Abstract
Objective:
This article reviews the pathogenesis, diagnosis, and treatment of patients with primary gastric lymphoma, with special attention to the changing role of surgery.
Summary Background Data:
Primary gastric lymphomas are non-Hodgkin lymphomas that originate in the stomach and are divided into low-grade (or indolent) and high-grade (or aggressive) types. Low-grade lesions nearly always arise from mucosa-associated lymphoid tissue (MALT) secondary to chronic Helicobacter pylori (H. pylori) infection and disseminate slowly. High-grade lesions may arise from a low grade-MALT component or arise de novo and can spread to lymph nodes, adjacent organs and tissues, or distant sites.
Methods:
A review of the relevant English-language articles was performed on the basis of a MEDLINE search from January 1984 to August 2003.
Results:
About 40% of gastric lymphomas are low-grade, and nearly all these low-grade lesions are classified as MALT lymphomas. For low-grade MALT lymphomas confined to the gastric wall and without certain negative prognostic factors, H. pylori eradication is highly successful in causing lymphoma regression. More advanced low-grade lymphomas or those that do not regress with antibiotic therapy can be treated with combinations of H. pylori eradication, radiation therapy, and chemotherapy. Nearly 60% of gastric lymphomas are high-grade lesions with or without a low-grade MALT component. These lymphomas can be treated with chemotherapy and radiation therapy according to the extent of disease. Surgery for gastric lymphoma is now often reserved for patients with localized, residual disease after nonsurgical therapy or for rare patients with complications.
Conclusion:
The treatment of gastric lymphoma continues to evolve, and surgical resection is now uncommonly a part of the initial management strategy.
Gastric lymphomas consist of low- and high-grade non-Hodgkin lymphomas. Most low-grade lesions arise from mucosa-associated lymphoid tissue (MALT) associated with Helicobacter pylori (H. Pylori) infection and can often be treated solely with H. pylori eradication. High-grade gastric lymphomas are effectively treated with chemotherapy and radiation therapy. Surgical resection is rarely indicated in the initial management of patients with primary gastric lymphoma.
Over the past decade, the management of patients with gastric lymphoma has undergone significant changes with a shift toward nonsurgical treatment. Factors influencing this shift include a better understanding of the etiology of this disease and recent clinical studies demonstrating the efficacy of Helicobacter pylori (H. pylori) eradication therapy, radiation therapy, and chemotherapy. Numerous studies have shown that localized low-grade, mucosa-associated lymphoid tissue (MALT) gastric lymphomas can regress after H. pylori eradication therapy.1 If such therapy fails, relatively low doses (30 Gy) of external beam radiation can control gastric MALT lymphoma in up to 100% of cases.2 Combinations of chemotherapy and radiation therapy can effectively treat low- and high-grade gastric lymphomas.3 This article will present an overview of the diagnosis and workup of patients with gastric lymphoma, review the current literature on treatment options, and provide an algorithm for treatment.
Lymphomas of the stomach are of the non-Hodgkin type. In 2003, there were an estimated 53,400 new cases of non-Hodgkin lymphoma (NHL) and 28,300 deaths in the United States.4 Over the past 4 decades, there has been a steady increase in the incidence of NHL of approximately 3% per year. Deaths from NHL have also steadily increased, but more slowly than the incidence.
NHL usually originates in lymph node basins but can also occur in extranodal sites. The gastrointestinal tract is the most common site of extranodal NHL and accounts for 10 to 15% of all NHL cases.5 Gastrointestinal NHL occurs in the stomach in nearly half of cases.6 Based on these figures, one could estimate that about 3000 new cases of primary gastric lymphoma are diagnosed each year in the United States. There are a number of risk factors for gastrointestinal NHL, including infection with human immunodeficiency virus (HIV), immunosuppression after solid organ transplantation, celiac disease, and inflammatory bowel disease.7 The primary risk factor for gastric lymphoma is infection with H. pylori.8 Some authors have reported a small but significant increased risk of other malignancies in patients with gastric lymphoma, particularly adenocarcinoma of the stomach.9,10
The median age of diagnosis for gastric lymphoma is approximately 60 years old, and the disease affects an equal number of men and women.11 The most common presenting symptom is abdominal pain and loss of appetite.3 Other symptoms include weight loss, gastrointestinal bleeding, and vomiting. B symptoms (weight loss, fever, night sweats) are present in about 12% of patients. Given that these symptoms are nonspecific, there is often a delay in diagnosis. In 1 study, 96% of patients were symptomatic, and the median time from the onset of symptoms to diagnosis was about 3 months.3
GRADE AND PATHOGENESIS
The grading of gastric lymphoma is extremely important in both the prognosis and treatment of this disease (Fig. 1). Most of gastric lymphomas are of B-cell origin and divided into low-grade and high-grade tumors. In the new World Health Organization classification, low-grade NHL is referred to as indolent NHL and high grade NHL is referred to as aggressive NHL.12 For the remainder of this article, we will use the terms low-grade and high-grade because these are the terms used most commonly in the past literature.
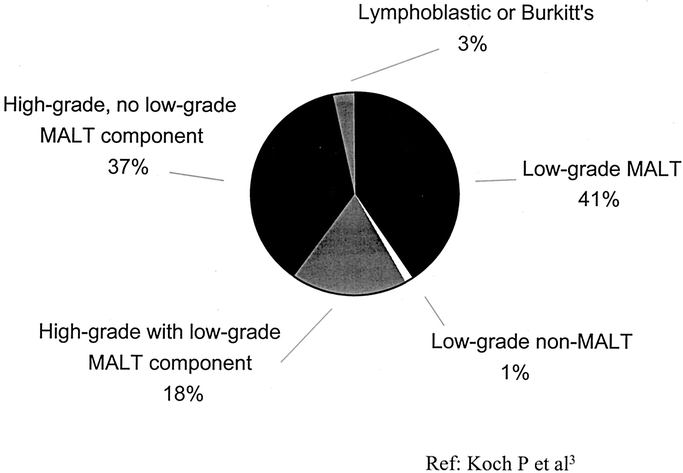
FIGURE 1. Primary gastric lymphoma according to grade.
Low-grade gastric lymphomas are nearly always derived from MALT and are thus termed low-grade MALT lymphomas. High-grade tumors contain a low-grade MALT component in about one third of cases. These lesions likely represent progression of disease from low-grade to high-grade. The remaining two thirds of high-grade lesions have no low-grade MALT component; it is controversial whether these tumors arose from low-grade lesions with subsequent obliteration of the low-grade component or whether these tumor were de novo high-grade.
MALT represents specialized lymphoid tissue associated with certain epithelia, with the most well-known examples being Peyer's patches in the ileum and Waldeyer's ring (tonsils and adenoids) in the nasopharynx and oropharynx.13 In the gastrointestinal tract, MALT is thought to respond to intraluminal antigens and generate mucosal immunity. The gastric mucosa is usually devoid of lymphoid tissue. Currently, it is thought that MALT develops in the gastric mucosa usually in response to chronic H. pylori infection. H. pylori produces specific antigens that initiate an inflammatory response. H. pylori-specific T cells produce interleukin-2 and other cytokines that induce the proliferation of B cells.14 This ultimately leads to the development of a reactive oligoclonal and then monoclonal lymphoproliferative lesion of B cells and subsequent malignant transformation of this monoclonal population to a low-grade MALT lymphoma.15 The time between oligoclonal proliferation to malignant transformation is unclear, but it may take years.
The morphologic characteristics of low-grade MALT lymphoma are that of a monotonous infiltrate of small and medium-sized lymphoid cells with a variable component of plasma cells (Fig. 2A). Isaacson specified 2 histologic criteria for low-grade MALT lymphoma: (1) replacement of gastric glands by uniform infiltrates comprised of cells resembling follicle center centrocytes, small lymphocytes, or monocytoid B cells; and (2) clear evidence of lymphoid destruction of gastric glands.16 In addition, polymerase chain reaction (PCR) can be used to demonstrate monoclonality. Centers vary on the requirements of lympho-epithelial lesions or monoclonality as mandatory for the diagnosis of low-grade MALT lymphoma.17 Transformation from low-grade to high-grade lymphoma appears histologically as increased numbers of transformed blast cells that eventually form sheets or clusters and ultimately grow to efface any residual preceding low-grade tumor (Fig. 2B).15
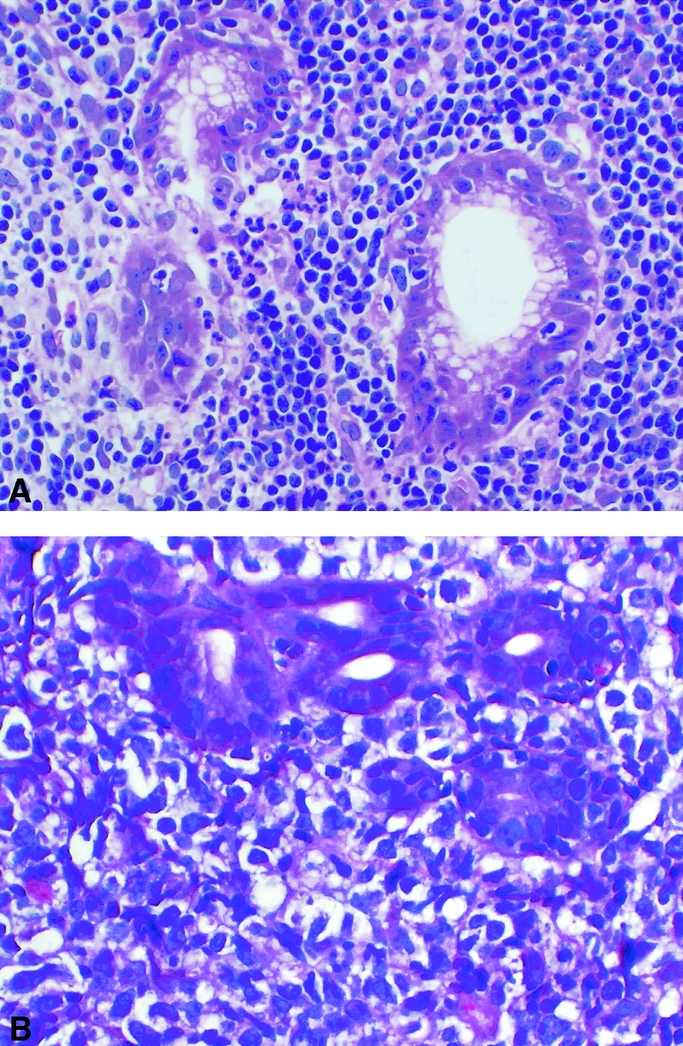
FIGURE 2. Histologic slides of gastric lymphoma. (A) Low-grade MALT lymphoma showing infiltration of lymphoid cells and destruction of gastric glands. (B) High-grade lymphoma showing sheets of transformed blast cells.
Low-grade MALT lymphomas are fairly indolent tumors that often remain localized for extended periods of time,18,19 while high-grade MALT lymphomas proliferate and disseminate more rapidly. The frequency of lymph node involvement also correlates with the grade of the lymphoma. In one series of 37 patients, only 15% of low-grade MALT lymphomas had spread to lymph nodes while 75 to 100% of high-grade lymphomas had spread to lymph nodes.20 Even in the subset of low-grade MALT lymphomas with a small (<20%) high-grade component, 83% had involved lymph nodes.
WORKUP
As with most other diseases, the workup of patients suspected of having gastric lymphoma begins with a history and physical examination. In addition to eliciting the symptoms such as abdominal pain and anorexia, sites of pain outside the abdomen should be determined. On examination, one should palpate all lymph node regions as well as the abdomen for hepatosplenomegaly or masses. Laboratory evaluation should include a complete blood count, chemistry panel, LDH level, and serum protein electrophoresis. Diagnosis can usually be established by upper endoscopy with biopsy. Three main patterns are generally seen on endoscopy: ulcerative, diffuse infiltration, or polypoid mass.21 Thus, endoscopic findings can range from the appearance of gastritis and superficial ulcers to diffuse thickening and irregularities of mucosal folds and a submucosal mass-like effect.11,15 Frankly exophytic masses with the appearance of carcinoma can also be found.22 Multiple biopsies of suspicious areas should be obtained along with biopsies of the antrum to assess for H. pylori infection. Endoscopic ultrasound can be useful to determine the depth of tumor invasion and presence of enlarged perigastric lymph nodes.21
Once the diagnosis of gastric lymphoma is established, an extent of disease workup is required to clinically stage the patient and determine treatment and prognosis. This workup should include a bone marrow biopsy as well as CT scan of the chest, abdomen, and pelvis. Abdominal CT scan abnormalities can be found in about 70% of patients and include gastric wall thickening and lymphadenopathy.23 These abnormalities are more common with high-grade lymphoma than with low-grade lymphoma (100% versus 51%). Positive emission tomography (PET) scans have been increasingly used in the staging of patients with lymphoma. Recent studies have demonstrated PET scans to be superior to Gallium-67 scintigraphy and equal to or superior to CT scans for the staging of lymphoma.24,25 However, MALT lymphomas are frequently not [18F]fluorodeoxyglucose-avid.
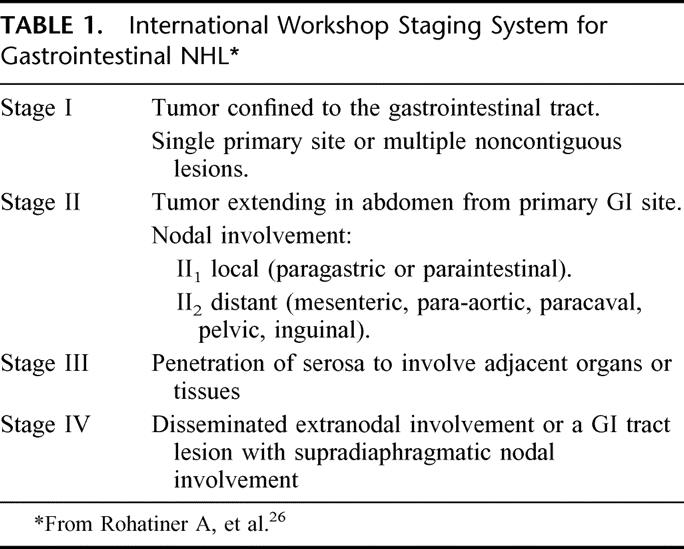
Primary gastric lymphoma and other gastrointestinal lymphoma have been staged using a number of staging systems, with the most commonly used system being the modification of the Ann Arbor staging system for lymphomas as suggested by Musshoff.7 The recent International Workshop recommended the staging system demonstrated in Table 1. 3,26 The distinction between late-stage primary gastric lymphoma and advanced NHL with secondary gastric involvement can be difficult. In general, patients designated as having primary gastric lymphoma should have disease predominantly confined to the stomach with clinical features suggestive of gastric pathology.7
TABLE 1. International Workshop Staging System for Gastrointestinal NHL
H. PYLORI ERADICATION FOR LOW-GRADE MALT LYMPHOMA
Patients with low-grade MALT lymphoma usually present with stage I or II disease and have slow progression.15 The prognosis for these patients overall is quite good, with the 10-year survival ranging between 80 and 90%.27 As most of these patients do well with a variety of treatment modalities, quality of life issues must be considered along with efficacy in the determination of optimal treatment.
As discussed earlier, there is good evidence that most gastric MALT lymphomas are caused by chronic infection with H. pylori. In mouse models, infection of mice with Helicobacter felis led to the development of gastric MALT lymphoma in 26% of cases,28 and some of these lymphoma progressed from low-grade to high-grade lesions. In another mouse model, infection with Helicobacter heilmannii resulted in gastric lymphoma in 14 to 89% of animals.29 One analysis of surgical specimens of patients with gastric MALT lymphoma showed H. pylori gastritis in 92 to 98% of cases.29–31 The duration of H. pylori infection is also a contributing factor. A case-control study showed a higher incidence of H. pylori infection in patients with gastric MALT lymphoma many years before the development of the lymphoma.8
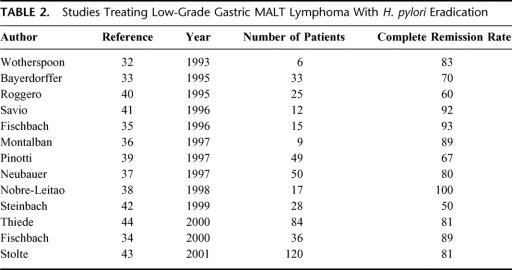
The most convincing evidence that H. pylori infection causes gastric MALT lymphoma comes from the demonstration that eradication of H. pylori leads to regression of gastric MALT lymphomas. In 1993, Weatherspoon et al were the first to report the regression of low-grade MALT lymphoma in 5 of 6 patients following eradication of H. pylori.32 Since that time, over 20 studies have reported successful treatment of gastric MALT lymphoma with H. pylori eradication (Table 2), 32–44 with numbers of patients ranging from 6 to 120 and the complete remission rates ranging from 35 to 100%. Current treatment of initial H. pylori eradication includes a 2-week regimen of (1) lansoprazole or omeprazole, clarithromycin, and metronidazole, (2) lansoprazole or omeprazole, bismuth, metronidazole, and tetracycline, or (3) H2 blocker, bismuth, metronidazole, and tetracycline, with eradication rates ranging between 80 and 95%.45
TABLE 2. Studies Treating Low-Grade Gastric MALT Lymphoma With H. pylori Eradication
The largest series by Stolte et al reported a complete lymphoma remission rate of 81%.43 In this study, 120 patients with a histologically documented, unequivocal diagnosis of low-grade MALT lymphoma were enrolled. Endoscopic examination before and after treatment included biopsies from the antrum and body to grade gastritis and assess for H. pylori infection. Multiple biopsies were taken from the tumor or suspicious areas. Patients were staged by physical examination, ultrasound, and in some cases abdominal CT scan. Patients were treated with a 2-week course of H. pylori eradication therapy. Endoscopy and biopsy were repeated every 2 months until H. pylori eradication, and then every 6 months. Surgery, chemotherapy, and/or radiation therapy were recommended for patients with less than complete response to treatment. Ninety-seven patients (81%) had a complete response, 11 (9%) had a partial response, and 12 (10%) had no response. Nine (10%) of the 97 patients with a complete response had recurrence of their lymphoma; only one of these patients had prior reinfection with H. pylori. Of the 23 patients with no response or incomplete response, most received surgery and/or chemotherapy, and only 2 patients died of lymphoma.
Other studies have specifically addressed which patients are at risk for failing to respond to H. pylori eradication. Stage of disease is important. Patients with involvement of perigastric lymph nodes (stage II1) or beyond are unlikely to completely respond to H. pylori eradication. A multicenter French study of 34 patients with H. pylori-associated low-grade MALT lymphoma subjected all patients to endoscopic ultrasound. Seventy-nine percent of patients with stage I disease had a complete response to H. pylori eradication therapy, while none of the 10 patients with stage II disease had a response.46 Another study of 48 patients found that patients with perigastric lymph nodes on EUS achieved remission in only 33% of cases compared with 76% for patients with no perigastric lymph nodes.47 A t(11:18) chromosomal translocation found in the tumor also predicts failure of response to H. pylori eradication. Liu et al reviewed 111 patients and found that the t(11;18) translocation and stage beyond IE were risk factors for no response or relapse.48 Seventy-three percent of patients who were stage I and did not have the t(11:18) translocation had a complete response, while only 4% of stage I patients with the t(11:18) translocation had a complete response. Only 5% of the stage II patients with or without the t(11:18) translocation had a complete response. Other investigators have reported in small studies that lymphoma penetrating beyond the mucosa or submucosa are unlikely to respond to H. pylori eradication therapy.49,50
Patients with H. heilmanii infection can also develop MALT lymphomas,43 and these lymphomas can also be cured by eradication therapy.51 Rarely, patients with MALT lymphoma are not found to be infected by any strain of Helicobacter by current tests. If one feels that this could be due to false negative tests, H. pyloi eradication therapy could be attempted. However, patients who are truly Helicobacter-negative generally do not respond to antibiotics, often have the t(11:18) translocation, and should be treated with radiation and/or chemotherapy. There are also rare patients with low-grade gastric lymphomas that are not MALT lymphomas. These patients should not be treated with Helicobacter eradication but rather with radiation therapy and/or chemotherapy appropriate for their specific type of lymphoma.
If H. pylori eradication fails to induce lymphoma regression, localized gastric MALT lymphoma is highly sensitive to external beam radiation. Relatively low doses in the range of 30 Gy can have local control rates of up to 100%.2,52 More extensive disease can be treated with a combination of radiation therapy and chemotherapy. While chemotherapy is likely unable to cure MALT lymphoma, responses and remissions are common with single agents such as chlorambucil52 or fludarabine, or combination regimens such as cyclophosphamide, vincristine, and prednisone (CVP).3
In de Jong's review of treatment of low-grade MALT lymphoma at 19 centers, all centers used H. pylori eradication therapy for stage I disease and 8 of 19 centers used H. pylori eradication for stage II1 disease.17 These centers considered time to treatment failure (the interval of time after eradication of H. pylori after which the disease is considered not to respond) to be between 3 to 18 months. Choice of treatment in patients who failed H. pylori eradication therapy depended on the center. Hematology-oriented groups preferred nonsurgical therapies such as radiation or chemotherapy while gastroenterology-oriented groups preferred surgery with or without additional chemotherapy or radiation therapy.
The time interval between eradication of H. pylori and lymphoma regression usually ranges from 4 weeks to 14 months.49 Follow-up after treatment with H. pylori eradication should include upper endoscopy with biopsies every 3-6months. When H. pylori eradication therapy is not successful, an additional course of H. pylori eradication therapy can be considered. If the lymphoma persists, patients should go on to receive radiation therapy and/or chemotherapy. The duration of time one should wait for lymphoma response after H. pylori eradication is controversial. It seems reasonable for the subgroup of patients with a high success rate (ie, disease confined to gastric wall, no involved lymph nodes, no t(11:18) translocation) to undergo endoscopy and biopsy after H. pylori eradication every 3–6 months for 12 months. If after 12 months the lymphoma persists, patients should go on to radiation and possibly chemotherapy. For the subgroup of patients with a low success rate (ie, involved lymph nodes, t(11:18) translocation) one can consider radiation therapy and chemotherapy much earlier in their course, possibly at the time of initial H. pylori eradication therapy or after 3–6 months.
The recurrence of H. pylori infection after successful eradication is low,15 but recurrence of H. pylori can be followed by relapse of lymphoma. Also, lymphoma recurrence can occur without H. pylori reinfection.43 In addition, these patients are at increased risk of intestinal metaplasia and the development of adenocarcinoma.53 Thus these patients must be followed for an extended period of time.
In summary, for patients with low-grade MALT lymphoma, stage I patients with lesions limited to the gastric wall should be treated with H. pylori eradication and followed with endoscopic surveillance (Fig. 3A). If H. pylori eradication fails, a second course of H. pylori eradication treatment should be considered. Patients with lymph node involvement (stage II) or found to have the t(11;18) translocation may also be treated for H. pylori but are at high risk for failure. These high-risk patients are candidates for primary radiation therapy and/or chemotherapy early in the course of their treatment. Patients with stage III and IV disease have even less success with H. pylori eradication therapy alone than high-risk stage I and stage II patients. These patients should go on to radiation and/or chemotherapy at the same time as H. pylori eradication therapy.
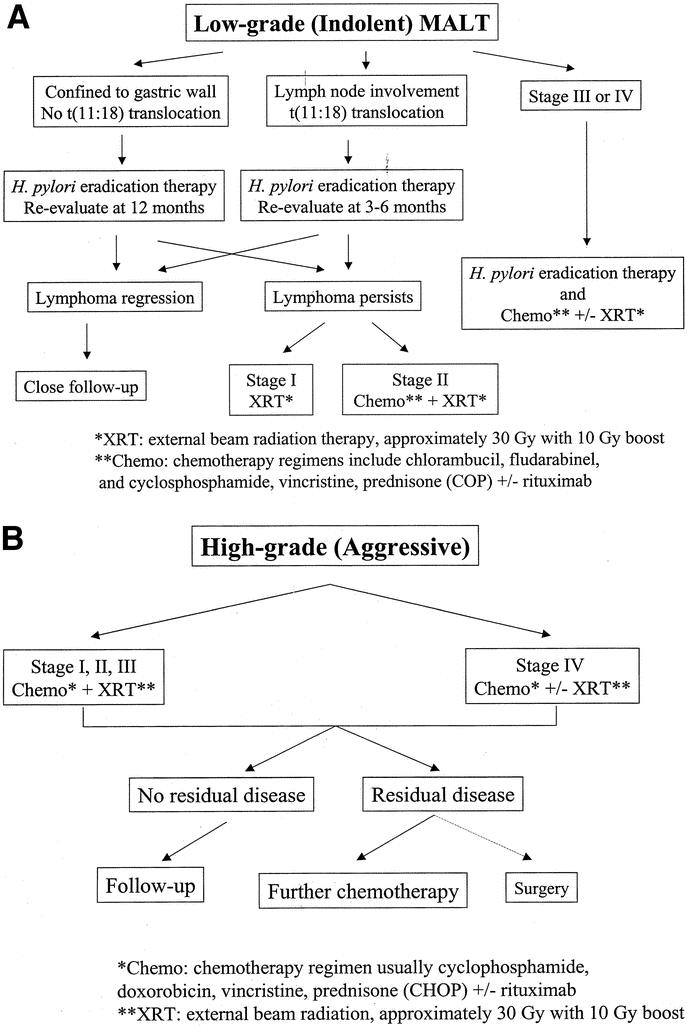
FIGURE 3. Algorithm for management of primary gastric lymphoma. (A) Low-grade; (B) high-grade.
SURGERY FOR EARLY STAGE GASTRIC LYMPHOMA
There has been little consensus as to the most appropriate treatment of patients with early stage gastric lymphoma who do not respond to H. pylori eradication or who are not candidates for H. pylori eradication (eg, patients with high-grade disease). Brands et al reviewed 100 papers analyzing 3157 patients with all stages of gastric lymphoma treated from 1974 to 1995.54 The overall survival during that time period increased from 37% to 87%. The recommended treatment of stage I disease was surgery alone in 30% of studies, surgery and radiation therapy in 32%, surgery and chemotherapy in 15%, and surgery, chemotherapy, and radiation therapy in 8%. Only 20% of studies recommended treatment without surgery. In patients with stage II to IV disease, about 50% of studies recommended surgery, chemotherapy, and radiation therapy, whereas less than 10% of studies recommended chemotherapy and radiation therapy without surgery.
In the past, surgery was important in the diagnosis, staging, and management of early stage gastric lymphoma. Several series demonstrated 5-year survival rates of over 90% with resection alone.55,56 Kodera, et al, performed gastrectomy and D2 lymphadenectomy alone on 60 patients with stage I and II gastric lymphoma and achieved a 5-year survival of greater than 90%.56 In our own experience, 10-year disease-free survival following surgery alone for stage I or II1 disease was 100%.55 However, complication rates for gastrectomy and lymphadenectomy can be high. Median length of stay in our series was 10 days, and the combined early and late complication rate was 26%. Complications occurred in 50% of patients undergoing proximal or total gastrectomy. Thus while surgery can clearly result in excellent survival for patients with disease confined to the operative specimen, it is associated with both short-term and long-term morbidity. In addition, other surgical series have reported 5-year survival for surgery alone to be much lower, in the 50 to 70% range.57–61
CHEMOTHERAPY AND RADIATION THERAPY WITHOUT SURGERY
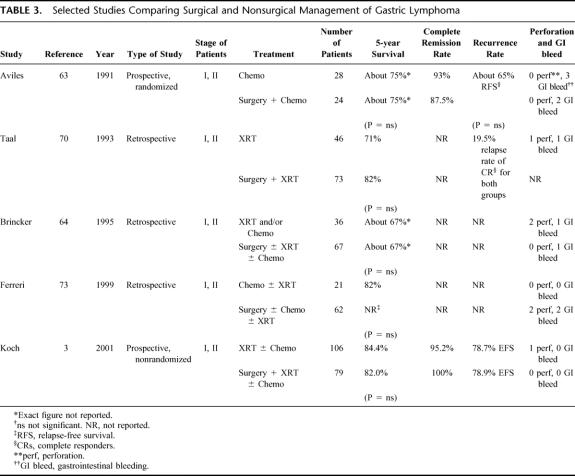
For nearly 2 decades, some investigators have suggested that surgery may not be necessary in the treatment of early gastric lymphoma. Maor et al at the MD Anderson Cancer Center reported in 1984 on the treatment of 9 patients with stage I and II gastric lymphoma with chemotherapy and radiation therapy without gastric resection.62 Only one patient relapsed. More convincing evidence that early gastric lymphoma could be adequately treated without surgery was put forth by Aviles et al in 1991 (Table 3). 63 This study prospectively randomized 52 patients with stage I or II gastric lymphoma to chemotherapy or surgery plus chemotherapy. The relapse-free survival and the overall survival were equivalent in both groups. Five-year overall survival was about 75% in both groups. Other series have also suggested that surgery may not be necessary for patients with early gastric lymphoma (Table 3).64–73 In the series from Milan, 83 patients with stage I or II high-grade gastric lymphoma were reviewed retrospectively.73 Twenty-one patients received chemotherapy alone or chemotherapy plus radiation. The remaining 62 patients received surgery with or without adjuvant therapy. There was no difference in survival between patients who received chemotherapy as primary treatment versus patients who received chemotherapy after surgery. Nonsurgical therapy resulted in a 5-year survival of 82%, 10-year survival of 64%, and stomach preservation rate of 100%.
TABLE 3. Selected Studies Comparing Surgical and Nonsurgical Management of Gastric Lymphoma
The question of whether or not surgery is required for early gastric lymphoma was best addressed by the German Multicenter Study Group.3 Their study was a prospective but nonrandomized study of 185 patients with stage I or II gastric lymphoma registered between 1992 and 1996. The choice of treatment was left to the participating center. One hundred six patients were enrolled in the surgery group, and 79 patients in the nonsurgery group. In the surgery group, patients underwent gastrectomy followed by radiation (for low-grade tumors) or chemotherapy and radiation therapy (for high-grade tumors). In the nonsurgery group, nearly all patients received chemotherapy and radiation therapy (low-grade, stage I lymphomas received radiation alone). Chemotherapy consisted of the COP regimen (cyclophosphamide, vincristine, and prednisone) for low-grade lesions and the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) for high-grade lesions.
Acute toxicities were reported for this study in an interim analysis.74 Most toxicities were grade I and II. The only grade III toxicities occurring in more than 7% of patients were diarrhea and constipation. There were no cases of perforation and 1 case of gastrointestinal hemorrhage occurring in a patient treated with primary chemotherapy. There were 2 treatment-related deaths from liver failure; 1 patient from each treatment arm.
With regard to stage, 52% of patients were stage I, 31% stage II1, and 17% stage II2. Forty-four percent of patients had low-grade MALT lymphoma, 1% low-grade non-MALT lymphoma, 17% high-grade with low-grade MALT component, and 38% high-grade lymphoma only. There was no significant difference in survival between the surgery and nonsurgery groups. The overall 5-year survival rate in the surgical and nonsurgical groups was 82% and 84%, respectively. Negative prognostic factors in the surgery group included age greater than 60 years, decreased performance status, elevated LDH, and incomplete resection. There were no significant negative prognostic factors in the nonsurgery group. Interestingly, stage and grade were not significant prognostic factors in either group.
There are several issues that need to be addressed to properly interpret the results from the German Multicenter Study Group. First, because the patients were not randomized, significant differences exist between the 2 treatment groups. For example, the surgical group had more stage II patients (59% versus 49%) and more high-grade patients (59% versus 51%). In addition, 48 patients enrolled in this study had stage I, low-grade MALT lymphomas, and it is unclear if any of these patients were candidates for H. pylori eradication alone. Despite these issues, this study provides additional evidence that surgery may not be necessary in the primary treatment of early-stage gastric lymphoma and that initial treatment with radiation and/or chemotherapy produces good results.
One argument against nonsurgical therapy for the treatment of gastric lymphoma has been the threat of perforation and hemorrhage. In the only prospective, randomized study of nonsurgical versus surgical treatment, Aviles et al found that of the 28 patients randomized to nonsurgical therapy, no patient experienced perforation.63 Three patients experienced bleeding compared with 2 patients in the surgical group. In the German prospective, nonrandomized study of 185 patients, no patient treated without surgery suffered perforation, and 1 patient had GI bleeding.3 The results from 3 other retrospective studies are listed in Table 3.64,70,73 One must consider that retrospective studies may have inherent biases. Despite this, the overall rate of perforation and bleeding in these selected studies is 1.7% and 2.1% for those treated without surgery and 0.9% and 2.2% for those treated with surgery. Thus the rate of perforation is very low, and the rate of GI bleeding is not significantly different from those treated with surgery.
While the efficacy of surgical and nonsurgical therapies for gastric lymphoma are similar, the recurrence pattern may be different. In the trial by the German Multicenter Study Group, after surgical resection 6 patients recurred: 3 systemically and 3 loco-regionally.3 After nonsurgical management, 7 patients recurred, all locally. Ferreri et al reported in their study of stage I and II patients that in the nonsurgical group 4 of 19 complete responders recurred – 2 locally and 2 systemically.73 Seventeen of 62 patients treated with surgery recurred – 2 locally and 15 systemically. These studies do not stratify location of recurrence by stage. Thus while surgical and nonsurgical options have similar results in terms of overall survival, recurrence patterns may differ in that patients treated without surgery have a greater chance of local recurrence, and patients treated with surgery tend to recur systemically.
In summary, for patients with early-stage, high-grade gastric lymphoma can be treated initially with chemotherapy and/or radiation therapy (Fig. 3B). Rituximab, a monoclonal antibody directed at the cell surface antigen CD20 on B cells, is also increasingly being used in conjunction with CHOP (R-CHOP).75 Residual disease can treated with further chemotherapy. Surgery is likely not necessary and should be reserved for rare complications such as bleeding or perforation and in uncommon cases of localized residual disease.
ADVANCED DISEASE
In unusual cases, patients with gastric lymphoma present with bleeding or obstruction, and sound clinical judgment is needed. Gastric lymphomas rarely present with severe hemorrhage. These patients require urgent endoscopy and possibly urgent surgery. For stable patients who present with microcytic anemia and chronic blood loss, nonoperative treatment can likely be used. For patients with complete or near complete obstruction, one highly successful strategy is to administer high-dose steroids (dexamethasome, 10 mg intravenously every 6 hours), which can lead to an almost uniformly prompt response. Radiation therapy and/or chemotherapy can be subsequently delivered. In rare cases of no response to steroids, surgical resection may be the best option. Minor obstructive symptoms can be further evaluated with noninvasive tests and a can be reached regarding surgical or medical management.
Patients with spread of gastric lymphoma to adjacent organs or tissues (stage III) or disseminated disease (stage IV) are best treated with systemic chemotherapy with or without radiation (Fig. 3B). The risk of perforation or gastrointestinal bleeding without initial surgical resection is quite low. Surgery may be indicated in rare cases for patients with a response to chemotherapy and/or radiation therapy who are left with localized residual disease in the stomach alone. In addition, surgery may be required for the palliation of symptoms such as bleeding or obstruction that do not resolve with nonoperative therapies. Primary surgical therapy is generally unwarranted because of a significant risk of complications and delay in initiation of systemic therapy.
SUMMARY
Gastric lymphoma remains an enigmatic disease, and treatment strategies continue to evolve. With current advances in nonoperative modalities, patients with gastric lymphoma can usually be diagnosed by endoscopy and biopsy and adequately staged without surgery. For patients with low-grade MALT lymphoma without negative features such as spread beyond the gastric wall or the t(11;18) chromosomal translocation, H. pylori eradication therapy is highly effective. This form of therapy mandates close follow-up both to document lymphoma regression and to detect relapses. For patients with more advanced low-grade lymphomas or any high-grade lymphoma, primary treatment has shifted away from gastric resection and toward primary chemotherapy and/or radiation therapy. Most contemporary treatment algorithms no longer include surgical resection in the primary treatment of gastric lymphoma and reserve surgery for the management of complications or unique cases of locally persistent disease.
ACKNOWLEDGMENTS
The authors thank Dr. Laura H. Tang for providing the histologic photographs.
Footnotes
Reprints: Martin S. Karpeh, MD, Department of Surgery State University of New York at Stony Brook Health Science Center T-18 Stony Brook, NY 11794-8191. E-mail: mkarpeh@notes.cc.sunysb.edu.
REFERENCES
- 1.Stolte M. Helicobacter pylori gastritis and gastric MALT-lymphoma. Lancet. 1992;339:745–746. [DOI] [PubMed] [Google Scholar]
- 2.Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998;16:1916–1921. [DOI] [PubMed] [Google Scholar]
- 3.Koch P, del Valle F, Berdel WE, et al. Primary gastrointestinal non-Hodgkin's lymphoma: II. Combined surgical and conservative or conservative management only in localized gastric lymphoma–results of the prospective German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001;19:3874–3883. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. [DOI] [PubMed] [Google Scholar]
- 5.D'Amore F, Brincker H, Gronbaek K, et al. Non-Hodgkin's lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol. 1994;12:1673–1684. [DOI] [PubMed] [Google Scholar]
- 6.Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin's lymphoma in a population-based registry. Br J Cancer. 1999;79:1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crump M, Gospodarowicz M, Shepherd FA. Lymphoma of the gastrointestinal tract. Semin Oncol. 1999;26:324–337. [PubMed] [Google Scholar]
- 8.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura S, Aoyagi K, Iwanaga S, et al. Synchronous and metachronous primary gastric lymphoma and adenocarcinoma: a clinicopathological study of 12 patients. Cancer. 1997;79:1077–1085. [PubMed] [Google Scholar]
- 10.Zucca E, Pinotti G, Roggero E, et al. High incidence of other neoplasms in patients with low-grade gastric MALT lymphoma. Ann Oncol. 1995;6:726–728. [DOI] [PubMed] [Google Scholar]
- 11.Arenas RB. Gastric lymphoma. In: Posner MC, Vokes EE, Weichselbaum RR, eds. Cancer of the Upper Gastrointestinal Tract. London: BC Decker, Inc.; 2002:322–335. [Google Scholar]
- 12.Jaffe ES, Harris NL, Vardiman JW, et al. Pathology and genetics: neoplasms of the hematopoietic and lymphoid tissues. In: Kleihues P, Sobin L, eds. World Health Organization Classification of Tumours. Lyon: IARC Press; 2001. [Google Scholar]
- 13.Armitage JO, Mauch PM, Harris NL, et al. Non-Hodgkin's lymphomas. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 14.Hussell T, Isaacson PG, Crabtree JE, et al. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571–574. [DOI] [PubMed] [Google Scholar]
- 15.Isaacson PG. Gastric MALT lymphoma: from concept to cure. Ann Oncol. 1999;10:637–645. [DOI] [PubMed] [Google Scholar]
- 16.Isaacson PG, Spencer J. Malignant lymphoma of mucosa-associated lymphoid tissue. Histopathology. 1987;11:445–462. [DOI] [PubMed] [Google Scholar]
- 17.De Jong D, Aleman BM, Taal BG, et al. Controversies and consensus in the diagnosis, work-up and treatment of gastric lymphoma: an international survey. Ann Oncol. 1999;10:275–280. [DOI] [PubMed] [Google Scholar]
- 18.Fischbach W, Goebeler-Kolve M, Starostik P, et al. Minimal residual low-grade gastric MALT-type lymphoma after eradication of Helicobacter pylori. Lancet. 2002;360:547–548. [DOI] [PubMed] [Google Scholar]
- 19.Sandmeier D, Benhattar J, Bouzourene H. The natural history of a gastric low grade B cell MALT lymphoma followed during 11 years without treatment. J Clin Pathol. 2002;55:548–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko YH, Han JJ, Noh JH, et al. Lymph nodes in gastric B-cell lymphoma: pattern of involvement and early histological changes. Histopathology. 2002;40:497–504. [DOI] [PubMed] [Google Scholar]
- 21.Taal BG, Burgers JM. Primary non-Hodgkin's lymphoma of the stomach: endoscopic diagnosis and the role of surgery. Scand J Gastroenterol Suppl. 1991;188:33–37. [DOI] [PubMed] [Google Scholar]
- 22.Seifert E, Schulte F, Weismuller J, et al. Endoscopic and bioptic diagnosis of malignant non-Hodgkin's lymphoma of the stomach. Endoscopy. 1993;25:497–501. [DOI] [PubMed] [Google Scholar]
- 23.Choi D, Lim HK, Lee SJ, et al. Gastric mucosa-associated lymphoid tissue lymphoma: helical CT findings and pathologic correlation. AJR Am J Roentgenol. 2002;178:1117–1122. [DOI] [PubMed] [Google Scholar]
- 24.Kostakoglu L, Goldsmith SJ. Positron emission tomography in lymphoma: comparison with computed tomography and Gallium-67 single photon emission computed tomography. Clin Lymphoma. 2000;1:67–74. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Shalom R, Mor M, Yefremov N, et al. The value of Ga-67 scintigraphy and F-18 fluorodeoxyglucose positron emission tomography in staging and monitoring the response of lymphoma to treatment. Semin Nucl Med. 2001;31:177–190. [DOI] [PubMed] [Google Scholar]
- 26.Rohatiner A, D'Amore F, Coiffier B, et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397–400. [DOI] [PubMed] [Google Scholar]
- 27.De Jong D, Boot H, van Heerde P, et al. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology. 1997;112:1466–1474. [DOI] [PubMed] [Google Scholar]
- 28.Enno A, O'Rourke JL, Howlett CR, et al. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. A mouse model of Helicobacter pylori-induced gastric lymphoma. Am J Pathol. 1995;147:217–222. [PMC free article] [PubMed] [Google Scholar]
- 29.O'Rourke JL, Enno A, Howlett CR, et al. Gastric B-cell lymphomas induced in the single mouse strain by various isolates of Helicobacter heilmannii. Similarities and differences. Gut 1995;37(Suppl 1):A7. [Google Scholar]
- 30.Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin's lymphomas. J Clin Pathol. 1994;47:436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, et al. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. [DOI] [PubMed] [Google Scholar]
- 32.Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. [DOI] [PubMed] [Google Scholar]
- 33.Bayerdorffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591–1594. [DOI] [PubMed] [Google Scholar]
- 34.Fischbach W, Dragosics B, Kolve-Goebeler ME, et al. Primary gastric B-cell lymphoma: results of a prospective multicenter study. The German-Austrian Gastrointestinal Lymphoma Study Group. Gastroenterology. 2000;119:1191–1202. [DOI] [PubMed] [Google Scholar]
- 35.Fischback W, Kolve ME, Engemann R, et al. Unexpected success of Helicobacter pylori eradication in low-grade lymphoma. Gastroenterology. 1996;110:A512. [Google Scholar]
- 36.Montalban C, Manzanal A, Boixeda D, et al. Helicobacter pylori eradication for the treatment of low-grade gastric MALT lymphoma: follow-up together with sequential molecular studies. Ann Oncol. 1997;8(Suppl 2):37–39. [PubMed] [Google Scholar]
- 37.Neubauer A, Thiede C, Morgner A, et al. Cure of Helicobacter pylori infection and duration of remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma. J Natl Cancer Inst. 1997;89:1350–1355. [DOI] [PubMed] [Google Scholar]
- 38.Nobre-Leitao C, Lage P, Cravo M, et al. Treatment of gastric MALT lymphoma by Helicobacter pylori eradication: a study controlled by endoscopic ultrasonography. Am J Gastroenterol. 1998;93:732–736. [DOI] [PubMed] [Google Scholar]
- 39.Pinotti G, Zucca E, Roggero E, et al. Clinical features, treatment and outcome in a series of 93 patients with low-grade gastric MALT lymphoma. Leuk Lymphoma. 1997;26:527–537. [DOI] [PubMed] [Google Scholar]
- 40.Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med. 1995;122:767–769. [DOI] [PubMed] [Google Scholar]
- 41.Savio A, Franzin G, Wotherspoon AC, et al. Diagnosis and posttreatment follow-up of Helicobacter pylori-positive gastric lymphoma of mucosa-associated lymphoid tissue: histology, polymerase chain reaction, or both? Blood. 1996;87:1255–1260. [PubMed] [Google Scholar]
- 42.Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med. 1999;131:88–95. [DOI] [PubMed] [Google Scholar]
- 43.Stolte M, Bayerdorffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002;50 Suppl 3:III19–III24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiede C, Wundisch T, Neubauer B, et al. Eradication of Helicobacter pylori and stability of remissions in low-grade gastric B-cell lymphomas of the mucosa-associated lymphoid tissue: results of an ongoing multicenter trial. Recent Results Cancer Res. 2000;156:125–133. [DOI] [PubMed] [Google Scholar]
- 45.Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330–2338. [DOI] [PubMed] [Google Scholar]
- 46.Ruskone-Fourmestraux A, Lavergne A, Aegerter PH, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001;48:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy M, Copie-Bergman C, Traulle C, et al. Conservative treatment of primary gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue: predictive factors of response and outcome. Am J Gastroenterol. 2002;97:292–297. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Ye H, Ruskone-Fourmestraux A, et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122:1286–1294. [DOI] [PubMed] [Google Scholar]
- 49.Sackmann M, Morgner A, Rudolph B, et al. Regression of gastric MALT lymphoma after eradication of Helicobacter pylori is predicted by endosonographic staging. MALT Lymphoma Study Group. Gastroenterology. 1997;113:1087–1090. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura S, Matsumoto T, Suekane H, et al. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgner A, Lehn N, Andersen LP, et al. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821–828. [DOI] [PubMed] [Google Scholar]
- 52.Gospodarowicz M, Tsang R. Mucosa-associated lymphoid tissue lymphomas. Curr Oncol Rep. 2000;2:192–198. [DOI] [PubMed] [Google Scholar]
- 53.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1–9. [DOI] [PubMed] [Google Scholar]
- 54.Brands F, Monig SP, Raab M. Treatment and prognosis of gastric lymphoma. Eur J Surg. 1997;163:803–813. [PubMed] [Google Scholar]
- 55.Bartlett DL, Karpeh MS Jr, Filippa DA, et al. Long-term follow-up after curative surgery for early gastric lymphoma. Ann Surg. 1996;223:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kodera Y, Yamamura Y, Nakamura S, et al. The role of radical gastrectomy with systematic lymphadenectomy for the diagnosis and treatment of primary gastric lymphoma. Ann Surg. 1998;227:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brooks JJ, Enterline HT. Primary gastric lymphomas. A clinicopathologic study of 58 cases with long-term follow-up and literature review. Cancer. 1983;51:701–711. [DOI] [PubMed] [Google Scholar]
- 58.Mittal B, Wasserman TH, Griffith RC. Non-Hodgkin's lymphoma of the stomach. Am J Gastroenterol. 1983;78:780–787. [PubMed] [Google Scholar]
- 59.Rosen CB, van Heerden JA, Martin JK Jr, et al. Is an aggressive surgical approach to the patient with gastric lymphoma warranted? Ann Surg. 1987;205:634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Secco GB, Fardelli R, Campora E, et al. Primary gastric lymphoma. J Surg Oncol. 1993;54:157–162. [DOI] [PubMed] [Google Scholar]
- 61.Weingrad DN, DeCosse JJ, Sherlock P, et al. Primary gastrointestinal lymphoma: a 30-year review. Cancer. 1982;49:1258–1265. [DOI] [PubMed] [Google Scholar]
- 62.Maor MH, Maddux B, Osborne BM, et al. Stages IE and IIE non-Hodgkin's lymphomas of the stomach. Comparison of treatment modalities. Cancer. 1984;54:2330–2337. [DOI] [PubMed] [Google Scholar]
- 63.Aviles A, Diaz-Maqueo JC, de la Torre A, et al. Is surgery necessary in the treatment of primary gastric non-Hodgkin lymphoma. Leuk Lymphoma. 1991;5:365–369. [DOI] [PubMed] [Google Scholar]
- 64.Brincker H, D'Amore F. A retrospective analysis of treatment outcome in 106 cases of localized gastric non-Hodgkin lymphomas. Danish Lymphoma Study Group, LYFO. Leuk Lymphoma. 1995;18:281–288. [DOI] [PubMed] [Google Scholar]
- 65.Burgers JM, Taal BG, van Heerde P, et al. Treatment results of primary stage I and II non-Hodgkin's lymphoma of the stomach. Radiother Oncol. 1988;11:319–326. [DOI] [PubMed] [Google Scholar]
- 66.Gobbi PG, Dionigi P, Barbieri F, et al. The role of surgery in the multimodal treatment of primary gastric non-Hodgkin's lymphomas. A report of 76 cases and review of the literature. Cancer. 1990;65:2528–2536. [DOI] [PubMed] [Google Scholar]
- 67.Haim N, Leviov M, Ben Arieh Y, et al. Intermediate and high-grade gastric non-Hodgkin's lymphoma: a prospective study of non-surgical treatment with primary chemotherapy, with or without radiotherapy. Leuk Lymphoma. 1995;17:321–326. [DOI] [PubMed] [Google Scholar]
- 68.Maor MH, Velasquez WS, Fuller LM, et al. Stomach conservation in stages IE and IIE gastric non-Hodgkin's lymphoma. J Clin Oncol. 1990;8:266–271. [DOI] [PubMed] [Google Scholar]
- 69.Rabbi C, Aitini E, Cavazzini G, et al. Stomach preservation in low- and high-grade primary gastric lymphomas: preliminary results. Haematologica. 1996;81:15–19. [PubMed] [Google Scholar]
- 70.Taal BG, Burgers JM, van Heerde P, et al. The clinical spectrum and treatment of primary non-Hodgkin's lymphoma of the stomach. Ann Oncol. 1993;4:839–846. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka Y, Takao T, Watanabe H, et al. Early stage gastric lymphoma: is operation essential? World J Surg. 1994;18:896–899. [DOI] [PubMed] [Google Scholar]
- 72.Tondini C, Balzarotti M, Santoro A, et al. Initial chemotherapy for primary resectable large-cell lymphoma of the stomach. Ann Oncol. 1997;8:497–499. [DOI] [PubMed] [Google Scholar]
- 73.Ferreri AJ, Cordio S, Paro S, et al. Therapeutic management of stage I-II high-grade primary gastric lymphomas. Oncology. 1999;56:274–282. [DOI] [PubMed] [Google Scholar]
- 74.Willich NA, Reinartz G, Horst EJ, et al. Operative and conservative management of primary gastric lymphoma: interim results of a German multicenter study. Int J Radiat Oncol Biol Phys. 2000;46:895–901. [DOI] [PubMed] [Google Scholar]
- 75.King KM, Younes A. Rituximab: review and clinical applications focusing on non-Hodgkin's lymphoma. Expert Rev Anticancer Ther. 2001;1:177–186. [DOI] [PubMed] [Google Scholar]