Abstract
Objective:
We sought to determine the expression of molecular markers in an animal model of cholangiocarcinoma compared with those in human cholangiocarcinoma.
Summary Background Data:
Cholangiocarcinoma is a rare disease characterized by early intrahepatic and extrahepatic spread, which seriously limits the efficacy of surgery. Establishing an experimental model to study the cholangiocarcinogenesis is desirable.
Methods:
Sprague-Dawley rats weighing 300 ± 50 g were used for the study group. The animals were given 0.3% thioacetamide in tap water continuously. Thirty mass-forming peripheral cholangiocarcinoma patients also were studied. Expression of epidermal growth factor receptor (EGFR), MUC1, MUC2, MUC5AC, MMP-2, MMP-9, and p53 in both human and experimental rat cholangiocarcinoma was examined using immunohistochemistry.
Results:
Using thioacetamide 0.3% as a hepatoxin to induce cholangiocarcinoma in rats, microfoci of cancerous cells were detected from 12 weeks, and all experimental rats displayed diffuse mass-forming cholangiocarcinoma after 24 weeks. EGFR was strongly expressed in 14 (47%) of 30 human cholangiocarcinoms and 24 (100%) of 24 rat cholangiocarcinomas, respectively. MUC1 was strongly expressed in all human and rat cholangiocarcinomas, whereas MUC2 and MUC5AC were focally and weakly expressed. MMP-2 and MMP-9 were strongly expressed in 22 (73%) of 30 human cholangiocarcinomas and 24 (100%) of 24 rat cholangiocarcinomas, respectively. p53 overexpression was detected in 9 (30%) of 30 human cholangiocarcinoma and none of the rat cholangiocarcinoma, respectively.
Conclusions:
The expression of EGFR, apomucins, MMPs, and p53 in rat cholangiocarcinoma was strongly homologous to human cholangiocarcinoma. Thioacetamide-induced cholangiocarcinoma in rats provides an excellent model for investigating cholangiocarcinogenesis in vivo.
Expression of epidermal growth factor receptor, apomucins, matrix metalloproteinases, and p53 in thioacetamide-induced rat cholangiocarcinoma is strongly homologous to those in human cholangiocarcinoma.
The incidence of intrahepatic cholangiocarcinoma is approximately 10% of primary liver cancers. The endemic area of cholangiocarcinoma is Southeast Asia, including Taiwan.1–5 Early hematogenous and lymphatic extrahepatic spread seriously limit the efficacy of surgery. The 5-year survival of cholangiocarcinoma patients after a curative resection is less than 30%.6 Although several studies have examined mutations of oncogenes and tumor suppressor genes in intrahepatic cholangiocarcinoma, the mechanism of cancer cells development and invasion remains unclear. Therefore, establishing an experimental model to extensively study cholangiocarcinogenesis is desirable.
This study presents an animal model of thioacetamide (CH3CSNH2)-induced intrahepatic cholangiocarcinoma in rats, as previously reported.7,8 Using this animal model, we systematically investigate the similarity of various protooncogenes and tumor suppressor gene between cholangiocarcinoma in experimental animals and humans.
MATERIALS AND METHODS
Patients
Thirty patients with mass-forming peripheral cholangiocarcinoma who had undergone curative hepatectomy were studied. Patients diagnosed with either periductal infiltrating cholangiocarcinoma or intraductal papillary tumor of the liver (IPNL)9 were excluded. Of the 30 cases of cholangiocarcinoma, 26 were histologically adenocarcinoma while the remaining 4 were adenosquamous carcinoma.
Animals
Sprague-Dawley rats weighing 300 ± 50 g were used in this study. The animals were fed a laboratory diet and water ad libitum. The animals were administered 0.3% thioacetamide in tap water continuously until sacrifice. Three experimental rats and 1 sham rat were sacrificed every 2 weeks during the study. The hepatic tissues were removed from the animals and processed for histology and protein study.
Immunohistochemical Stainings for Epidermal Growth Factor Receptor (EGFR), MUC1, MUC2, MUC5AC, MMP-2, MMP-9, and p53
Formalin-fixed, paraffin-embedded tissues, either from human or rat specimen, were cut into 4-μm sections and mounted on glu-coated slides. A modification of the avidin-biotin-peroxidase complex immunohistochemical method was performed. Slides were heated at 60°C for 60 minutes, then deparaffinized in xylene, and rehydrated in graded alcohols. Endogenous peroxidase was blocked by incubation in 0.3% hydrogen peroxidase in methanol, and slides were rehydrated and washed in phosphate-buffered saline (PBS), pH 7.4 for 15 minutes. Sections were then blocked with 10% normal goat serum in PBS with 1% bovine serum albumin for 15 minutes. The blocking serum was decanted and various primary antibodies (EGF-R, 1:200, monoclonal, mouse, antihuman, clone BC04, DAKO; MUC1, 1: 50, monoclonal, Novocastra, Newcastle upon Tyne, UK; MUC2, 1: 100, monoclonal, Novocastra; MUC5AC, 1: 50, Novocastra; MMP-2, 1:100, Zymed, South San Francisco, CA; MMP-9, 1:40, Novocastra; p53, 1:50, DAKO, Denmark) were applied, respectively, in PBS with 1% bovine serum albumin for 16 hours at 4°C. Slides were washed in PBS with 1% Tween 20 for 10 minutes. After 3 PBS rinses, biotinylated goat antirabbit immunoglobulin (Vector Labs, Burlingame, CA) at a dilution of 1/500 was applied for 30 minutes at room temperature. Following another PBS rinse, avidin DH: biotinylated horseradish peroxidase complex (Vector Labs) was applied for 30 minutes. After a final PBS rinse, the tissue sections were reacted with 0.06% diaminobenzidine (Sigma Chemical Co.) for 5 minutes, rinsed, counter-stained with hematoxylin, dehydrated with graded alcohols, cleared xylene, and coverslipped with permount.
Interpretation of EGFR, MUC1, MUC2, and MUC5AC, MMP-2, MMP-9, and p53 proteins was performed using a scale – (negative), + (weakly positive), ++ (moderately positive), and +++ (strongly positive) first, taking into account the percentage of stained cells and the intensity of staining. Then, those with scale – or + were stratified as immuno-negative; whereas those with scale ++ or +++ were stratified as immunopositive.
RESULTS
Using thioacetamide 0.3% as a hepatoxin to induce cholangiocarcinoma in SD rats, the following experimental results were obtained: microfoci of cancerous cells were detected from 12 weeks, while multiple whitish tumors were identified grossly from 16 weeks (Fig. 1), moreover, all experimental rats displayed diffuse mass-forming cholangiocarcinoma after 24 weeks. The mortality rate of experimental rats fed thioacetamide (0.3%) before 26 weeks approached zero.
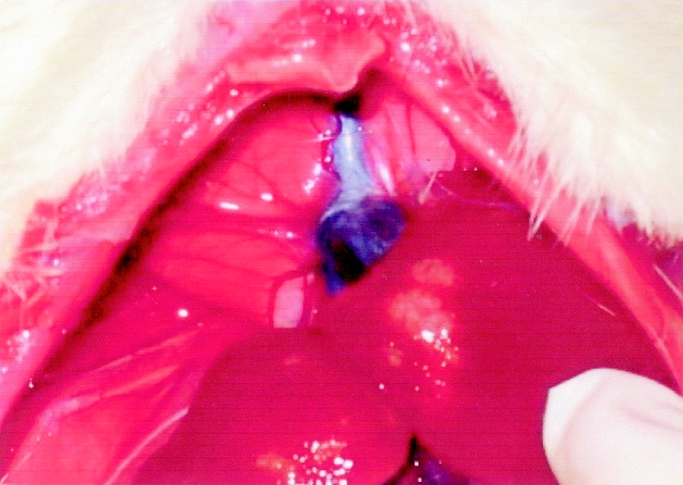
FIGURE 1. Multiple mass-forming peripheral cholangiocarcinoma in Sprague-Dawley rat induced by thioacetamide 0.3% at 16 weeks.
EGFR, MUC1, MUC2, MUC5AC, MMP-2, MMP-9, and p53 expression in rat and human cholangiocarcinoma was tabulated (Table 1). Briefly, EGFR was strongly expressed in 14 (47%) of 30 human cholangiocarcinoms, and 24 (100%)of 24 rat cholangiocarcinomas, respectively (Fig. 2). Additionaly, MUC1 was strongly expressed in all human and rat cholangiocarcinomas, while MUC2 and MUC5AC were focally and weakly expressed (Fig. 3). Moreover, MMP-2 and MMP-9 were strongly expressed in 22 (73%) of 30 human cholangiocarcinomas and 24 (100%) of 24 rat cholangiocarcinomas, respectively (Fig. 4). Finally, p53 was over-expressed in 9 (30%) of 30 human cholangiocarcinoma, with 4 adenosquamous carcinoma and 5 adenocarcinoma exhibiting p53 overexpression, while none of the rat cholangiocarcinomas displayed p53 overexpression (Fig. 5).
TABLE 1. Expression of EGFR, Apomucins, MMPs, and P53 in Human and Rat Cholangiocarcinoma
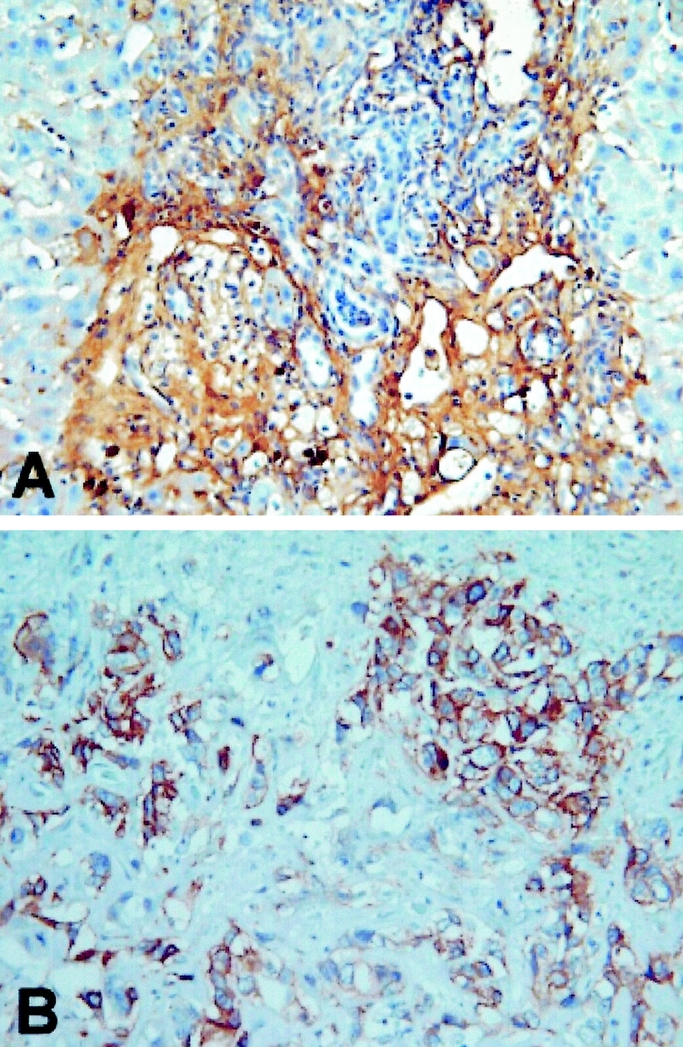
FIGURE 2. Strong expression of epidermal growth factor receptor in rat (A) and human (B) cholangiocarcinoma, respectively (original magnification, 400×).
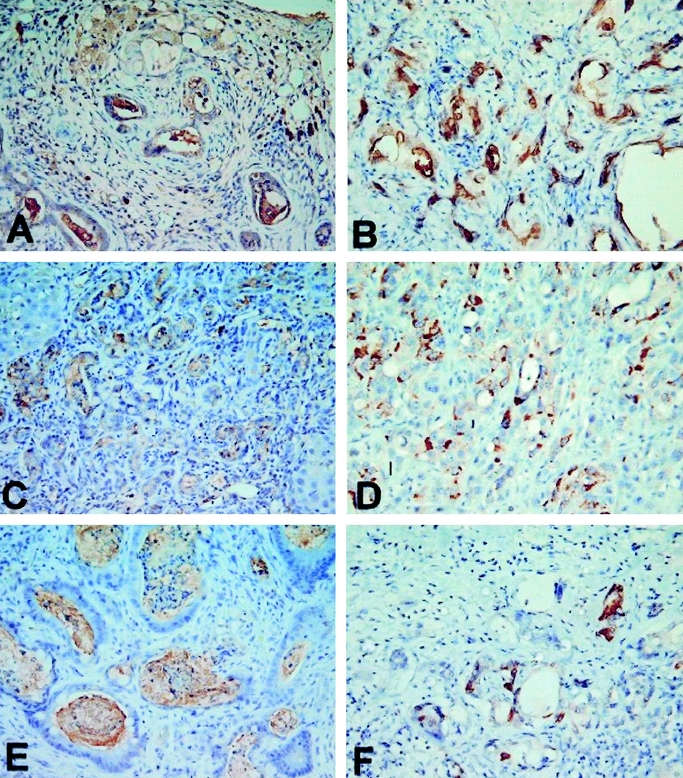
FIGURE 3. Strong expression of MUC1 in rat (A) and human (B) cholangiocarcinoma, respectively. Focal and weak expression of MUC2 in rat (C) and human (D) cholangiocarcinoma, respectively. Focal and weak expression of MUC5AC in rat (E) and human (F) cholangiocarcinoma, respectively (original magnification, 400×).
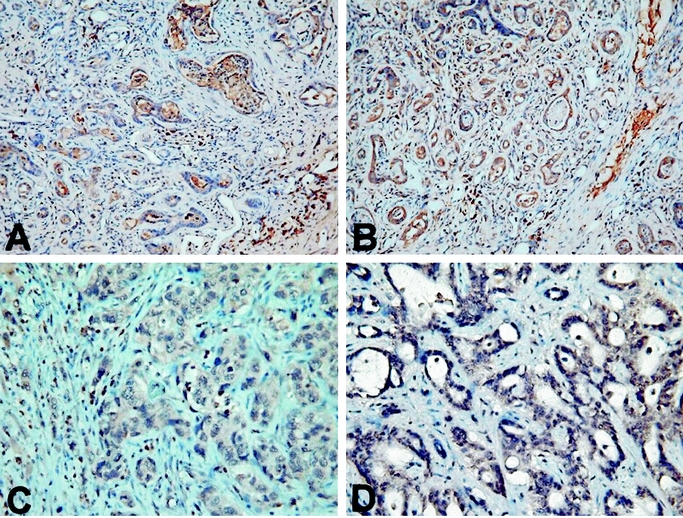
FIGURE 4. Strong expression of MMP-2 in rat (A) and human (B) cholangiocarcinoma, respectively. Strong expression of MMP-9 in rat (C) and human (D) cholangiocarcinoma, respectively (original magnification, 400×).
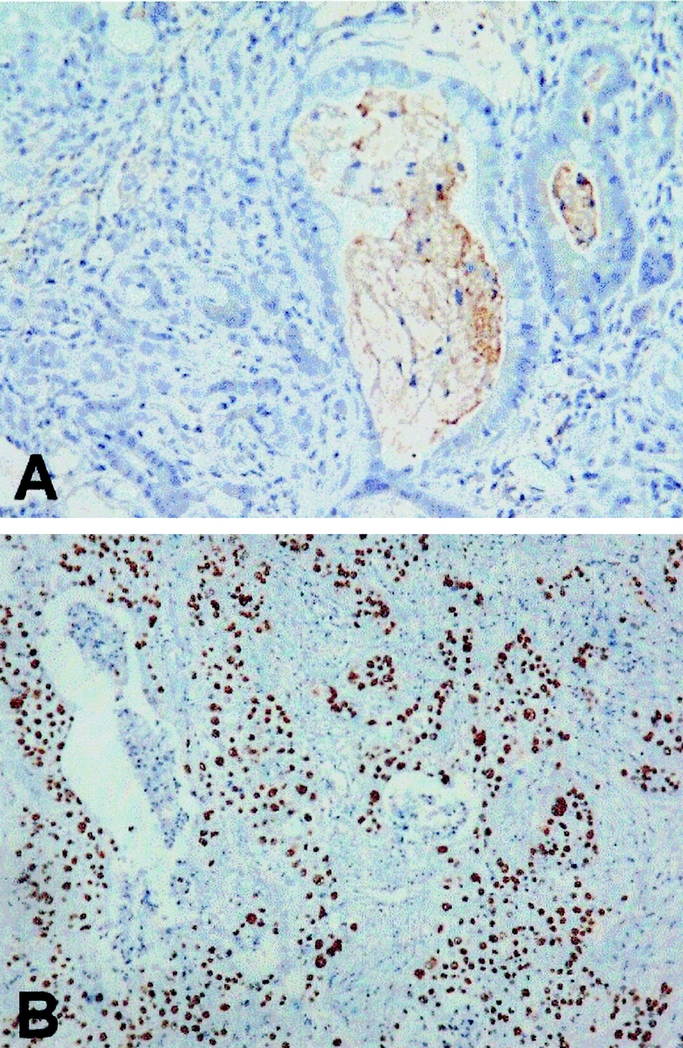
FIGURE 5. Negative p53 overexpression in rat cholangiocarcinoma (A); and strong p53 overexpression in human adenosquamous cholangiocarcinoma (B) (original magnification, 400×).
DISCUSSION
Human cholangiocarcinoma essentially can be divided into mass-forming, periductal infiltrating, and the intraductal type.6 Most peripheral cholangicarcinomas belong to the mass-forming type. The experimental animals fed thioacetamide (0.3%) here consistently developed diffuse mass-forming cholangiocarcinoma with moderate-poor differentiation. Notably, a minimal mortality rate during experimental study could be obtained. Most importantly, this study observed the entire cholangiiocarcinogenesis process from dysplasia (the precursor of the cancer) to the advanced cancerous stage.
Neoplasm formation is initiated and stimulated by hormones and growth factors. Epidermal growth factor (EGF), one of the polypeptide growth factors, stimulates proliferation and differentiation of various cell types. The effects of EGF are mediated through its specific cell-surface receptor (EGFR), which is a glycoprotein with a molecular weight of 170 kDa.10 EGFR has certain structural similarities to the avian erythroblastosis virus v-erb-B transforming protein (ERBB).11 The receptor comprises an external binding domain, a transmembrane region and an intracellular domain exhibiting tyrosine kinase function. In response to EGF, the receptor kinase phosphorylates intracellular substrate, after which the mitogenic signal is initiated.11 Although the molecular pathogenesis of cholangiocarcinoma remains poorly understood, increasing evidence exists that overexpression of protooncogene-encoded receptor tyrosine kinase ERBB-2 together with upregulation of cyclooxygenase-2 (COX-2) may be important to cholangiocarcinogenesis.12,13 The present study found EGFR expression in 100% of rat cholangiocarcinomas and 47% of human cholangiocarcinoma, respectively. Significantly, EGFR was expressed in 2 of 3 rats fed thioacetamide for 10 weeks, with bile ductule proliferation with cellular dysplasia being noted in both cases. Thus, EGFR appears to be an important protooncogene for cholangiocarcinogenesis. From these observations, combined targeting of EGFR and COX-2, in addition to other conventional chemotherapeutic agents, appears to have therapeutic potential for human cholangiocarcinoma.
Mucins are high molecular weight glycoproteins with O-linked oligosaccharides attached to the serine or threonine residues of the apomucin protein backbone.14 Fourteen MUC genes have been identified to date. Malignant transformation of epithelial cells was generally associated with the abnormal glycosylation of MUCs.15–17 Yonezawa et al showed high MUC1 expression in invasive cholangiocarcinoma, which has a poor prognosis, but rare MUC1 expression in biliary cytstadenocarcinoma, which has a favorable prognosis. Conversely, MUC2 is rarely expressed in invasive cholangiocarcinoma, but is expressed in cystadenocarcinoma.16,17 From the morphologic perspective, Suh et al showed that MUC1 was expressed in all 3 morphologic types; whereas MUC2 was highly expressed only in intraductal growth type and was never expressed in the mass-forming or periductal infiltrating type.18 The present study confirmed some of the above results, namely that MUC1 but not MUC2 is strongly expressed in mass-forming cholangiocarcinoma; furthermore, this study showed that MUC5AC, which is a gastric-type mucin, also was not expressed in mass-forming cholangiocarcinoma.
Both invasion and neovascularization of neogrowths require extracellular matrix breakdown and the subsequent migration of cells through the degraded structures. Because extracellular matrix remodeling is the major activity of a family of enzymes known as matrix metalloproteinases (MMPs), these enzymes have come under investigation for their contributions to the malignant phenotype. The MMP family currently has 23 known members, which are subdivided according to their structures and substrate specificities into membrane-type matrix metalloproteinases (MT-MMPs), collagenases, stromelysins and gelatinases.19–21 Excessive tissue destruction occurs when MMP activity goes unchecked, leading to a myriad of pathologic states including tumor invasion and metastasis. Among the MMP family, MMP-2 and MMP-9 were found to be expressed in 45% (5 of 11) and 27% (3 of 11) of human cholangiocarcinoma in an immunohistochemistry based survey by Terada et al22 Moreover, another study by Shirabe et al detected MMP-9 in 43% (16 of 37) of human cholangiocarcinoma.23 Additionally, Shirabe et al showed that MMP-9 in cholangiocarcinoma was a prognostic factor related to lymph node metastasis. The present study found that MMP-2 and MMP-9 were expressed in 73% of human cholangiocarcinoma, exceeding the levels of expression other’ investigations.22,23 Unexpectedly, MMP-2 and MMP-9 were expressed in all stages of rat cholangiocarcinomas, from as early as 12 weeks.
Inactivation of p53 caused by missense mutations or interaction with oncogenic viral proteins allows progression through the cell cycle without a physiological checkpoint, resulting a selective growth advantage for cancer cells.24,25 The majority of p53 gene mutations, abolishing the tumor suppressor activity, lead to its accumulation in tumor cells that can be detected by immunohistochemical method.26 It is generally accepted that p53 gene alteration play a key role in late-stage events of tumor pathogenesis. The reported frequencies of p53 overexpression in cholangiocarcinoma vary from 19% to 58%.26–30 Ohashi et al showed that p53overexpression was detected in 19% (4 of 21) of cholangiocarcinoma cases, and p53 overexpression showed no clear association with gross appearance.30 From the present data, p53 overexpression was prevalent in adenosquamous carcinoma (4 of 4) of human cholangiocarcinoma; whereas p53 overexpression was detected in only 19% (5 of 26) of cases of adenocarcinoma, indicating that most human cholangiocarcinomas might progress along a p53-independent pathway. Interestingly, no rat cholangiocarcinomas, which universally present as moderate-poorly differentiated adenocarcinoma, displayed p53 overexpression even in the very advanced stage (eg, 26 weeks), an observation that resemlbes to most human cholangiocarcinoma.
In conclusion, this study presented an animal model of mass-forming peripheral cholangiocarcinoma, and the model exhibited the advantages of convenience, cheapness, minimal mortality, and consistency. Investigating EGFR, apomucin, MMPs, and p53 revealed extremely homologous expression of these molecular markers in human and rat cholangiocarcinoma. Therefore, this study recommends thioacetamide-induced cholangiocarcinoma in rats as an excellent model for studying cholangiocarcinogenesis in vivo.
Footnotes
Reprints: Yi-Yin Jan, MD, Department of Surgery, Chang Gung Memorial Hospital, 5 Fu-Hsing Street, Taoyuan, Taiwan. E-mail: tsy471027@adm.cgmh.org.tw.
REFERENCES
- 1.Chen MF, Jan YY, Wang CS, et al. Intrahepatic duct stone associated with cholangiocarcinoma. Am J Gastroenterol. 1989;84:391–395. [PubMed] [Google Scholar]
- 2.Chen MF, Jan YY, Wang CS, et al. A reappraisal of cholangiocarcinoma in patients with hepatolithiasis. Cancer. 1993;71:2461–2465. [DOI] [PubMed] [Google Scholar]
- 3.Chen MF, Jan YY, Chen TC. Clinical studies of mucin-producing cholangiocellular carcinoma: a study of 22 histopathology-proven cases. Ann Surg. 1998;227:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh TS, jan YY, Tseng JH, et al. Malignant perihilar biliary obstruction: magnetic resonance cholangiopancreatographic findings. Am J Gastroenterol. 2000;95:432–440. [DOI] [PubMed] [Google Scholar]
- 5.Jan YY, Yeh TS, Chen MF. Cholangiocarcinoma presenting as pyogenic liver abscess. Is its outcome influenced by concomitant hepatolithiasis? Am J Gastroenterol. 1998;93:253–255. [DOI] [PubMed] [Google Scholar]
- 6.Khan A, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl VI):vi1–vi9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzhugh OG, Nielson AA. Liver tumors in rats fed thiourea or thioacetamide. Science. 1948;108:626–628. [DOI] [PubMed] [Google Scholar]
- 8.Bader AA, Mathew TC, Abul H, et al. Cholangiocarcinoma and liver cirrhosis in relation to changes due to thioacetamide. Mol Cell Biochem. 2000;208:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Chen TC, Nakanuma Y, Zen Y, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651–658. [DOI] [PubMed] [Google Scholar]
- 10.Korc M, Meltzer P, Trent J. Enhanced expression of epidermal growth factor receptor correlates with alterations chromosome 7 in human pancreatic cancer. Proc Nat Acad Sci USA. 1986;83:5141–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downward J, Yaden Y, Mates E, et al. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequence. Nature. 1984;307:521–527. [DOI] [PubMed] [Google Scholar]
- 12.Endo K, Yoon BI, Pairojkul C, et al. ERBB-2 overexpression and cyclooxygenase-2 regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439–450. [DOI] [PubMed] [Google Scholar]
- 13.Sirica AE, Lai GH, Endo K, et al. Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma: potential therapeutic targets. Seminars Liver Dis. 2002;22:303–313. [DOI] [PubMed] [Google Scholar]
- 14.Degand P, Pigny P, Van SI, et al. MUC genes: a superfamily of genes? Toward a functional classification of human apomucins. J Soc Biol. 1999;193:85–99. [PubMed] [Google Scholar]
- 15.Sasaki M, Nakanuma Y, Kim Y. Characterization of apomucin expression in intrahepatic cholangiocarcinoma and their precursor lesions: an immunohistochemical study. Hepatology. 1996;24:1074–1078. [DOI] [PubMed] [Google Scholar]
- 16.Yonezawa S, Sueyoshi K, Nomoto M, et al. MUC2 gene expression is found in noninvasive tumors but not in invasive tumors of the pancreas and liver: its close relationship with prognosis of the patients. Hum Pathol. 1997;28:344–352. [DOI] [PubMed] [Google Scholar]
- 17.Higashi M, Yonezawa S, Ho JJL, et al. Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology. 1990;30:1347–1355. [DOI] [PubMed] [Google Scholar]
- 18.Suh KS, Chang SH, Lee HJ, et al. Clinical outcomes and apomucin expression of intrahepatic cholangiocarcinoma according to gross morphology. J Am Coll Surg. 2002;195:782–789. [DOI] [PubMed] [Google Scholar]
- 19.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. [DOI] [PubMed] [Google Scholar]
- 20.Sato H, Seiki M. Regulatory mechanism of 92-kDa type IV collagenase gene expression which is associated invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- 21.Ueno H, Nakamura H, Inoue M, et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997;57:2055–2060. [PubMed] [Google Scholar]
- 22.Terada T, Okada Y, Nakanuma Y. Expression of immunoreactive matrix metalloproteinase and tissue inhibitors of matrix metalloproteinases in human normal liver and primary liver tumors. Hepatology. 1996;23:1341–1344. [DOI] [PubMed] [Google Scholar]
- 23.Shirabe K, Shimada M, Kajiyama K, et al. Expression of matrix metalloproteinase-9 in surgically resected intrahepatic cholangiocarcinoma. Surgery. 1999;126:842–846. [PubMed] [Google Scholar]
- 24.Levine AJ, Momand J, Finlay CA. The p53 tumor suppressor gene. Nature. 1991;351:453–456. [DOI] [PubMed] [Google Scholar]
- 25.Harris CC. Structure and function of the p53 tumor suppressor gene: clues for rational cancer therapeutic strategies. J Natl Cancer Inst. 1996;88:1442–55. [DOI] [PubMed] [Google Scholar]
- 26.Diamantis I, Karamitopoulou E, Perentes E, et al. P53 protein immunoreactivity in extrahepatic bile duct and gallbladder cancer: correlation with tumor grade and survival. Hepatology. 1995;22:774–779. [PubMed] [Google Scholar]
- 27.Teh M, Wee A, Raju GC. An immunohistochemical study of protein in gallbladder and extrahepatic bile duct/ampullary carcinoma. Cancer. 1994;74:1542–1545. [DOI] [PubMed] [Google Scholar]
- 28.Washington K, Gottfried MR. Expression of p53 in adenocarcinoma of the gallbladder and bile ducts. Liver. 1996;16:99–104. [DOI] [PubMed] [Google Scholar]
- 29.Tannapfel A, Engeland K, Weinans L, et al. Expression of p73, a novel protein related to the p53 tumor suppressor p53, and apoptosis in cholangiocarcinoma of the liver. Br J Cancer. 1999;80:1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohashi K, Nakajima Y, Kanehiro H, et al. Ki-ras mutations and p53 protein expressions in intraheoatic cholangiocarcinomas: relation to gross tumor morphology. Gastroenterology. 1995;109:1612–1617. [DOI] [PubMed] [Google Scholar]