Abstract
Objective:
Our objective was to perform a prospective study of surgical treatment of hilar cholangiocarcinoma according to newly established guidelines for performing safe and curative resections.
Summary Background Data:
The poor survival rate after resection of hilar cholangiocarcinoma is considered to be mainly the result of in-hospital death and positive ductal margins.
Methods:
Between July 1999 and December 2002, 40 of 42 surgically explored patients with hilar cholangiocarcinoma underwent resection. They were managed with preoperative biliary decompression, portal embolization, cholangiographic evaluation, and a choice of surgical procedures and techniques.
Results:
Hospital or 30-day mortality and morbidity rates were 0% and 48%, respectively. Hepatic failure was not encountered. Histopathologic examination revealed no positive ductal margins in all 40 patients, but 2 showed positive separation margins from the right hepatic artery. The overall 3-year survival rate and median survival time were 40% and 27 months. Survival of patients with Bismuth type III or IV tumors or of patients who underwent right hepatectomy was significantly better. Survival of patients who underwent concomitant vascular resection was similar to survival of those who did not. Univariate analysis indicated the type of hepatectomy, histopathologic grade, Bismuth classification, concomitant hepatic artery resection, and International Union Against Cancer stage as significant prognostic factors.
Conclusions:
No postoperative mortality and no positive ductal margins were achieved according to the above guidelines in a high-volume expert center. Long-term results, however, have not been significantly improved. A survival analysis of the patient series with homogeneous conditions derived from a short study period suggests the need for additional strategies including right hepatectomy for Bismuth type I or II tumors.
Surgical resection of hilar cholangiocarcinoma was achieved with no postoperative mortality and no positive ductal margins in 40 consecutive cases during a 3.5-year period in a high-volume expert center. To improve long-term results some, additional strategies are proposed.
The use of major hepatectomy in the surgical treatment of hilar cholangiocarcinoma has increased resectability and improved long-term results.1–3 The reported 3-year and 5-year survival rates of 30 to 40% and 20 to 30%, respectively, however, are still far from encouraging.4–14 The poor survival rates have mostly been attributed to in-hospital death from postoperative hepatic failure7,15–18 and to positive surgical margins, particularly in the stumps of the remnant hepatic ducts.19–21 More extensive hepatic resection to obtain negative ductal margins increases the risk of postoperative hepatic failure, whereas less extensive hepatectomy, which is performed because of fear of hepatic failure, increases the risk of positive ductal margins. Therefore, balancing these conflicting considerations has been difficult.
To address this problem, we began a prospective study in July 1999 on surgical treatment of hilar cholangiocarcinoma using new departmental guidelines. The results of this study are reported here and new potential strategies are proposed to improve long-term survival.
PATIENTS AND METHODS
A prospective study on surgical treatment of hilar cholangiocarcinoma was conducted, starting in July 1999, employing the following guidelines: (1) Preoperative biliary decompression to reduce the serum bilirubin concentration below 2 mg/dL and to control segmental cholangitis22; (2) Preoperative portal embolization of the liver to be resected when subsequent right hepatectomy or left trisegmentectomy (resection of Couinaud's segments 2, 3, 4, 5, and 8) were planned; (3) Precise cholangiographic evaluation of longitudinal cancer spread with or without cholangioscopy; (4) Planning for hilar resection including the hilar and cystic plate23,24 with or without hemihepatectomy plus caudate lobectomy; (5) Skeletonization of the portal vein and hepatic artery with nodal clearance around the head of the pancreas; (6) Portal vein resection and reconstruction before hepatic parenchymal dissection and hepatic ductal division25,26; (7) Transhepatic direct approach to the limits separating the hepatic ducts from the vasculature and ductal division.
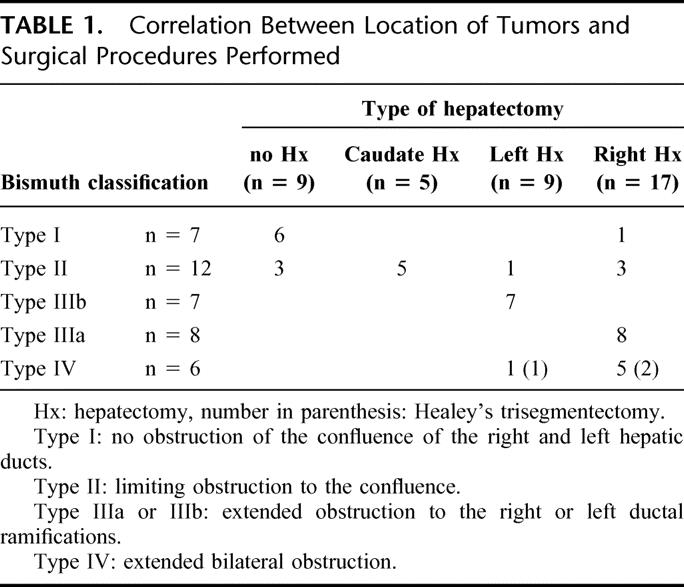
For 3.5 years, until December 2002, 43 patients with hilar cholangiocarcinoma were admitted and evaluated for surgical resection. Because our institute is a referral center, it is likely that the patients had been screened for surgery in previous hospitals. Only one patient was considered ineligible for surgery due to poor functional hepatic reserve associated with a large hematoma resulting from percutaneous biliary puncture. Two patients did not undergo tumor resection because of peritoneal implantation or extensive vascular involvement. Altogether, 40 patients underwent resection, giving a resectability rate of 95%, and were thus included in this study. Age ranged from 42 to 82 years, with a median of 68 years, and the male/female ratio was 34/6. The location of the lesions was classified according to the Bismuth classification,27 as follows: type I, no obstruction of the confluence of the right and left hepatic ducts; type II, limited obstruction to the confluence; type IIIa or IIIb, extended obstruction to the right or left ductal ramifications; and type IV- extended bilateral obstruction. A single chief surgeon (S.K.) led all operations. No definite chemotherapy or radiotherapy was added after surgery.
Survival rates after surgery were calculated using the Kaplan-Meier method, including any death. Statistical comparison of survival rates was analyzed by use of the log-rank test. Multivariate analysis was made using the Cox proportional hazards model. A P value less than 0.05 was considered significant.
RESULTS
Operative Procedures Performed
Depending on the longitudinal cancer spread, 4 types of operative procedures were employed: right hepatectomy (n = 17) mainly for Bismuth type IIIa or IV tumors, left hepatectomy (n = 9) mainly for type IIIb tumors, isolated caudate lobectomy (n = 5) for type II tumors, and hilar resection alone (n = 9) for type I or II tumors (Table 1). All hepatectomies included caudate lobectomy and hilar resection. Healey's trisegmentectomy (resection of Couinaud's segments 4, 5, 6, 7, and 8; or 2, 3, 4, 5, and 8) was performed in 3 cases, in which preoperative evaluation indicated longitudinal spread extending beyond the separating limits of the hepatic ducts from the vasculature in the conventional right or left hepatectomy. Frozen section was not used intraoperatively for histologic assessment of proximal ductal margins.
TABLE 1. Correlation Between Location of Tumors and Surgical Procedures Performed
Concomitant vascular resection was carried out because of macroscopic involvement in 14 patients, with portal vein resection in 8 patients, hepatic artery resection in 8 patients, and both procedures in 2 patients. Pancreatoduodenectomy was necessary for intrapancreatic extension in 7 patients with Bismuth type I or II tumors. Operative time ranged from 357 to 956 minutes, with a median of 593 minutes. Blood loss ranged from 270 to 2980 mL, with a median of 1240 mL, and red blood cell transfusion of 1 to 4 units was performed in 14 patients, with a median of 2 units transfused.
Pathologic Findings
The histopathologic grade of tumors was determined according to the International Union Against Cancer (UICC) staging system (2002).28 The grade was well-differentiated carcinoma (G1) in 15 patients, moderately differentiated carcinoma (G2) in 17 patients, and poorly differentiated carcinoma (G3) in 8 patients. Four, 12, 14, and 10 patients had T1, T2, T3, and T4 tumors, respectively. Four, 9, 8, 7, 10, and 2 patients had stage IA, IB, IIA, IIB, III, and IV tumors, respectively.28 Perineural invasion and lymph node metastasis were found in 27 and 15 patients, respectively. Histologic invasion of the portal trunk or its major branches and the hepatic artery were found in 12 and 5 patients, respectively.
Detailed examination using serial longitudinal sections revealed no positive ductal margins in any of the 40 patients, including 8 in which the distance between the cancerous and surgical margins were within 5 mm. Positive separation margins from the right hepatic artery, however, were demonstrated in 2 patients. Thus, R0 curative resection was achieved in 38 patients (95%).
Operative Morbidity and Mortality
There was no in-hospital nor 30-day mortality postoperatively. Complications developed in 19 patients (48%). The most frequent was bile leak in 9 patients, followed by pancreatic fistula in 4 patients, and intraperitoneal abscess in 3. The maximum postoperative concentration of serum bilirubin ranged from 0.8 to 9.6 mg/dL, with a median of 3.5 mg/dL, and hepatic failure was not encountered.
Postoperative Survival
The 3-year survival rate and median survival period were 40% and 27 months for all 40 resected patients and 44% and 35 months for the 38 patients who underwent R0 resection. Survival in patients with Bismuth type III or IV tumors was significantly better than for patients with type I or II tumors (P = 0.016, Fig. 1). Also, survival of patients treated with right hepatectomy was better than of patients who underwent left hepatectomy (P = 0.013), isolated caudate lobectomy (P = 0.023), and hilar resection alone (P < 0.001, Fig. 2). Survival curves for patients with and without vascular resection were similar (P = 0.768, Fig. 3), as were curves for patients with and without histologic invasion of the portal vein (P = 0.620, Fig. 4). Univariate analysis revealed several significant prognostic factors, namely type of hepatectomy, histopathologic grade, Bismuth classification, concomitant hepatic artery resection, and UICC stage (Table 2). Multivariate analysis using the 5 significant factors from the univariate analysis showed the following 3 factors to be independent prognostic factors: type of hepatectomy (P = 0.001; 95% confidence interval [CI]: 0.212–0.684), histopathologic grade (P = 0.014; 95% CI: 1.222–6.051), and UICC stage (P = 0.034; 95% CI: 1.063–4.705); however, the statistical significance is limited owing to the small number of this patient series.
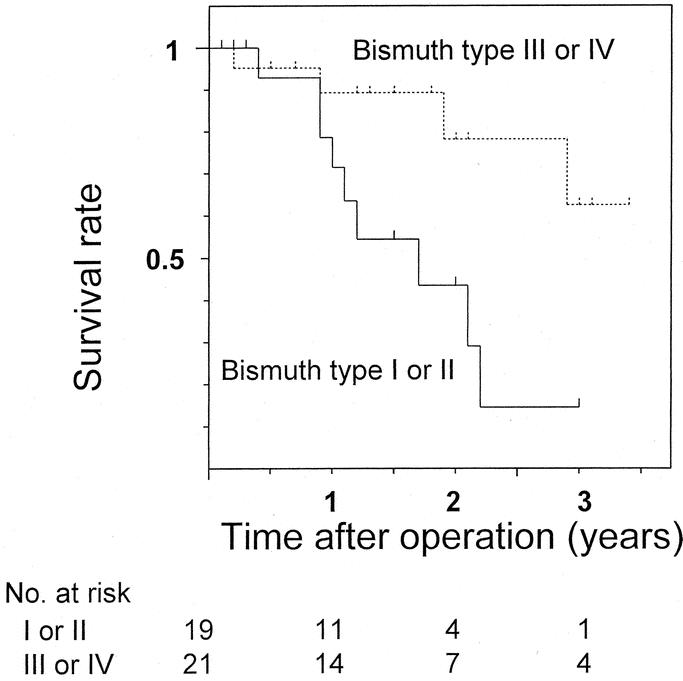
FIGURE 1. Postoperative survival in resected patients with Bismuth type I or type II hilar cholangiocarcinoma and with type III or IV tumors. There was a significant difference between the 2 (P = 0.016).
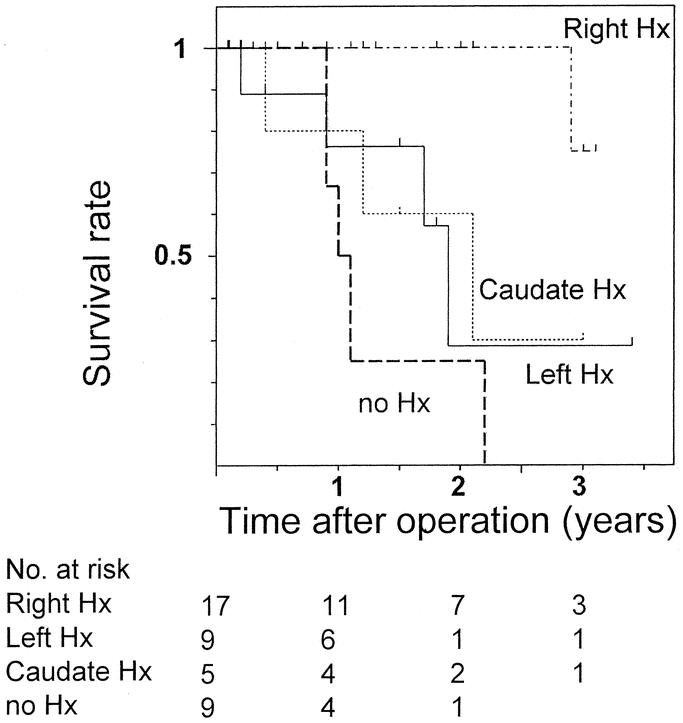
FIGURE 2. Postoperative survival in resected patients with hilar cholangiocarcinoma according to surgical procedures. Survival in patients treated with right hepatectomy was significantly better in patients who underwent left hepatectomy (P = 0.013), isolated caudate lobectomy (P = 0.023), or no hepatectomy, ie, hilar resection alone (P < 0.001). Hx: hepatectomy.
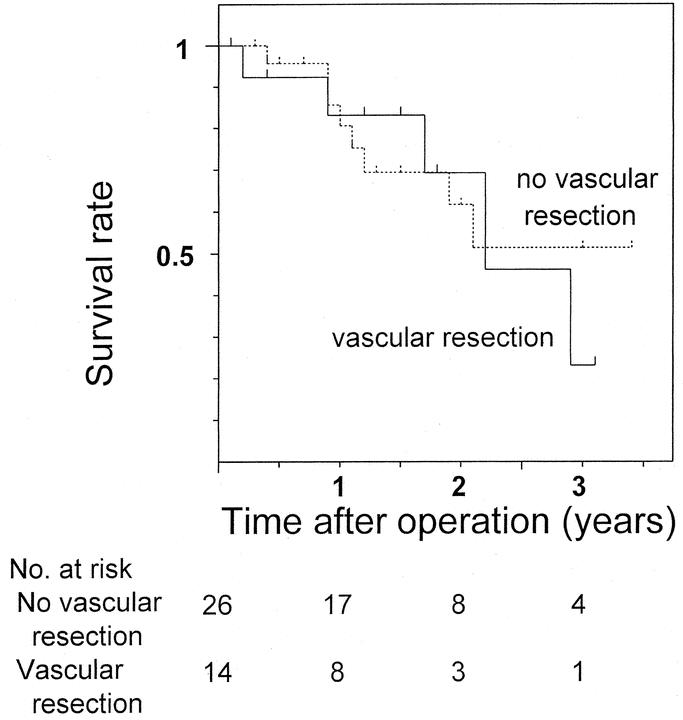
FIGURE 3. Comparison of postoperative survival in resected patients with hilar cholangiocarcinoma who underwent concomitant vascular resection to those who did not (P = 0.768).
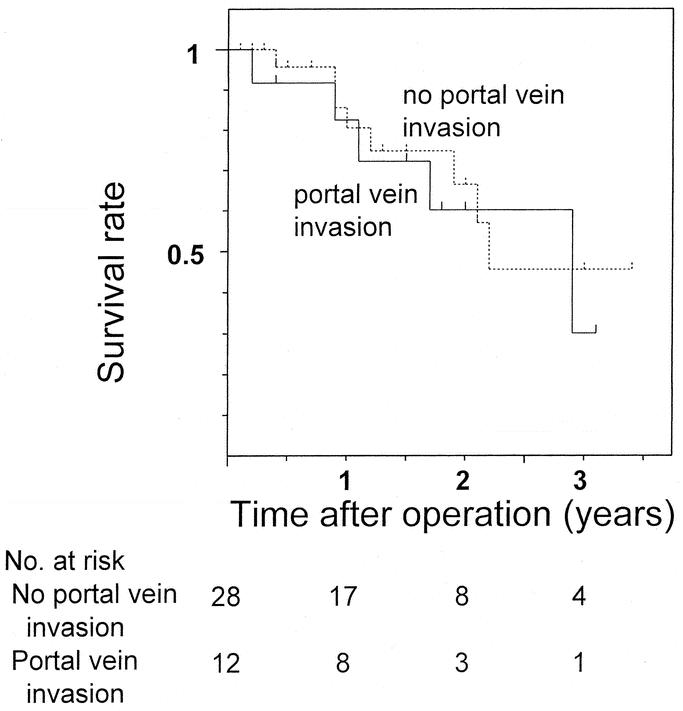
FIGURE 4. Comparison of postoperative survival in resected patients with hilar cholangiocarcinoma who had histologic portal vein invasion to those who did not (P = 0.620).
TABLE 2. Univariate Survival Analysis of Clinicopathologic Factors
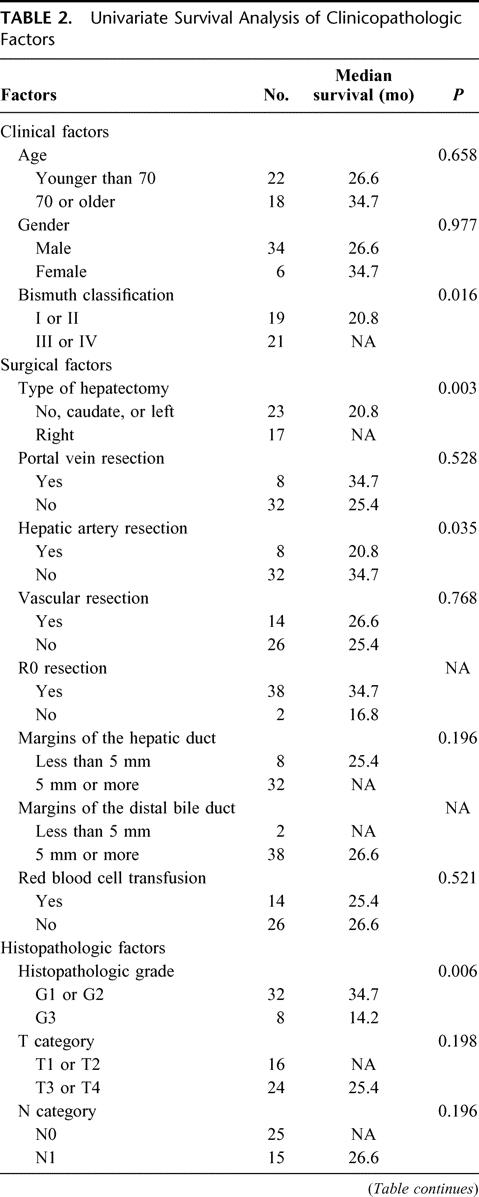
TABLE 2. (continued)
The mode of recurrence in the 9 patients who died of disease after R0 resection were peritoneal seeding in 5 patients, hepatic metastasis in 2, and nodal recurrence and local recurrence in one patient each.
DISCUSSION
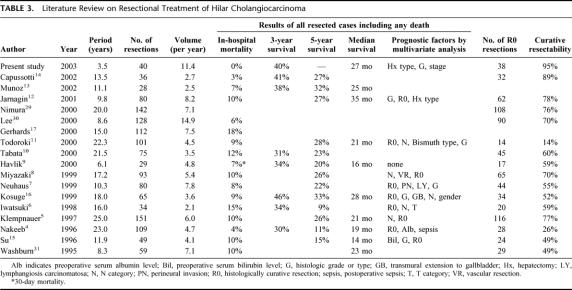
The newly established guidelines for surgical treatment of hilar cholangiocarcinoma have enabled us to achieve no postoperative mortality and no positive ductal margins in 40 consecutive resections over the last 3.5 years. These results have never been reported before (Table 3). 4–17,29–31 Absence of hepatic failure after major hepatectomy, the most frequent cause of postoperative in-hospital death, is probably attributable to reduced jaundice, recovery of damaged liver function, and early treatment of segmental cholangitis22 by suitable biliary decompression32 as well as hypertrophy of and functional transition to the future remnant liver by preoperative portal embolization.33,34 Accurate evaluation of intraductal longitudinal spread by precise cholangiography,29 choice of appropriate hepatectomy, and hepatic ductal division at the separating limits from the vasculature are mandatory to ensure negative ductal margins. In cases of portal vein resection, the preceding portal reconstruction enabled ductal division at the separating limits from the vasculature by full mobilization of the remnant portal branch, as in a conventional hepatectomy without portal vein resection.25,26 We do not routinely use frozen sections to assess the remnant ductal stumps because the determination, whether positive or negative, is sometimes difficult even in permanent sections. Furthermore, even if the remnant is positive, additional ductal resection at the separating limits is not feasible.
TABLE 3. Literature Review on Resectional Treatment of Hilar Cholangiocarcinoma
Contrary to expectations, despite no postoperative mortality and no positive ductal margins, long-term results were not significantly improved, as shown by the 3-year survival rate of 40% and the median survival period of 27 months, which are similar to the results previously reported (Table 3). One possible explanation is that the present study included patients with more advanced disease, represented by the high rate (35%) of concomitant vascular resection. However, this is unlikely due to the comparable survival rates for patients with and without concomitant vascular resection (Fig. 3), as well as the survival rates for patients with and without histologic portal vein invasion (Fig. 4). Survival rates in patients who underwent concomitant vascular resection have been reported to be worse than in patients who did not.8,12,29 This can be attributed to associated high mortality or high positive rates of remnant ductal margins when hepatic ductal division precedes portal reconstruction. The present study suggests that concomitant vascular resection with no postoperative mortality and no positive ductal margins has a similar outcome in patients with vascular invasion to those without.
The diversity of clinical backgrounds derived from a long study period may influence analytic process and results. The uniformity of no postoperative mortality and no positive ductal margins and the homogeneity of diagnostic or therapeutic factors ensured by the short period in this prospective study enabled a pure analysis of the impact of surgical or tumor factors on patient survival. Interestingly, the analysis demonstrated that the survival in patients with Bismuth type III or IV tumors were significantly better than in patients with type I or II tumors (Fig. 1). Also, the survival of patients treated with right hepatectomy was significantly better than of patients who underwent left hepatectomy, isolated caudate lobectomy, or hilar resection alone (Fig. 2). Most likely the right hepatectomy enables en-bloc resection of the hepatic ductal confluence and its surrounding structures because the confluence lies on the right side of the hepatic hilum. In the 2 patients in this study who did not have R0 resection, left hepatectomy or hilar resection alone were performed and the separation margins from the right hepatic artery were positive. Right hepatectomy facilitates en-bloc resection including the right hepatic artery, which runs close to the ductal confluence and has the potential to increase radicality. Although we aggressively employed concomitant vascular resection, the maneuvers needed to isolate the portal system or hepatic artery from the tumor might have caused microscopic seeding. Analysis of tumor recurrence after R0 resection indicated a low frequency of local recurrence and a high frequency of peritoneal seeding, contrary to previous reports describing locoregional recurrence as the most common mode.35 Considering that no patients had tumors exposing the serosal surface, seeding recurrence is likely to result from intraoperative separating maneuvers or spillage of bile possibly containing viable cancer cells. Thus, en-bloc resection of the vessels without separating maneuvers near the tumor may be advisable.
In conclusion, no postoperative mortality and no positive ductal margins have been achieved according to the guidelines in a high-volume expert center. To improve long-term results, additional strategies are proposed including right hepatectomy for Bismuth type I or II tumors, the application of routine resection of the right hepatic artery in a left hepatectomy for type IIIb tumors, and the prevention of intraoperative bile spillage as well as preoperative spillage caused by percutaneous biliary drainage.
Footnotes
Reprints: Satoshi Kondo, MD, Department of Surgical Oncology, Hokkaido University Graduate School of Medicine, N15 W7, Kita-ku, Sapporo 060-8638, Japan. E-mail: kondows@med.hokudai.ac.jp.
REFERENCES
- 1.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;4:533–544. [DOI] [PubMed] [Google Scholar]
- 2.Launois B, Terblanche J, Lakehal M, et al. Proximal bile duct cancer: high resectability rate and 5-year survival. Ann Surg. 1999;230:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saldinger PF, Blumgart LH. Resection of hilar cholangiocarcinoma: a European and United States experience. J Hepatobiliary Pancreat Surg. 2000;7:111–114. [DOI] [PubMed] [Google Scholar]
- 4.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klempnauer J, Ridder GJ, von Wasielewski R, et al. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 1997;15:947–954. [DOI] [PubMed] [Google Scholar]
- 6.Iwatsuki S, Todo S, Marsh JW, et al. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187:358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki M, Ito H, Nakagawa K, et al. Parenchyma-preserving hepatectomy in the surgical treatment of hilar cholangiocarcinoma. J Am Coll Surg. 1999;189:575–583. [DOI] [PubMed] [Google Scholar]
- 9.Havlik R, Sbisa E, Tullo A, et al. Results of resection for hilar cholangiocarcinoma with analysis of prognostic factors. Hepatogastroenterology. 2000;47:927–931. [PubMed] [Google Scholar]
- 10.Tabata M, Kawarada Y, Yokoi H, et al. Surgical treatment for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:148–154. [DOI] [PubMed] [Google Scholar]
- 11.Todoroki T, Kawamoto T, Koike N, et al. Radical resection of hilar bile duct carcinoma and predictors of survival. Br J Surg. 2000;87:306–313. [DOI] [PubMed] [Google Scholar]
- 12.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munoz L, Roayaie S, Maman D, et al. Hilar cholangiocarcinoma involving the portal vein bifurcation: long-term results after resection. J Hepatobiliary Pancreat Surg. 2002;9:237–241. [DOI] [PubMed] [Google Scholar]
- 14.Capussotti L, Muratore A, Polastri R, et al. Liver resection for hilar cholangiocarcinoma: in-hospital mortality and longterm survival. J Am Coll Surg. 2002;195:641–647. [DOI] [PubMed] [Google Scholar]
- 15.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerhards MF, van Gulik TM, de Wit LT, et al. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma: a single center experience. Surgery. 2000;127:395–404. [DOI] [PubMed] [Google Scholar]
- 18.Nagino M, Kamiya J, Uesaka K, et al. Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg. 2001;25:1277–1283. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg. 1998;227:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebata T, Watanabe H, Ajioka Y, et al. Pathological appraisal of lines of resection for bile duct carcinoma. Br J Surg. 2002;89:1260–1267. [DOI] [PubMed] [Google Scholar]
- 21.Okazaki Y, Horimi T, Kotaka M, et al. Study of the intrahepatic surgical margin of hilar bile duct carcinoma. Hepatogastroenterology. 2002;49:625–627. [PubMed] [Google Scholar]
- 22.Kanai M, Nimura Y, Kamiya J, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119:498–504. [DOI] [PubMed] [Google Scholar]
- 23.Couinaud C. Surgical Anatomy of the Liver Revisited. Paris: C. Couinaud; 1989.
- 24.Kawarada Y, Das BC, Taoka H. Anatomy of the hepatic hilar area: the plate system. J Hepatobiliary Pancreat Surg 2000;7:580–586. [DOI] [PubMed] [Google Scholar]
- 25.Kondo S, Katoh H, Hirano S, et al. Portal vein resection and reconstruction prior to hepatic dissection during right hepatectomy and caudate lobectomy for hepatobiliary cancer. Br J Surg. 2003;90:694–697. [DOI] [PubMed] [Google Scholar]
- 26.Kondo S, Katoh H, Hirano S, et al. Wedge resection of the portal bifurcation concomitant with left hepatectomy plus biliary reconstruction for hepatobiliary cancer. J Hepatobiliary Pancreat Surg. 2002;9:603–606. [DOI] [PubMed] [Google Scholar]
- 27.Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39–47. [DOI] [PubMed] [Google Scholar]
- 28.International Union Against Cancer (UICC). TNM Classification of Malignant Tumors. 6th ed. New York: Wiley-Liss; 2002. [Google Scholar]
- 29.Nimura Y, Kamiya J, Kondo S, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–162. [DOI] [PubMed] [Google Scholar]
- 30.Lee SG, Lee YJ, Park KM, et al. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7:135–141. [DOI] [PubMed] [Google Scholar]
- 31.Washburn WK, Lewis WD, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch Surg. 1995;130:270–276. [DOI] [PubMed] [Google Scholar]
- 32.Nimura Y, Kamiya J, Kondo S, et al. Technique of inserting multiple biliary drains and management. Hepatogastroenterology. 1995;42:323–331. [PubMed] [Google Scholar]
- 33.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 34.Nagino M, Kamiya J, Kanai M, et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000;127:155–160. [DOI] [PubMed] [Google Scholar]
- 35.Gazzaniga GM, Filauro M, Bagarolo C, et al. Surgery for hilar cholangiocarcinoma: an Italian experience. J Hepatobiliary Pancreat Surg. 2000;7:122–127. [DOI] [PubMed] [Google Scholar]