, Walls G, et al. Intraoperative neuromonitoring of surgery for benign goiter. Am J Surg. 2002;183:673–678. [DOI] [PubMed] [Google Scholar]
- 12.Eltzschig HK, Posner M, Moore FD Jr. The use of readily available equipment in a simple method for intraoperative monitoring of recurrent laryngeal nerve function during thyroid surgery: initial experience with more than 300 cases. Arch Surg. 2002;137:452–456. [DOI] [PubMed] [Google Scholar]
- 13.Hermann M, Freissmuth M. Neuromonitoring of the recurrent nerve: validation and merits. Eur Surg. 2003;35:228–235. [Google Scholar]
- 14.Hermann M, Alk G, Roka R, et al. Laryngeal recurrent nerve injury in surgery for benign thyroid diseases: effect of nerve dissection and impact of individual surgeon in more than 27,000 nerves at risk. Ann Surg. 2002;235:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz A, Nemiroff P. Anastomoses and bifurcations of the recurrent laryngeal nerve: report of 1177 nerves visualized. Am Surg. 1993;59:188–191. [PubMed] [Google Scholar]
- 16.Eisele DW. Intraoperative electrophysiologic monitoring of the recurrent laryngeal nerve. Laryngoscope. 1996;106:443–449. [DOI] [PubMed] [Google Scholar]
- 17.Hemmerling TM, Schmidt J, Bosert C, et al. Intraoperative monitoring of the recurrent laryngeal nerve in 151 consecutive patients undergoing thyroid surgery. Anesth Analg. 2001;93:396–399. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich T, Staemmler A, Hansch U, et al. Intraoperative electrophysiological monitoring of the recurrent laryngeal nerve in thyroid gland surgery—a prospective study. Zentralbl Chir. 2002;127:414–420. [DOI] [PubMed] [Google Scholar]
- 19.Hamelmann WH, Meyer T, Timm S, et al. A critical estimation of intraoperative neuromonitoring (IONM) in thyroid surgery. Zentralbl Chir. 2002;127:409–413. [DOI] [PubMed] [Google Scholar]
- 20.Timmermann W, Dralle H, Hamelmann W, et al. Does intraoperative nerve monitoring reduce the rate of recurrent nerve palsies during thyroid surgery? Zentralbl Chir. 2002;127:395–399. [DOI] [PubMed] [Google Scholar]