Abstract
Chondromodulin I (chm-I), a type II transmembrane protein, is highly expressed in the avascular zones of cartilage but is downregulated in the hypertrophic region, which is invaded by blood vessels during enchondral ossification. In vitro and in vivo assays with the purified protein have shown chondrocyte-modulating and angiogenesis-inhibiting functions. To investigate chm-I function in vivo, we generated transgenic mice lacking chm-I mRNA and protein. Null mice are viable and fertile and show no morphological changes. No abnormalities in vascular invasion and cartilage development were detectable. No evidence was found for a compensating function of tendin, a recently published homologue highly expressed in tendons and also, at low levels, in cartilage. Furthermore, no differences in the expression of other angiogenic or antiangiogenic factors such as transforming growth factor β1 (TGF-β1), TGF-β2, TGF-β3, fibroblast growth factor 2, and vascular endothelial growth factor were found. The surprising lack of phenotype in the chm-I-deficient mice suggests either a different function for chm-I in vivo than has been proposed or compensatory changes in uninvestigated angiogenic or angiogenesis-inhibiting factors. Further analysis using double-knockout technology will be necessary to analyze the function of chm-I in the complex process of enchondral ossification.
Angiogenesis is a complex, highly regulated physiological process. A coordinated sequence of endothelial cell division and selective degradation of vascular basement membranes and the surrounding extracellular matrix with migration of endothelial cells results in new capillary growth from existing vessels. The initiation of angiogenesis is associated with the expression of a number of angiogenic growth factors of which vascular endothelial growth factor (VEGF) and basic fibroblast growth factor 2 (FGF-2) are the most potent. However, to regulate and balance angiogenesis, which in adults is mainly restricted to tissue repair, angiogenesis inhibitors are important and are indispensable for the maintenance of vessel-free tissues such as cartilage (14).
Chondromodulin I (chm-I), a type II transmembrane glycoprotein of 335 amino acids (17, 24), is mainly found in cartilage and is expressed by resting, proliferating, and early hypertrophic chondrocytes (16). After translation, the chm-I precursor is cleaved by furin proteases at the RERR-ELVR site. The membrane-bound precursor has only a short half-life, while the cleaved, mature chm-I is abundant in the extracellular matrix. In addition, an extracellular cleavage event releases a shorter 9-kDa form of chm-I (4, 24).
Direct involvement of chm-I in angiogenesis was shown in vitro when recombinant mature chm-I inhibited proliferation and tube morphogenesis of endothelial cells in cell culture experiments (18). Injection of mature chm-I into growing mouse osteoblastomas inhibited angiogenesis and resulted in profound inhibition of tumor growth in vivo (15). A second effect of chm-I is its modulating effect on chondrocytes. chm-I increases proliferation and differentiation of chondrocytes in vitro (17). Its expression pattern and the angiogenesis-inhibiting function shown in vitro suggested an important function for chm-I in skeletal development.
Enchondral ossification takes place in the growth plates at the distal ends of the long bones between the later epiphysis and diaphysis. Resting chondrocytes start to proliferate, differentiate into hypertrophic chondrocytes, and finally undergo apoptosis. chm-I is highly expressed in the avascular cartilage regions of the growth plate. Its expression is abolished in the area of late hypertrophic chondrocytes, where blood vessel invasion takes place (30). The cartilage matrix is here degraded and replaced with the typical trabecular bone matrix produced by osteoblasts. Blood vessels provide a conduit for the recruitment of cells involved in cartilage resorption and bone deposition (14). Based on the in vitro data, it was suggested that chm-I was involved in the regulation of chondrocyte proliferation and the regulation of vessel invasion into the hypertrophic zone of the growth plate. Expression of chm-I is downregulated in rescued cbfa-1 knockout mice, in contrast to that of VEGF, which is upregulated in the same mice, suggesting that chm-1 and VEGF have complementary roles (34).
Besides being detected in cartilage, chm-I has been detected by Northern hybridization in the eye and the thymus (7). In situ hybridization and reverse transcription-PCR (RT-PCR) analysis revealed chm-I expression in the retina and the ciliary body (12). Immunostaining gave evidence for chm-I in the aqueous humor and the vitreous body but not in Bruch's membrane, which serves as a barrier against vessel invasion from the sclera into the retina (12). Therefore, chm-I has also been implicated in the regulation of retinal vascularization during development and the maintenance of an intact retina and vitreous body.
To investigate chm-I function in vivo, we generated a chm-I knockout mouse strain. Here, we demonstrate that chm-I-null mice are viable and fertile and do not exhibit any abnormalities during enchondral ossification and eye development. A compensatory upregulation of tendin, a close homologue, or other factors involved in angiogenesis was not found.
MATERIALS AND METHODS
Generation of chm-1-deficient mice.
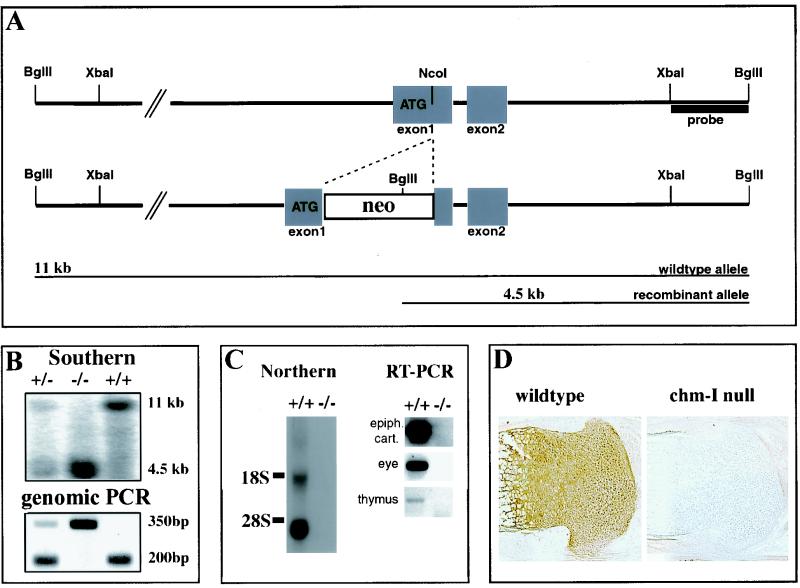
The complete open reading frame of chm-I was amplified by RT-PCR, sequenced, and then used for the hybridization of 129/Sv mouse P1-derived artificial chromosome (PAC) library RPCI21 (25). Five of the positive PACs were ordered from the Human Genome Mapping Project resource center, Cambridge, United Kingdom, and investigated for the presence of exon 1. PAC 354N10 was then used for the construction of the targeting vector (Fig. 1). A 13-kb XbaI fragment and a 3.8-kb HindIII fragment were subcloned into the pBS/KS vector, and the region around exon 1 was sequenced. A cassette with an internal ribosomal entry site (IRES), a lacZ reporter gene with a nuclear localization signal, and a floxed neomycin cassette were introduced into the NcoI site of exon 1.
FIG. 1.
Targeted disruption of mouse chm-I. (A) Structure of wild-type allele, targeting construct, and recombinant locus. Boxes, exons. The expected fragment sizes after BglII digestion are 11 kb for the wild-type allele and 4.5 kb for the recombinant allele. (B) (Top) Southern blot analysis of mouse tail DNA isolated from the progeny of a mating between heterozygous parents. DNAs were digested with BglII and hybridized with the probe indicated in panel A. (Bottom) PCR genotyping with primers for wild-type and recombinant alleles. +/+, wild-type mouse; +/−, heterozygous mouse; −/−, homozygous mutant mouse. (C) (Left) Northern blot analysis of total RNA from limb cartilage derived from newborn wild-type and homozygous mutant mice. The filter was hybridized with a cDNA probe specific for chm-I. No RNA is detectable in the mutant mice. (Right) RT-PCR with chm-I-specific primers, cartilage, thymuses, and whole eyes of homozygous mutant and homozygous wild-type mice gives no evidence for chm-I mRNA in the chm-I-deficient mouse. (D) Immunohistochemistry of newborn tibial growth plate with a chm-I-specific antibody confirms complete deletion of the chm-I protein in the mutant mouse.
The targeting construct was electroporated into passage 11 R1 embryonic stem (ES) cells and selected with G-418 at a concentration of 500 μg/ml (ES medium) (11). To check for homologous recombination, Southern blots from BglII-digested ES cell clones were hybridized with a 1.6-kb XbaI-BglII fragment and homologous recombined clones were identified by the detection of a band of 4.5 kb in addition to the 12-kb wild-type band. Targeted ES cells were injected into blastocysts to generate chimeras, which were subsequently mated with C57BL/6 females. Genotyping of heterozygous backcrosses was performed by PCR using a forward primer in the 5′ untranslated region (ATTCGTTGTAGGGACAGAGG) and allele-specific primers in intron 1 (TGGACCTGGCACCCACAGG) for the wild-type allele and in the internal ribosome entry site of the lacZ gene (AACTCACAACGTGGCACTGG) for the recombinant allele.
Skeletal analysis.
Skeletons of 14.5- and 15.5-day-old embryos and newborn mice were prepared and stained with alcian blue and alizarin red as described previously (1). For X-ray analysis, 6-month-old chm-I-null and control mice were anesthetized with Avertin and X-ray images were taken with a Siemens Polymat 70 at 48 kV and 0.2 mA.
Histology, immunohistochemistry, and electron microscopy.
For histological analysis, limbs and trunks dissected at various embryonic and adult stages were fixed overnight in fresh 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS; pH 7.2) or in 95% ethanol-5% glacial acetic acid. Samples from mice analyzed after birth were decalcified in 10% EDTA-PBS for 3 days. After paraffin embedding, sections were cut 5 to 6 μm thick and stained with hematoxylin-eosin (HE) or safranin O-van Kossa (3).
Immunostaining was performed by the avidin-biotin complex procedure using a commercial available kit (Vectastain). Primary antibodies against matrilin 1, matrilin 3, collagens II, IX, X, and XI, aggrecan, perlecan, endomucin, and chm-I have been previously described (2, 4, 6, 22). Electron-microscopic analysis of newborn cartilage tissue was performed as previously described (1).
RNA isolation, Northern blot analysis, and RT-PCR.
Chondrocyte RNA was isolated from newborn mouse rib and limb cartilage with the Qiagen RNeasy kit. For Northern analysis, 20 μg of total RNA was size fractionated on a 0.8% agarose-2.2 M formaldehyde gel, transferred to a Hybond N+ membrane (Amersham), and hybridized with 32P-labeled cDNA probes specific for mouse chm-I (nucleotides 529 to 1044). Two micrograms of total RNA isolated from retina, eye, thymus, and epiphyseal and rib cartilage were transcribed into cDNA (Superscript II; Amersham). Semiquantative RT-PCR was performed with primers for the PTHrPR gene (PTHrPR-F, AAACAGTCATGTGACTGGGC; PTHrPR-R, TTGGACAGTGCTGAGTCCC), transforming growth factor β1 (TGF-β1) gene (TGFβ1-F, TACTATGCTAAAGAGGTCACCC; TGFβ1-R, TCCTTGGTTCAGCCACTGCC), TGF-β2 gene (TGFβ2-F, TTTGCAGGTATTGATGGCACC; TGFβ2-R, ATCCATTTCCATCCAAGATCCC), TGF-β3 gene (TGFβ3-F, AACTAGCTATCTCAGGTCCC; TGFβ3-R, ACTTCAGTCTGTGCATCTGG), FGF-1 gene (FGF1-F, TATCACGTCACTGTGTGCTTGG; FGF1-R, TTCATTTGAACAGCATTCTCTGG), FGF-2 gene (FGF2-F, TTCCTGCGCATCCATCCCG; FGF2-R, AGTGCCACATACCAACTGGAG), and VEGF gene (VEGF-F, ACTGTGAGCCTTGTTCAGAGC; VEGF-R, TAAGGACTGTTCTGTCAACGG).
In situ hybridization.
Digoxigenin (DIG)-UTP (Boehringer Mannheim)-labeled sense and antisense riboprobes were generated from plasmids containing rat PTHrPR, mouse Indian hedgehog Ihh, and mouse Col2a1 and ColX cDNA probes. Limbs from newborn and embryonic day 15 (E15) mouse embryos were fixed overnight in 4% PFA in Tris-buffered saline solution (TBS; 50 mM Tris, 150 mM NaCl), (pH 9.5), subsequently dehydrated, and embedded in paraffin. Five- to 6-μm-thick sections were dewaxed and rehydrated. After three rinses with TBS (pH 7.4), the sections were refixed with 4% PFA in TBS (pH 9.5) for 20 min at room temperature. The sections were then rinsed three times with TBS and digested for 20 min at 37°C with 10 μg of proteinase K/ml in 0.1 M Tris-150 mM NaCl (pH 8.5). After three rinses with TBS, the sections were dehydrated by ascending ethanol washes. Air-dried sections were finally covered with 50 μl of hybridization solution (5 μg of RNA probe/ml) and sealed with coverslips. After a 1-min heat treatment at 95°C, sections were hybridized overnight in a humidified chamber at 50°C. The next day, coverslips were removed in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and the slides were subsequently washed three times for 20 min each at 55°C in 50% formamide-1× SSC and two times for 15 min at room temperature in 1× SSC. For the detection of DIG-labeled transcripts, the sections were incubated for 1 h with an alkaline phosphatase-coupled DIG antibody (dilution, 1:500; La Roche) in 10% sheep serum in TBS. After three TBS rinses and an equilibration for 5 min, sections were developed according to the manufacturer's recommendation for 20 min to 2 h and subsequently mounted.
Biochemical analysis of cartilage.
Cartilage samples were dissected from limbs or ribs of wild-type and homozygous mutant newborn mice and extracted with radioimmunoprecipitation buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100) for 2 h at 4°C. Unextracted material was removed by centrifugation at 14,000 × g for 10 min at 4°C. Proteins were separated on sodium dodecyl sulfate polyacrylamide gels (15%) and blotted onto polyvinylidene difluoride membranes (Amersham). The blots were subsequently hybridized with polyclonal antibodies specific for VEGF, FGF-2, transforming growth factor β2 (TGF-β2), and TGF-β3 (Santa Cruz Biotechnology). Bound antibodies were hybridized to horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Sigma) and detected by using the ECL (enhanced chemiluminescence) kit (Amersham).
Analysis of eye vascularization.
Whole eyes from 3-, 7-, and 14-day-old chm-I-deficient and wild-type mice were fixed overnight in 4% PFA in PBS (pH 7.4) and embedded in paraffin. HE staining of consecutive 4-μm-thick sections was analyzed by light microscopy. For retinal whole-mount immunostaining, dissected retinas were fixed in 4% PFA in PBS (pH 7.4) for 12 h, washed in PBS, and treated for 1 h at room temperature in 100% methanol. After washes in PBS, the retinas were blocked for 1 h in PBS-10% fetal calf serum (FCS) and subsequently incubated with the anti-rat PECAM antibody in PBS-10% FCS for 12 h (dilution, 1:100). After frequent washes over 6 h with PBS, the tissue was incubated with a CY3-coupled secondary antibody in PBS-1% FCS for 1 h (dilution, 1:100). After frequent washes for 6 h, the retinas were incised at the edges and mounted for immunofluorescence microscopy. The retinas were analyzed by comparing the diameters and densities of the retinal vessel network.
Analysis of thymus development.
Thymuses from E16, newborn, and 2-week-old mice were dissected and filtered through a cell strainer. Single-cell suspensions were first incubated with fluorescein isothiocyanate-conjugated anti-CD4 (Gk1.5) and phycoerythrin-conjugated anti-CD8 (53-6.7; PharMingen) monoclonal antibodies. Dead cells were excluded from analysis on a FACScalibur (Becton Dickinson) by propidium iodide (1 μg/ml; Sigma) counterstaining.
RESULTS
Generation of chm-I-null mice.
chm-I was inactivated by the insertion of an internal ribosomal entry site-LacZ-neomycin cassette into exon 1 (Fig. 1A). Out of 120 ES cell clones surviving G-418 selection, 18 homologous recombinant clones were identified by Southern blot analysis of BglII-digested genomic DNA (data not shown). Chimeric males were generated and subsequently crossed with C57/B6 females to produce heterozygous offspring. Southern and/or PCR analysis of 219 offspring revealed a Mendelian ratio of genotypes (Fig. 1B). Northern blot analysis of total RNA derived from limb cartilage was performed and revealed a complete lack of chm-I mRNA. RT-PCR experiments on transcribed cDNAs confirmed the complete ablation of chm-I mRNA in the eye and the thymus (Fig. 1C). No chm-I protein was detected in cartilage by immunohistochemistry (Fig. 1D).
Analysis of skeletal development.
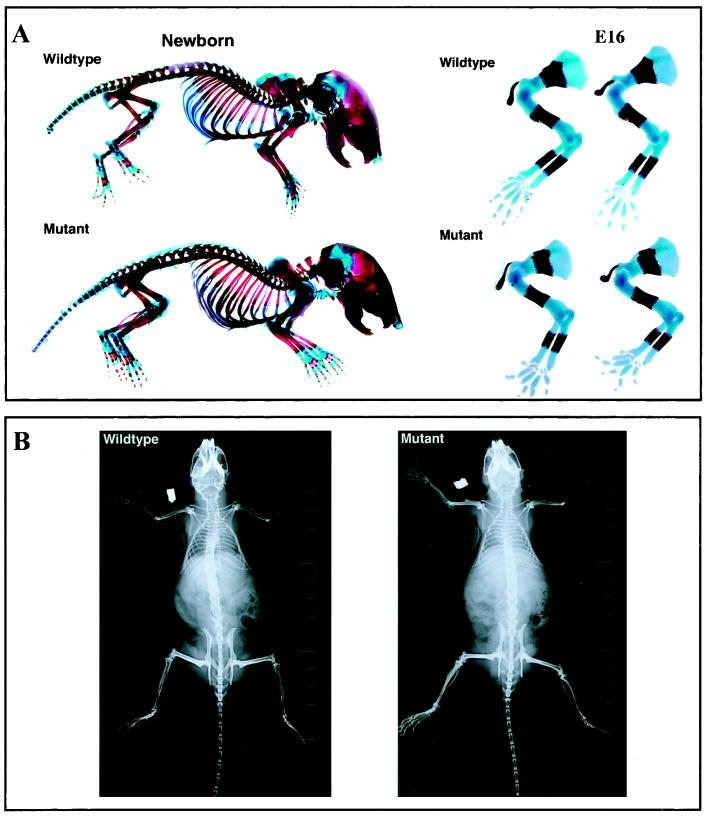
chm-I-deficient mice showed no obvious abnormalities, were fertile, and had a normal life span. Whole-skeleton staining of E14, E16, and newborn mice (Fig. 2A and data not shown) gave no evidence for size differences or malformations. In particular, no differences in the time point or extent of mineralization of the shafts of long bones were observed. X-ray analysis of 6-month-old mice showed no skeletal abnormalities and normal bone density (Fig. 2B). To investigate the involvement of chm-I in enchondral ossification, we performed histological analysis of the long bones of E14, E14.5, E15, E16, and newborn mice. In addition, the long bones of 3-day-old, 1-week-old, and 6-month-old mice were investigated. Vascular invasion into the cartilage in the growth plate was analyzed by staining with endomucin as a marker for endothelial cells (6). No differences in vascular invasion were observed in E15 mouse embryos. Likewise, no differences in chondrocyte differentiation were distinguishable by collagen II and collagen X immunostaining (Fig. 3A). These results were confirmed by careful examination of tibial, femoral, and humoral sections of E14 to E16 mice (data not shown).
FIG. 2.
Analysis of the skeleton in wild-type and chm-I-deficient mice. (A) No gross abnormalities are detectable in alcian blue and alizarin red skeletal staining of newborn mice. The relation of the bony shafts of E16 forelimbs to the complete bone length is unchanged in chm-I mutant mice. (B) X-ray examination of 6-month-old mutant and wild-type mice shows no differences in bone morphology and bone density.
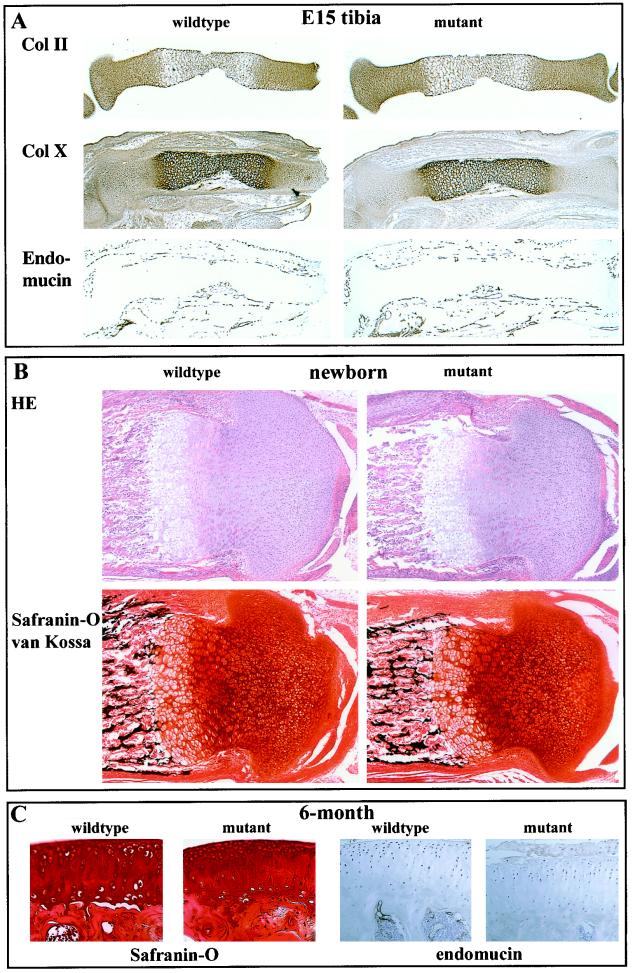
FIG. 3.
Immunostaining of E15 and newborn cartilage. (A) Consecutive sections of tibias from wild-type and chm-I-deficient E15 littermates were stained with specific antibodies against type II and type X collagens and endomucin. Collagen distributions and levels of vascular invasion were comparable. (B) HE and safranin O-van Kossa staining of the tibias from newborn littermates of heterozygous crossings show no obvious morphological differences or altered proteoglycan content. (C) Consecutive sections of the tibias from wild-type and chm-I-deficient 6-month-old littermates were stained with safranin O. The articular cartilage and the articular surface are normal and give no indications for degeneration.
At the newborn stage, HE staining showed normal growth plate morphology in chm-I-deficient mice. Results of safranin O-van Kossa staining of the cartilage matrix for proteoglycans and minerals, respectively, for wild-type and chm-I-deficient mice were indistinguishable (Fig. 3B). The distributions of collagen types II, IX, X, and XI were identical based on staining, and no differences for a selection of known extracellular matrix molecules including matrilin 1 and 3, perlecan, aggrecan, fibromodulin, and collagen I staining were detectable (data not shown). Although we did not detect any changes at the newborn stage and although chm-I expression decreases in adult mice, we further analyzed the chm-I mutant for changes in the adult stage (6-month-old mice). Based on HE sections and immunostaining with matrilin 3, collagen II, and endomucin and staining with safranin O (Fig. 3C and data not shown), the morphology of the long bones and the remnants of the growth plate were normal. The articular cartilage was normally developed, and no degenerative changes were visible at the articular surface at all stages analyzed.
In contrast to the in vitro experiments, which described a strong angiogenesis-inhibiting function of chm-I, we were unable to find any indication of increased angiogenesis in chm-I-deficient mice. Time point, progression, and extent of vascular invasion during bone development remained normal after deletion of the chm-I gene.
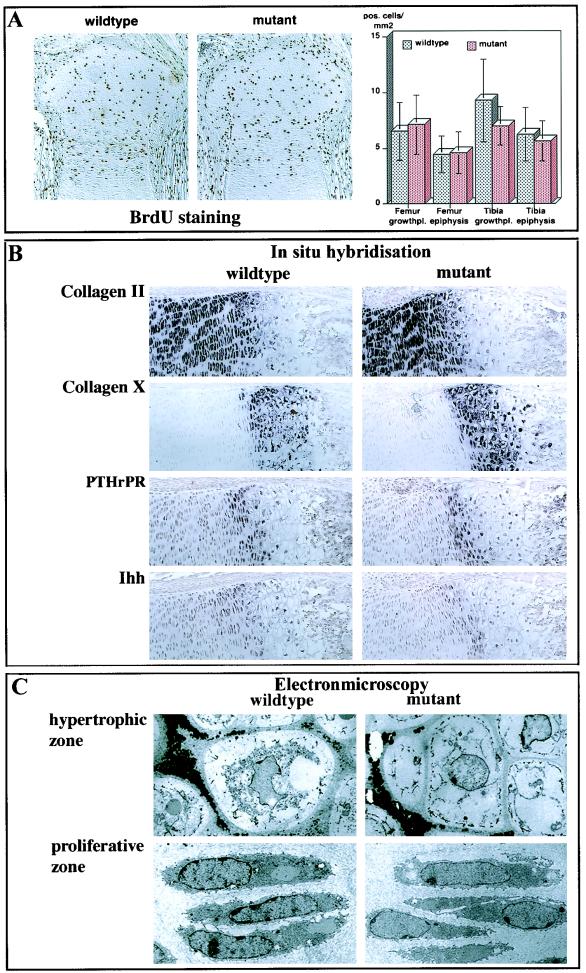
In vitro experiments also proposed a function for chm-I in the proliferation and differentiation of chondrocytes during enchondral ossification. Therefore, we investigated proliferation and differentiation in chm-I-deficient mice. However, no changes in the proliferation measured by bromodeoxyuridine (BrdU) incorporation assays (Fig. 4A) and KI-67 immunostaining could be detected (data not shown). In situ hybridization with probes for Col2a1, ColX, PTHrPR, and Ihh as markers for chondrocyte differentiation revealed no changes in the distribution or amount of mRNA transcripts in newborn growth plates (Fig. 4B). Ultrastructural analysis of tibial newborn growth plates of chm-I-null mice showed normal chondrocyte morphology and the presence of a normal collagen fibrillar network in the extracellular matrix (Fig. 4C).
FIG. 4.
Analysis of chondrocyte proliferation and differentiation. (A) Consecutive sections of newborn knee joints were stained with BrdU (left). BrdU-positive cells in the femoral and tibial growth plates and epiphysis were counted. No significant differences in the number of positive cells per square millimeter section could be observed (right). (B) In situ hybridization with probes specific for Col2a1, Col X, PTHrPR, and Ihh probes showed similar distributions of these markers in the proliferative, early hypertrophic, and hypertrophic zones in wild-type and mutant newborn mouse tibial sections. (C) Electron microscopy revealed no changes in cell morphology or extracellular matrix in the hypertrophic or proliferative zone.
Analysis of eye and thymus development.
We also investigated whether the elimination of the chm-I gene leads to changes in eye or thymus development and morphology. We analyzed the retinal vascularization of newborn and 3-, 7-, and 14-day-old chm-I-deficient mice by HE staining of sections through whole-eye and whole-mount immunostaining with PECAM. The eyes appeared normal, corneas and lenses were without any abnormalities, and no pathological vascularization was observed (data not shown).
To investigate the influence of chm-I on T-lymphocyte development, we performed fluorescence-activated cell sorter analysis investigating the expression of CD4 and CD8 on T cells isolated from E16, newborn, and 2-week-old mice. No differences in the proportions of CD4− to CD8−, CD4+ to CD8−, CD4− to CD8+, and CD4+ to CD8+ cells in all these stages were observed. By histological methods no differences in the cortex/matrix proportion or vessel density in the thymus could be observed (data not shown).
Analysis of factors involved in angiogenesis.
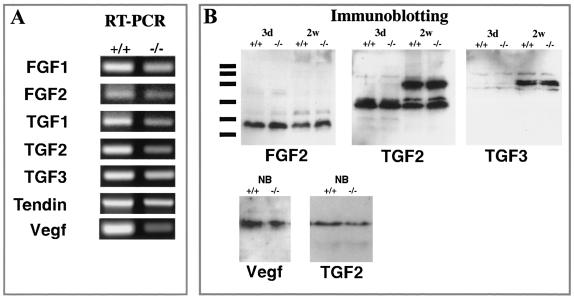
To find an explanation for the unexpected lack of phenotype in the chm-I-deficient mice, we investigated the expression levels of other factors that might compensate for the missing chm-I protein. We performed RT-PCR experiments on RNA isolated from mutant and control mice (Fig. 5A). Although tendin is a possible candidate that may compensate for the loss of chm-I due to its high degree of homology, it was not upregulated based on RT-PCR experiments. No changes could be seen for genes for FGF-1 and -2, VEGF, and TGF-β1 to -β3, factors known to be involved in chondrocyte differentiation or the regulation of angiogenesis in cartilage. To confirm these results on the protein level, we analyzed total-protein extracts from isolated limb epiphyseal cartilage and rib cartilage tissue from mutant and control mice at the newborn stage. Immunoblotting was performed for FGF2, VEGF, TGF-β2, and TGF-β3 after nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. No significant differences were observed (Fig. 5B).
FIG. 5.
Analysis of epiphyseal cartilage by RT-PCR and immunoblotting. (A) RT-PCR experiments gave no evidence for changed expression of genes for FGF-1, FGF-2, TGF-β1, TGF-β2, TGF-β3, tendin, or VEGF. (B) Immunoblotting for FGF-2, TGF-β2, TGF-β3, and VEGF with total protein extracts from cartilage also gave no evidence for a changed expression of these growth factors.
DISCUSSION
During endochondral bone formation, chondrocytes in the avascular cartilage differentiate and form hypertrophic cartilage, which undergoes erosion and vascularization followed by bone deposition. Whereas chondrocytes of the avascular cartilage produce inhibitors of angiogenesis, hypertrophic chondrocytes produce angiogenic stimulators that initiate vascularization. The expression pattern of chm-I during development and in vitro and in vivo experiments with mature chm-I suggested an important role for chm-I in the proliferation and differentiation of chondrocytes, as well as in the vascular invasion of growth plates (15, 16, 18). Recent studies of chm-I expression in rats provided further evidence for a regulatory role of chm-I during vasculogenesis in the retina and the vitreous body (12). To test chm-I function in vivo, we generated chm-I-deficient mice.
Mice lacking chm-1 expression are viable and fertile and do not show any signs of abnormal cartilage development or enchondral bone formation in vivo. Neither the onset of primary ossification nor vascular invasion nor proliferation of chondrocytes is altered in the mutant mice. Collagen type II and X deposition and the expression of PTHrPR and Ihh are undisturbed, suggesting that the lack of chm-I does not disturb cartilage maturation and terminal differentiation. In addition to normal development and vascularization of cartilage tissue, development of both eye and thymus is unaffected by the loss of chm-I.
The lack of phenotype and the undisturbed vasculogenesis are unexpected and might be explained by differences between the in vivo and the in vitro situation or by the action of factors compensating for the loss of chm-I in vivo but not in vitro.
It has been shown that a finely tuned balance between chondrocyte-derived signals that repress cartilage maturation and endothelial signals that promote late differentiation of chondrocytes is essential for normal endochondral ossification (5). This cross talk does not exist when isolated endothelial cells or chondrocytes, respectively, are treated with chm-1. Therefore, chm-1 may exert effects under such in vitro conditions that are counteracted in vivo.
In addition it is conceivable that the amount and form of the chm-I protein used in vivo or in vitro might provoke different cellular responses. The mature chm-I, as used in the in vitro experiments, might not undergo the physiological proteolytic cleavage that occurs in vivo and thus might show different functions in the endothelial cell culture system (4, 16, 17). Furthermore, the antiangiogenesis function of chm-I has also been observed in vivo when the growth of transplanted chondrosarcomas and adenocarcinomas was inhibited after local administration of recombinant mature chm-I (15). The locally applied amount of chm-I (0.1 μg/μl) presumably exceeds physiological levels and is not comparable to chm-I levels during development. It could therefore be possible that the in vitro observations are pharmacologically relevant but have no physiological importance.
It is also possible that the consequences of the chm-I loss are compensated for by close homologues, which have redundant chm-I function in cartilage development. The recently identified protein tendin is a close homologue of chm-I. It is strongly expressed in tendons, but low levels are found in a broad range of tissues including eye, thymus, and cartilage (7, 31, 36). Due to its homology and the overlapping expression pattern, tendin is a possible candidate for redundant and/or compensatory effects in the chm-I-deficient mouse. Tendin and chm-I, however, diverge in the amino acid sequence at the chm-I furin protease recognition site RERR as well as in the second cleavage site of chm-I (4). A different cleavage of both proteins may give rise to subtle but important differences in their localizations within the extracellular matrix. In addition, we observed no upregulation of tendin in the chm-I-deficient mouse using RT-PCR experiments. Redundancy between tendin and chm-I or compensation by tendin therefore seems to be unlikely. We will, however, further address this question by generating tendin and chm-I double-knockout mice. Using careful sequence analysis of available mouse and human databases, we found no indications of further homologues of chm-I.
Finally, the loss of a potent angiogenesis inhibitor and chondrocyte growth factor in the network of growth plate-regulating proteins might be compensated for by down- or upregulation of other factors to reachieve a normal balance. It has been shown recently that the expression of chm-I is downregulated by the administration of FGF-2 or TGF-β1 in vitro (29). To investigate whether chm-I is able to influence the expression of angiogenic and chondrocyte growth-promoting factors, we investigated the expression levels of FGF-2, TGF-β1 to -β3, and VEGF genes. FGF signaling plays a key role in the negative control of endochondral ossification. Although FGF-2 inhibits hypertrophy of cultured chondrocytes, it also indirectly acts as a stimulant of chondrocyte hypertrophy by its angiogenesis-promoting function (5). RT-PCR and Western assays revealed, however, that the expression of the FGF-2 gene is not changed in chm-1-deficient mice and suggested no feedback regulation of chm-I or FGF-2 or compensatory changes to balance the loss of chm-I function. The expression levels of genes for TGF-β isoforms 1 to 3, which modulate chondrocyte maturation and which inhibit endothelial proliferation (9, 20, 23, 26, 33, 35) were also unchanged. Finally, we observed no altered expression of the gene for VEGF, which has strong angiogenic effects on endothelial cells and which is highly expressed in the hypertrophic zone of human cartilage (8, 13, 19). These findings clearly suggest that proteins with major roles in angiogenesis and cartilage growth are unchanged in chm-1-deficient mice.
VEGF knockouts show a significant growth plate phenotype, whereas deletion of other angiogenic factors expressed during bone development, including TGF-β1 to -β3 and endostatin, does not result in phenotypic changes in the growth plate (10, 21, 27, 28, 32). The normal bone development observed after elimination of single factors sheds light on the stringency and compensatory reserves of the developing bone. An integrated analysis of protein and RNA profiling of the cartilage of the above-mentioned mice and the chm-I-deficient mouse might lead to a better understanding of the interplay of cartilage and endothelial growth factors.
Acknowledgments
We thank Kathryn Rodgers for carefully reading the manuscript.
R.F. was supported by the Swedish Cancer Foundation and the Fonds der Chemischen Industrie. O.B. is the recipient of a grant from the Deutsche Forschungsgesellschaft (grant no. Br2053/1-1).
REFERENCES
- 1.Aszódi, A., D. Chan, E. Hunziker, J. F. Bateman, and R. Fässler. 1998. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J. Cell Biol. 143:1399-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aszódi, A., J. F. Bateman, E. Hirsch, M. Baranyi, E. B. Hunzicker, Z. Bösze, and R. Fässler. 1999. Normal skeletal development of mice lacking matrilin-1: redundant function of matrilins in cartilage? Mol. Cell. Biol. 19:7841-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aszódi, A., L. Módis, A. Páldi, A. Rencendorj, I. Kiss, and Z. Bösze. 1994. The zonal expression of chicken cartilage matrix protein in the developing skeleton of transgenic mice. Matrix Biol. 14:181-190. [DOI] [PubMed] [Google Scholar]
- 4.Azizan, A., N. Holaday, and P. J. Neame. 2001. Post-translational processing of bovine chondromodulin-I. J. Biol. Chem. 276:23632-23638. [DOI] [PubMed] [Google Scholar]
- 5.Babarina, A. V., U. Mollers, K. Bittner, P. Vischer, and P. Bruckner. 2001. Role of the subchondral vascular system in endochondral ossification: endothelial cell-derived proteinases derepress late cartilage differentiation in vitro. Matrix Biol. 20:205-213. [DOI] [PubMed] [Google Scholar]
- 6.Brachtendorf, G., A. Kuhn, U. Samulowitz, R. Knorr, E. Gustafsson, A. J. Potocnik, R. Fässler, and D. Vestweber. 2001. Early expression of endomucin on endothelium of the mouse embryo and on putative hematopoietic clusters in the dorsal aorta. Dev. Dyn. 222:410-419. [DOI] [PubMed] [Google Scholar]
- 7.Brandau, O., A. Meindl, R. Fässler, and A. Aszódi. 2001. A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev. Dyn. 221:72-80. [DOI] [PubMed] [Google Scholar]
- 8.Colnot, C. I., and J. A. Helms. 2001. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech. Dev. 100:245-250. [DOI] [PubMed] [Google Scholar]
- 9.Dangelo, M., D. P. Sarment, P. C. Billings, and M. Pacifici. 2001. Activation of transforming growth factor beta in chondrocytes undergoing endochondral ossification. J. Bone Miner. Res. 16:2339-2347. [DOI] [PubMed] [Google Scholar]
- 10.Eklund, L., J. Piuhola, J. Komulainen, R. Sormunen, C. Ongvarrasopone, R. Fässler, A. Muona, M. Ilves, H. Ruskoaho, T. E. Takala, and T. Pihlajaniemi. 2001. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc. Natl. Acad. Sci. USA 98:1194-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fässler, R., and M. Meyer. 1995. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 9:1896-1908. [DOI] [PubMed] [Google Scholar]
- 12.Funaki, H., S. Sawaguchi, K. Yaoeda, Y. Koyama, E. Yaoita, S. Funaki, M. Shirakashi, Y. Oshima, C. Shukunami, Y. Hiraki, H. Abe, and T. Yamamoto. 2001. Expression and localization of angiogenic inhibitory factor, chondromodulin-I, in adult rat eye. Investig. Ophthalmol. Vis. Sci. 42:1193-1200. [PubMed] [Google Scholar]
- 13.Garcia-Ramirez, M., N. Toran, P. Andaluz, A. Carrascosa, and L. Audi. 2000. Vascular endothelial growth factor is expressed in human fetal growth cartilage. J. Bone Miner. Res. 15:534-540. [DOI] [PubMed] [Google Scholar]
- 14.Harper, J., and M. Klagsbrun. 1999. Cartilage to bone—angiogenesis leads the way. Nat. Med. 5:617-618. [DOI] [PubMed] [Google Scholar]
- 15.Hayami, T., C. Shukunami, K. Mitsui, N. Endo, K. Tokunaga, J. Kondo, H. E. Takahashi, and Y. Hiraki. 1999. Specific loss of chondromodulin-I gene expression in chondrosarcoma and the suppression of tumor angiogenesis and growth by its recombinant protein in vivo. FEBS Lett. 458:436-440. [DOI] [PubMed] [Google Scholar]
- 16.Hiraki, Y., H. Inoue, K. Iyama, A. Kamizono, M. Ochiai, C. Shukunami, S. Iijima, F. Suzuki, and J. Kondo. 1997. Identification of chondromodulin I as a novel endothelial cell growth inhibitor. Purification and its localization in the avascular zone of epiphyseal cartilage. J. Biol. Chem. 272:32419-32426. [DOI] [PubMed] [Google Scholar]
- 17.Hiraki, Y., H. Tanaka, H. Inoue, J. Kondo, A. Kamizono, and F. Suzuki. 1991. Molecular cloning of a new class of cartilage-specific matrix, chondromodulin-I, which stimulates growth of cultured chondrocytes. Biochem. Biophys. Res. Commun. 175:971-977. [DOI] [PubMed] [Google Scholar]
- 18.Hiraki, Y., T. Kono, M. Sato, C. Shukunami, and J. Kondo. 1997. Inhibition of DNA synthesis and tube morphogenesis of cultured vascular endothelial cells by chondromodulin-I. FEBS Lett. 415:321-324. [DOI] [PubMed] [Google Scholar]
- 19.Horner, A., N. J. Bishop, S. Bord, C. Beeton, A. W. Kelsall, N. Coleman, and J. E. Compston. 1999. Immunolocalisation of vascular endothelial growth factor (VEGF) in human neonatal growth plate cartilage. J. Anat. 194:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horner, A., P. Kemp, C. Summers, S. Bord, N. J. Bishop, A. W. Kelsall, N. Coleman, and J. E. Compston. 1998. Expression and distribution of transforming growth factor-beta isoforms and their signaling receptors in growing human bone. Bone 23:95-102. [DOI] [PubMed] [Google Scholar]
- 21.Kaartinen, V., J. W. Voncken, C. Shuler, D. Warburton, D. Bu, N. Heisterkamp, and J. Groffen. 1995. Abnormal lung development and cleft palate in mice lacking TGF-beta-3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 11:415-421. [DOI] [PubMed] [Google Scholar]
- 22.Liu, C., Z. M. Shao, L. Zhang, P. Beatty, M. Sartippour, T. Lane, E. Livingston, and M. Nguyen. 2001. Human endomucin is an endothelial marker. Biochem. Biophys. Res. Commun. 288:129-136. [DOI] [PubMed] [Google Scholar]
- 23.Matsunaga, S., T. Yamamoto, and K. Fukumura. 1999. Temporal and spatial expressions of transforming growth factor-betas and their receptors in epiphyseal growth plate. Int. J. Oncol. 14:1063-1067. [DOI] [PubMed] [Google Scholar]
- 24.Neame, P. J., J. T. Treep, and C. N. Young. 1990. An 18-kDa glycoprotein from bovine nasal cartilage. Isolation and primary structure of small, cartilage-derived glycoprotein. J. Biol. Chem. 265:9628-9633. [PubMed] [Google Scholar]
- 25.Osoegawa, K., M. Tateno, P. Y. Woon, E. Frengen, A. G. Mammoser, J. J. Catanese, Y. Hayashizaki, and P. J. de Jong. 2000. Bacterial artificial chromosome libraries for mouse sequencing and functional analysis. Genome Res. 10:116-128. [PMC free article] [PubMed] [Google Scholar]
- 26.Pepper, M. S., R. Montesano, J. D. Vassalli, and L. Orci. 1991. Chondrocytes inhibit endothelial sprout formation in vitro. Evidence for involvement of a transforming growth factor-β. J. Cell. Physiol. 146:170-179. [DOI] [PubMed] [Google Scholar]
- 27.Proetzel, G., S. A. Pawlowski, M. V. Wiles, M. Yin, G. P. Boivin, P. N. Howles, J. Ding, M. W. J. Ferguson, and T. Doetschman. 1995. Transforming growth factor-beta-3 is required for secondary palate fusion. Nat. Genet. 11:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanford, L. P., I. Ormsby, A. C. Gittenberger-de Groot, H. Sariola, R. Friedman, G. P. Boivin, E. L. Cardell, and T. Doetschman.1997. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124:2659-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shukunami, C., and Y. Hiraki. 1998. Expression of cartilage-specific functional matrix chondromodulin-I mRNA in rabbit growth plate chondrocytes and its responsiveness to growth stimuli in vitro. Biochem. Biophys. Res. Commun. 249:885-890. [DOI] [PubMed] [Google Scholar]
- 30.Shukunami, C., K. Iyama, H. Inoue, and Y. Hiraki. 1999. Spatiotemporal pattern of the mouse chondromodulin-I gene expression and its regulatory role in vascular invasion into cartilage during endochondral bone formation. Int. J. Dev. Biol. 43:39-49. [PubMed] [Google Scholar]
- 31.Shukunami, C., Y. Oshima, and Y. Hiraki. 2001. Molecular cloning of tenomodulin, a novel chondromodulin-I related gene. Biochem. Biophys. Res. Commun. 280:1323-1327. [DOI] [PubMed] [Google Scholar]
- 32.Shull, M. M., I. Ormsby, A. B. Kier, S. Pawlowski, R. J. Diebold, M. Yin, R. Allen, C. Sidman, G. Proetzel, D. Calvin, et al. 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tada, K., T. Fukunaga, Y. Wakabayashi, S. Masumi, Y. Sato, H. Izumi, K. Kohno, and M. Kuwano. 1994. Inhibition of tubular morphogenesis in human microvascular endothelial cells by co-culture with chondrocytes and involvement of transforming growth factor beta: a model for avascularity in human cartilage. Biochim. Biophys. Acta 1201:135-142. [DOI] [PubMed] [Google Scholar]
- 34.Takeda, S., J.-P. Bonnamy, M. J. Owen, P. Ducy, and G. Karsenty. 2001. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 15:467-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorp, B. H., I. Anderson, and S. B. Jakowlew. 1992. Transforming growth factor-beta 1, -beta 2 and -beta 3 in cartilage and bone cells during endochondral ossification in the chick. Development 114:907-911. [DOI] [PubMed] [Google Scholar]
- 36.Yamana, K., H. Wada, Y. Takahashi, H. Sato, Y. Kasahara, and M. Kiyoki. 2001. Molecular cloning and characterization of CHM1L, a novel membrane molecule similar to chondromodulin-I. Biochem. Biophys. Res. Commun. 280:1101-1106. [DOI] [PubMed] [Google Scholar]