Abstract
Objective:
To evaluate the first 100 adult right lobe living donor liver transplants (LDLT) in a single center to determine whether the results have improved with technical modifications and better experience.
Summary Background Data:
Right lobe LDLT has been increasingly performed for adults with end-stage liver disease. Numerous modifications in technique have been introduced, and a learning curve is likely in view of its complexity.
Methods:
One hundred consecutive adult right lobe LDLTs performed between May 1996 and May 2002 were retrospectively studied by comparing the first 50 (group 1) with the last 50 cases (group 2). The median follow-up was 37 (27 to 79) months for group 1 and 15 (7 to 27) months for group 2.
Results:
The characteristics of donors and liver grafts were similar. In group 2, fewer recipients were intensive care unit (ICU)-bound or had hepatorenal syndrome before transplantation, and there was a lower disease severity as shown by a lower Child-Pugh score and Model for End-Stage Liver Disease (MELD) score. Significant improvements were found in the operation time, blood loss, ICU stay, and postoperative complication rate of the donors and in the operation time, transfusion requirements, number of reoperations, ICU stay, and hospital stay of the recipients in group 2. The hospital mortality rate of recipients was reduced from 16% to 0% (P = 0.006). Graft survival rates at 12 months and 24 months were improved from 80% and 74%, respectively, in group 1 to 100% and 96%, respectively, in group 2 (P = 0.002). After adjusting for differences in recipient risk factors (ICU-bound, hepatorenal syndrome, Child-Pugh score, and MELD score) in a multivariate Cox model, recipients in group 2 had significantly lower risk of graft loss (relative risk compared with group 1, 0.13; 95% CI, 0.03 to 0.66; P = 0.014).
Conclusions:
There is a learning curve in adult right lobe LDLT. The results have significantly improved with technical refinement and better experience.
A single-center experience with 100 right lobe living donor liver transplants showed that the outcome of both donors and recipients have dramatically improved with better experience and skill. There is a learning curve in this operation.
The technique and practice of living donor liver transplantation (LDLT) have undergone major changes recently as a result of the extension of its application to adult recipients with the use of right lobe transplantation. Right lobe LDLT involves one of the most complicated and technically demanding surgical procedures and has created tremendous controversy with regard to the ethical issue of the safety of a healthy living donor.1 When the first series was reported in 1997,2 there was a high morbidity and reoperation rate in the recipients, and its role was poorly defined. Since then, the number of right lobe LDLTs has increased rapidly3,4 and numerous advances have been introduced. These included a better understanding of the minimum graft size requirement5,6 and anatomic variants of the right lobe,7,8 improved selection criteria for donors and recipients,9,10 as well as technical modifications particularly in venous outflow and biliary reconstruction.11–13 In addition, in view of the complexity of the procedure, it is likely that there will be a learning curve in the skill and experience of the transplant team. We have previously reported the impact of various modifications in technique and management on the results of right lobe LDLT.14–17 While each of these modifications might have contributed to the improvement in results, it is more important to evaluate the global effects of all these refinements together with the learning curve of the transplant team. The aim of the present study was to review our experience in the first 100 right lobe LDLTs performed at Queen Mary Hospital to determine how much the results have improved as a consequence of these advances in techniques, management, and experience.
METHODS
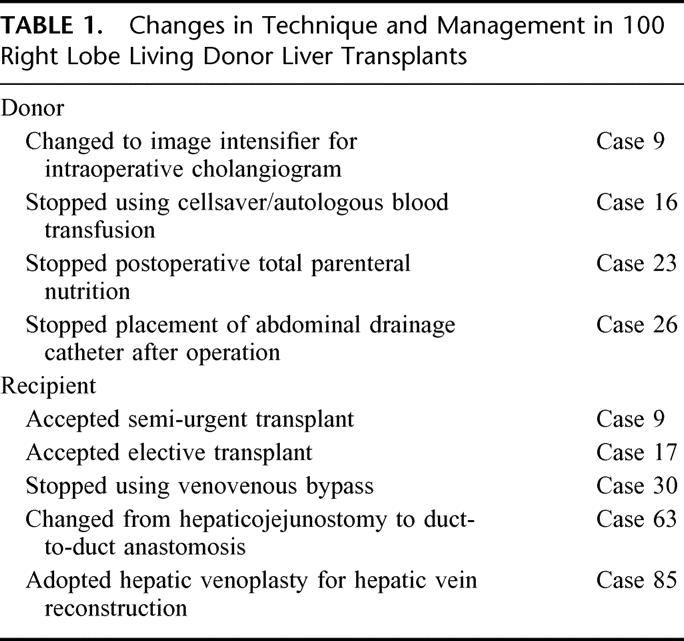
From May 1996 to May 2002, 100 LDLTs using right lobe grafts were performed at Queen Mary Hospital, Hong Kong. The same team of surgeons performed all the donor and recipient operations throughout this period. Our initial experience and techniques with the first 7 cases were reported in 1997.2 Since then, various modifications in our selection criteria, surgical techniques, and perioperative management of donors and recipients have been introduced (Table 1).
TABLE 1. Changes in Technique and Management in 100 Right Lobe Living Donor Liver Transplants
Recipient and Donor Selection
Unlike other transplant programs, we initiated our program of right lobe LDLT only in high-urgency patients who were intensive care unit (ICU)-bound before transplantation.18 The extremely high pretransplant mortality in such desperate situation provided the justification for the donor's risk. With the favorable results in the early experience, the technique was extended to semi-urgent hospital-bound patients since case 9 and to elective cases since case 17. The selection criteria and evaluation of the recipients were the same as those in cadaver donors; in general, we did not accept extended indications for LDLT. The donor selection criteria and evaluation had largely remained unchanged and the primary selection criterion was the donor's voluntary intent.18
Operative Techniques
In all except one case, the liver graft consisted of the whole right lobe (Couinard segments V, VI, VII, and VIII) with the inclusion of the middle hepatic vein (Fig. 1). In the only exception (case 85), a right lobe graft without the middle hepatic vein was harvested because the donor's left lobe was unusually small and a major branch of left hepatic vein joined the middle hepatic vein.19 Important modifications in the donor operation included the placement of a metal clip to mark the proposed site of right hepatic duct division before intraoperative cholangiogram with the use of image intensifier (from case 9),15 discontinuation of the use of cellsaver and autologous blood transfusion (from case 16), and discontinuation of the placement of abdominal drainage catheter (from case 26).
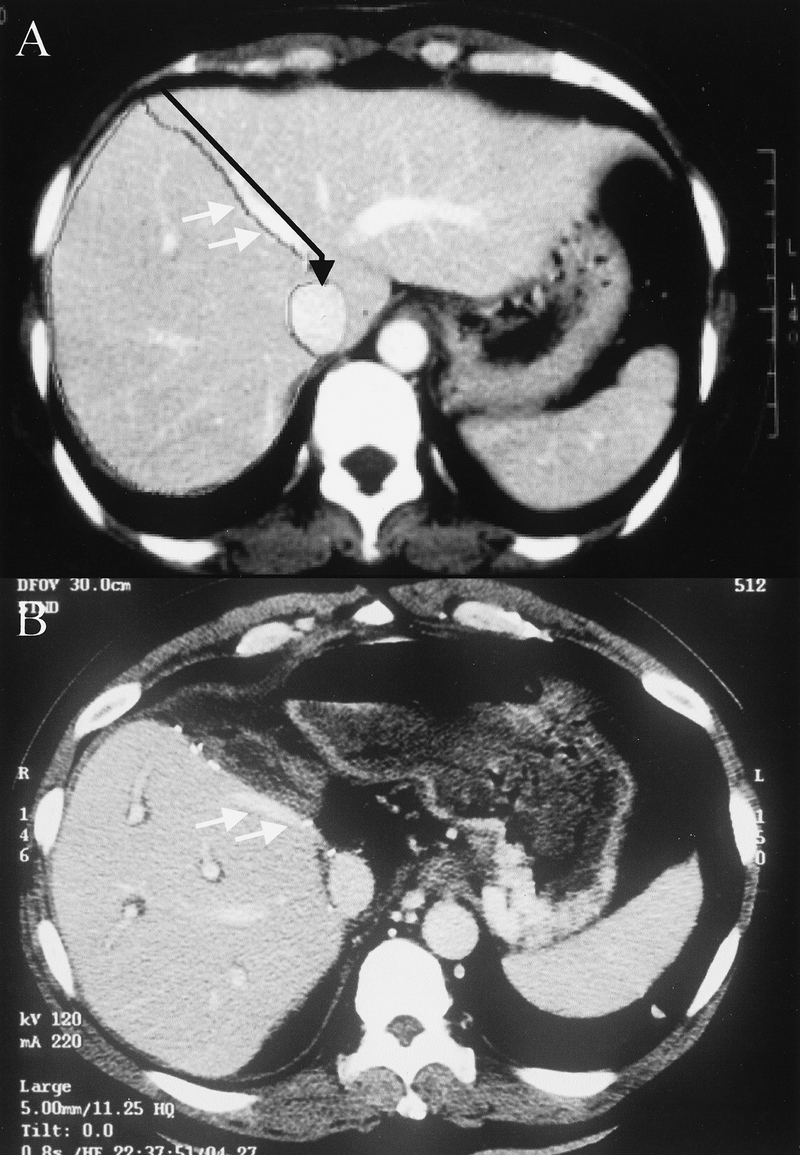
FIGURE 1. Computed tomography of (A) donor before transplantation and (B) recipient after transplantation. The line of resection (black arrow) is on the plane of the middle hepatic vein (white arrows) following almost exactly the dividing line between the right and left lobes of the liver as marked for preoperative volumetric study. The graft includes segments V, VI, VII, and VIII.
With regard to the recipient operation, venovenous bypass was routinely used initially; however, from case 30, all LDLTs were performed without venovenous bypass16 to avoid its complications and shorten the operation time. As a result of frequent biliary complications, biliary reconstruction was changed from hepaticojejunostomy to duct-to-duct anastomosis from case 63.15 A hepatic venoplasty technique was employed for hepatic venous outflow reconstruction since case 85.17 The right and middle hepatic veins of the graft were joined together to form a triangular cuff for a single anastomosis to the recipient's vena cava without the need of any interposition graft. With this technique, the recipient hepatectomy was simplified and venous outflow obstruction obviated.
Postoperative Management
In our early experience, postoperative total parenteral nutrition was routinely given to all donors, but this was discontinued from case 23. For the recipients, postoperative immunosuppression initially consisted of a triple regimen of cyclosporine, steroid, and azathioprine with the addition of OKT3 induction for patients with renal failure. Since case 8, the immunosuppression regimen has been changed to a double regimen of tacrolimus and steroid with the addition of mycophenolate mofetil and interleukin-2 receptor antagonist recently.
Statistical Analysis
For this study, we retrospectively compared the first 50 right lobe LDLTs (group 1) performed between May 1996 and September 2000 with the last 50 (group 2) performed between September 2000 and May 2002. This time period factor was taken to represent both the global effect of the refinements in technique and management as well as the learning curve of the transplant team. The statistical tests used included the Mann-Whitney U test, χ2 test, and Fisher exact test, and continuous variables were expressed as mean ± SEM. Graft survival and recipient survival rates were computed with life table and compared using log-rank test. The primary outcome measure was the graft survival. To adjust for the differences in recipient's pretransplant characteristics as a result of the changes in recipient selection between the 2 time periods, the graft survival of the 2 groups were compared with stratification according to risk factors that might be associated with a worse outcome. These risk factors included preoperative ICU-bound, presence of hepatorenal syndrome, a Child-Pugh score >9 and a Model for End-Stage Liver Disease (MELD)20 score >30. Finally, these risk factors together with the time period factor were put into a Cox proportional hazards model for multivariate analysis to determine whether the time period had an independent effect on the graft survival. The Child-Pugh score and MELD score were included as continuous variables; because these scores were applicable only to patients with chronic liver disease, recipients who received right lobe LDLT for fulminant hepatic failure were not included in this Cox model. Statistical analyses were performed by SPSS for Windows (SPSS, Inc, Chicago, IL) computer software program, and a P value < 0.05 was considered significant.
Hepatorenal syndrome was defined as oliguria and uremia in association with urine sodium level <10 mmol/L. MELD score before transplantation was calculated without adjustment for underlying etiology or malignancy. The standard liver weight of the recipients was calculated according to Urata's formula,21 and the graft weight was measured on the back table after flushing with University of Wisconsin solution. The recipient warm ischemic time was determined from the time the graft implantation was started to the time of portal reperfusion. Graft was considered to be lost if the recipient died or received retransplantation. No donor or recipient was lost to follow-up, and their follow-up status was continued until the end of December 2002. The median follow-up was 37 months (range, 27 to 79 months) for group 1 and 15 months (range, 7 to 27 months) for group 2, and all survivors had a minimum follow-up of 6 months. All data were collected prospectively by a research assistant.
RESULTS
Characteristics of Recipients
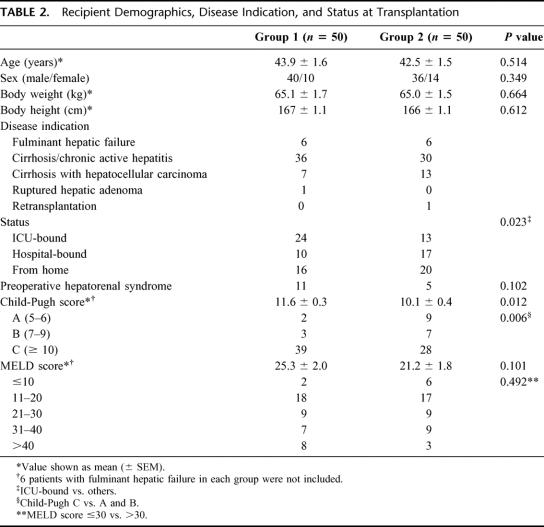
The demographics and disease indications for liver transplantation were comparable between the 2 groups (Table 2) except that there were more patients with hepatocellular carcinoma in group 2 (P = 0.134). Six patients in each group underwent LDLT because of fulminant hepatic failure. As a result of our initial policy of restricting right lobe LDLT to ICU-bound patients only, there were significant changes in the pretransplant status of the recipients. Fewer patients in group 2 were ICU-bound (24 patients versus 13 patients; P = 0.023) or had hepatorenal syndrome (11 patients versus 5 patients; P = 0.102) before transplantation. After excluding 6 patients in each group who underwent LDLT for fulminant hepatic failure, patients in group 2 had a less severe pretransplantation disease severity. The Child-Pugh score was significantly lower (11.6 ± 0.3 versus 10.1 ± 0.4; P = 0.012), and fewer patients were in Child-Pugh class C category (39 patients versus 28 patients; P = 0.006). The MELD score also tended to be lower (25.3 ± 2.0 versus 21.2 ± 1.8; P = 0.101).
TABLE 2. Recipient Demographics, Disease Indication, and Status at Transplantation
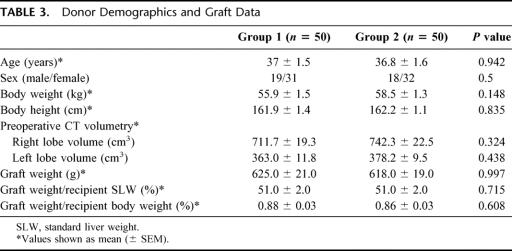
Characteristics of Donors and Liver Grafts
There was no significant difference in the donors’ demographics (Table 3). The body weight of the donor was less than that of the recipient in 36 cases in group 1 and 35 cases in group 2. The volume of the right lobe graft and that of the left lobe liver remnant as estimated by computed tomography with volumetry were similar in the 2 groups. The graft weight and its ratio to the recipients’ body weight or standard liver weight were also comparable.
TABLE 3. Donor Demographics and Graft Data
Operation Data
Donor Operation
There was a significant reduction in the operation blood loss (608 ± 47 mL versus 454 ± 34 mL; P = 0.02) and operation time (614 ± 20 minutes versus 486 ± 9 minutes; P < 0.001) in the donor operation of group 2. Despite the discontinuation of the use of cell saver and autologous transfusion, none of the donors in group 2 required transfusion of any blood products in the perioperative period.
Recipient Operation
There was a significant reduction in the recipient warm ischemic time (75 ± 5 minutes versus 61 ± 2 minutes; P = 0.001) and total operation time (942 ± 27 minutes versus 740 ± 16 minutes; P < 0.001). The use of various blood products was reduced by approximately 50% (Fig. 2). Red cell transfusion was reduced by 61%, fresh frozen plasma by 44% and platelets by 44%.
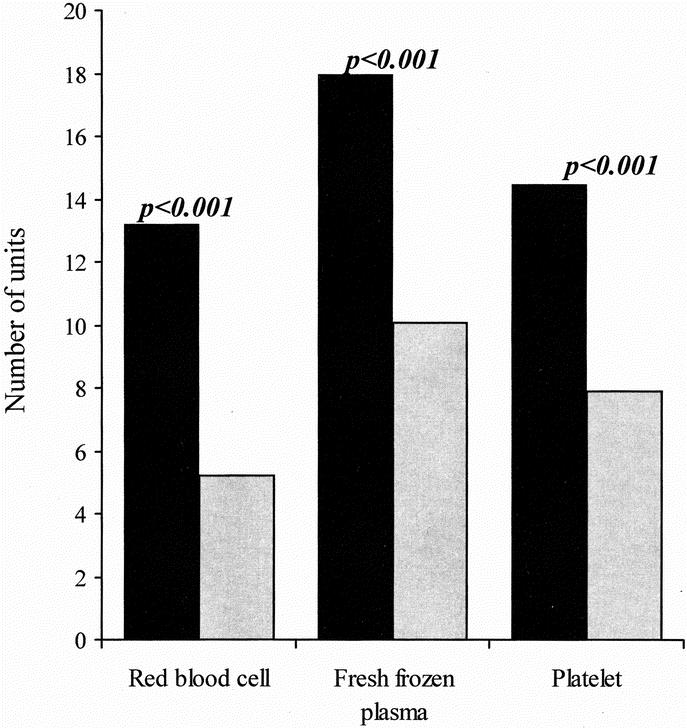
FIGURE 2. Comparison of the amount of blood products used during the recipient operation.
Outcome of Donors
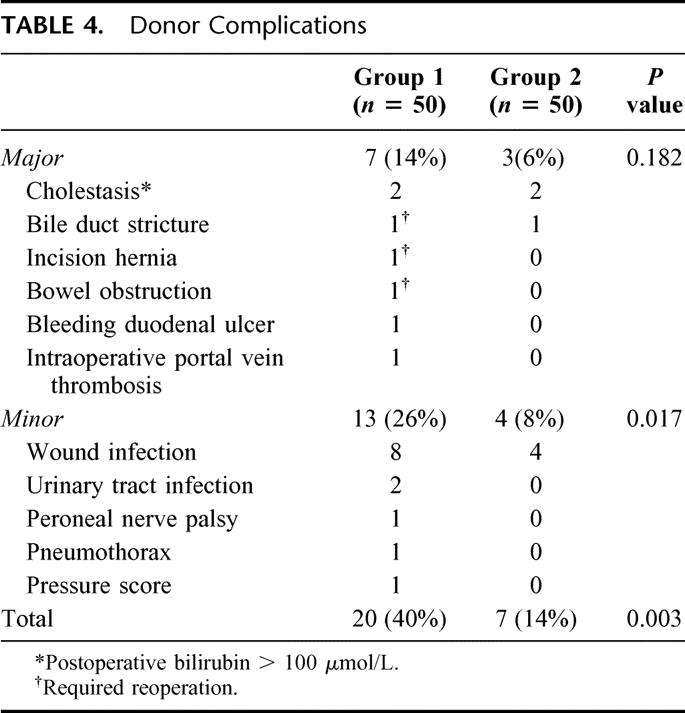
The major complication rate after donor operation was reduced from 14% to 6% (P = 0.182). Three donors in group 1 and none of the donors in group 2 required reoperation after discharge from hospital. A significant reduction was found in both the minor complication rate (26% versus 8%; P = 0.017) as well as the overall complication rate (40% versus 14%; P = 0.003) (Table 4). There was a 42% reduction in the ICU stay (2.4 ± 0.2 days versus 1.4 ± 0.1 day; P < 0.001) and a 19% reduction in hospital stay (12.4 ± 1.0 day versus 10 ± 0.5 day; P = 0.146).
TABLE 4. Donor Complications
Outcome of Recipients
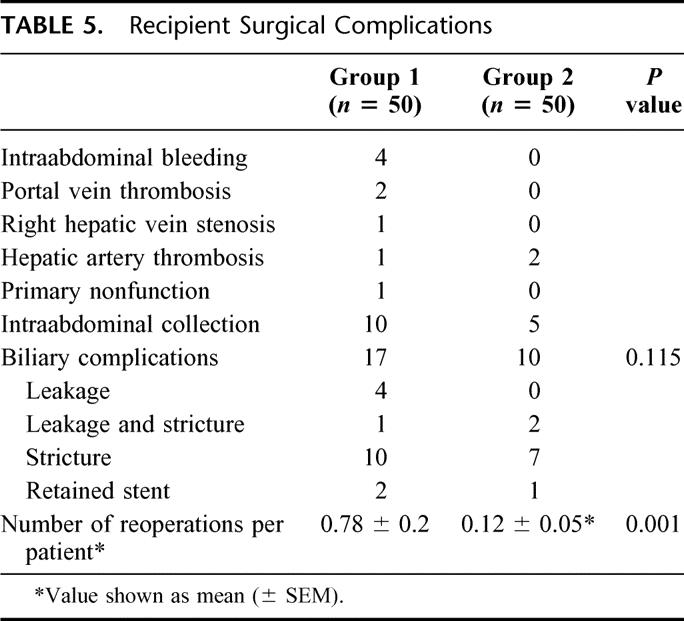
The postoperative surgical complications in the recipients (Table 5) were less frequent in group 2, and the number of reoperations per recipient was reduced by 85% (0.78 ± 0.2 versus 0.12 ± 0.05; P = 0.001). There were 3 retransplants using cadaver grafts in group 1 (primary nonfunction, 1; portal vein thrombosis, 2) and 1 using a right lobe graft in group 2 (recurrent hepatitis B, 1), giving an overall retransplant rate of 4%. Eight patients (16%) in group 1 died in hospital, but there was no hospital mortality (0%) in group 2 (P = 0.006). The postoperative ICU stay was reduced by 50% (10.8 ± 1.7 days versus 5.4 ± 0.9 day; P = 0.001) and the hospital stay by 32% (39.4 ± 4.9 days versus 27 ± 2.5 days; P = 0.038).
TABLE 5. Recipient Surgical Complications
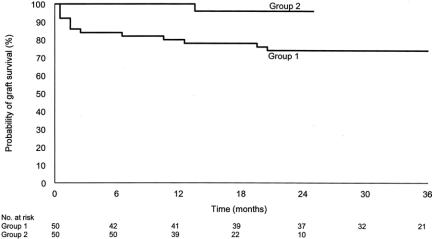
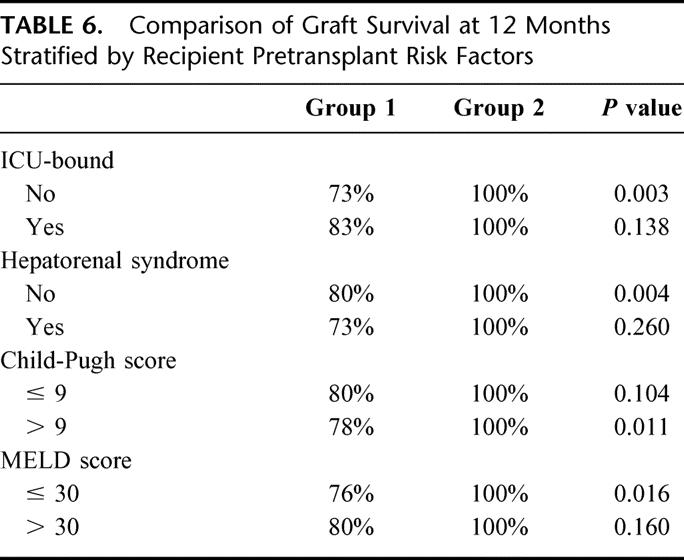
The graft survival rates at 12 months and 24 months were 100% and 96%, respectively, for group 2 compared with 80% and 74%, respectively, for group 1 (P = 0.002) (Fig. 3). The corresponding recipient survival rates were both 100% for group 2 compared with 80% and 74%, respectively, for group 1 (P < 0.001). Comparison of graft survival between the 2 groups stratified according to Child-Pugh score, MELD score, preoperative ICU-bound, and presence of hepatorenal syndrome revealed that the graft survival had similar improvement in almost every subgroup. Six patients with fulminant hepatic failure in each group (5 in group 1 and 6 in group 2 were alive at the latest follow-up) were then excluded from further analysis of graft survival, which aimed at adjusting for pretransplantation severity of chronic liver disease. With multivariate analysis, the time period factor in group 2 was the only independent predictive factor for graft survival in the final Cox model. Patients who received right lobe LDLT in group 2 had a relative risk of graft loss of 0.13 (95% CI, 0.03 to 0.66; P = 0.014) compared with those in group 1, thus confirming the improvement in results irrespective of the patient factors.
FIGURE 3. Comparison of graft survival between groups 1 and 2. (log-rank test P = 0.002).
DISCUSSION
The results of this study show that the outcome of both donors and recipients in right lobe LDLT had significantly improved in a single center experience with the first 100 cases performed over a 6-year period. When the second 50 cases were compared with the first 50 cases, the risk of graft loss was reduced by almost 90%, and this was independent of the recipient's risk factors of disease severity. In addition, there were significant reductions in postoperative complications and a better utilization of hospital resources in both the donor and recipient operations. This time period factor represented both the global effects of the modifications in technique and management as well as the learning curve of the transplant team. The modifications in technique and management were quantifiable, but the learning curve effect related to the better skill and experience of the transplant team was not. While we have arbitrarily divided the patients into 2 equal halves for the purpose of the present study, it is important to recognize that the improvement must be a continuous process.
Subjecting a healthy donor to the risks of life-threatening surgery can be justified only when the potential recipient has a compelling need for an early liver transplant, and this in turn is reflected by the pretransplantation mortality. Medical urgency and the severity of the underlying liver disease are predictors of pretransplantation mortality.20,22 Unfortunately, pretransplantation disease severity also affects posttransplantation survival. The presence of hepatorenal syndrome,23 the need for ICU care before transplantation,24,25 or a high MELD score26 or Child-Pugh score24 have been shown to be indicators of a poor outcome after liver transplantation. The effect may potentially be even more significant in right lobe LDLT because of the issue of graft size requirement.10,27 As a result of the acceptance of semi-urgent and subsequently elective patients for right lobe LDLT, recipients in group 2 had lower disease severity. The selection of better-risk recipients must have contributed to the improvement in hospital resource utilization such as blood product transfusion and postoperative ICU or hospital stay of recipients. Nonetheless, analysis of the graft survival stratified according to each of these risk factors showed that the graft survival rate at 12 months was close to 80%, even in the poor-risk recipients in the early experience, and the improvement in survival was comparable. By adjusting for recipient risk factors in a Cox model, the time period factor remained the only independent factor that accounted for the better survival rate. The improvement was therefore genuine.
The safety of the donor has been the crux of discussion in right lobe LDLT. A survey on the outcome of 1508 living donors in 5 transplant centers in Asia showed that complications tended to be more common and serious in right lobe donors.4 Another survey from the United States of 449 right lobe liver donors revealed a biliary complication rate of 6%, reoperation rate of 4.5%, and a mortality rate of 0.2%.28 The present study showed that even for hepatobiliary surgeons with extensive experience in hepatic resection, there was a learning curve in this complicated donor operation. To simplify the donor operation and to avoid the possibility of procedure-related risks, we had stopped using cellsaver, autologous blood transfusion, abdominal drainage, and postoperative total parenteral nutrition at various stages. Despite these, no donor required blood transfusion because the average operation blood loss was reduced to less than 500 mL in the last 50 cases. The major complication rate was reduced to 6%, and there was no reoperation. The significant reduction in the incidence of minor complications, particularly wound infection, was most likely related to the reduction in operation time.
The technique of cadaver liver transplantation has been well-established, and new programs are usually established by transplant surgeons with adequate training and experience without having to go through the learning curve in the past.29 In contrast, right lobe LDLT is a novel procedure with a relatively short history. For transplant centers including ours that pioneered this operation, a learning curve is not unexpected. Even for transplant centers that are going to start LDLT programs in the near future, a learning curve is still likely because the technique of right lobe LDLT has yet to be standardized and formal training in LDLT is lacking. The exact number of operations required for this learning curve is not defined, and the use of 50 cases in the present study was admittedly arbitrary. It would vary widely according to the transplant team's experience in both liver transplantation and hepatic resection. Apart from the learning curve effect, the volume-on-outcome effect in liver transplantation30 is likely to be more significant in right lobe LDLT because of its greater complexity. In a recent survey on 42 centers that had performed adult LDLT in the United States up to the end of 2000,28 only 14 centers had performed more than 10 such procedures each, and complications in the donors were more frequent in the centers performing the fewest operations. Nonetheless, 32 of 42 centers that had not performed such a transplantation planned to start doing so within the next 12 months. A case control study of 728 LDLTs in adults reported to UNOS showed that recipients of LDLT had more stable liver disease but the graft survival at 2 years was 64.4% only compared with 73.3% for cadaver liver grafts.31 Many of the LDLT programs are probably still in the very early learning phase, and the continuous proliferation of programs will dilute the volume of each other and delay the completion of this process. To facilitate a more rapid learning curve, it may be worthwhile to identify a limited number of centers of excellence with the best field strength for this challenging operation.
As far as graft survival is concerned, the progress in the advance of right lobe LDLT in our experience is reaching a steady state. The refinements in technique and skill have minimized various postoperative complications and have resulted in an excellent graft survival rate of over 90% even in the high-risk recipients (Table 6). The major technical hurdle in the recipients, however, is biliary complication. Our results indicated that biliary complications remained a common problem after right lobe LDLT, even in the latter part of our experience. Further studies are needed to continue to address this issue.
TABLE 6. Comparison of Graft Survival at 12 Months Stratified by Recipient Pretransplant Risk Factors
In conclusion, through the lessons learned in our first 100 right lobe LDLTs, we have dramatically improved the results of this operation. There is a learning curve in right lobe LDLT and liver transplant centers planning to start LDLT programs should take this into consideration.
Footnotes
Reprints: Professor Chung-Mau Lo, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong. E-mail: chungmlo@hkucc.hku.hk.
REFERENCES
- 1.Surman OS. The ethics of partial-liver donation. N Eng J Med. 2002;346:1038. [DOI] [PubMed] [Google Scholar]
- 2.Lo CM, Fan ST, Liu CL, et al. Adult-to-Adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trotter JF, Wachs M, Everson GT, et al. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. [DOI] [PubMed] [Google Scholar]
- 4.Lo CM. Complications and long-term outcome of living liver donors: a survey of 1508 cases in five Asian centers. Transplantation. 2003;75:S12–S15. [DOI] [PubMed] [Google Scholar]
- 5.Lo CM, Fan ST, Liu CL, et al. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999;68:1112–1116. [DOI] [PubMed] [Google Scholar]
- 6.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismaatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–327. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Tanaka K, Kiuchi T, et al. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation. 2002;73:1896–1903. [DOI] [PubMed] [Google Scholar]
- 8.Marcos A, Ham JM, Fisher RA, et al. Surgical management of anatomical variations of the right lobe in living donor liver transplantation. Ann Surg. 2000;231:824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcos A, Fisher RA, Ham JM, et al. Selection and outcome of living donors for adult to adult right lobe transplantation. Transplantation. 2000;69:2410–2415. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Haim M, Emre S, Fishbein TM, et al. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient's disease. Liver Transpl. 2001;7:948–953. [DOI] [PubMed] [Google Scholar]
- 11.Lee SG, Park KM, Hwang S, et al. Modified right liver graft from a living donor to prevent congestion. Transplantation. 2002;74:54–59. [DOI] [PubMed] [Google Scholar]
- 12.Ishiko T, Egawa H, Kasahara M, et al. Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg. 2002;236:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grewal HP, Shokouh-Amiri MH, Vera S, et al. Surgical technique for right lobe adult living donor liver transplantation without venovenous bypass or portocaval shunting and with duct-to-duct biliary reconstruction. Ann Surg. 2001;233:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg. 2000;231:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan ST, Lo CM, Liu CL, et al. Biliary reconstruction and complications of right lobe live donor liver transplantation. Ann Surg. 2002;236:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan ST, Yong BH, Lo CM, et al. Right lobe living donor liver transplantation with or without veno-venous bypass. Br J Surg. 2003;90:48–56. [DOI] [PubMed] [Google Scholar]
- 17.Lo CM, Fan ST, Liu CL, et al. Hepatic venoplasty in living-donor liver transplantation using right lobe graft with middle hepatic vein. Transplantation. 2003;75:358–360. [DOI] [PubMed] [Google Scholar]
- 18.Lo CM, Fan ST, Liu CL, et al. Applicability of living donor liver transplantation to high-urgency patients. Transplantation. 1999;67:73–77. [DOI] [PubMed] [Google Scholar]
- 19.Fan ST, Lo CM, Liu CL, et al. The safety and necessity of including the middle hepatic vein in the right lobe graft in adult-to-adult live donor liver transplantation. Ann Surg. 2003;238:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 21.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 22.Freeman RB Jr, Edwards EB. Liver transplant waiting time does not correlate with waiting list mortality: implications for liver allocation policy. Liver Transpl. 2000;6:543–552. [DOI] [PubMed] [Google Scholar]
- 23.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–1185. [DOI] [PubMed] [Google Scholar]
- 24.Baliga P, Merion RM, Turcotte JG, et al. Preoperative risk factor assessment in liver transplantation. Surgery. 1992;112:704–710. [PubMed] [Google Scholar]
- 25.Eghtesad B, Bronsther O, Irish W, et al. Disease gravity and urgency of need as guidelines for liver allocation. Hepatology. 1994;20:56S–62S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onaca NN, Levy MF, Sanchez EQ, et al. A correlation between the pretransplantation MELD score and mortality in the first two years after liver transplantation. Liver Transpl. 2003;9:117–123. [DOI] [PubMed] [Google Scholar]
- 27.Testa G, Malago M, Nadalin S, et al. Right-liver living donor transplantation for decompensated end-stage liver disease. Liver Transpl. 2002;8:340–346. [DOI] [PubMed] [Google Scholar]
- 28.Brown RS Jr., Russo MW, Lai M, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–825. [DOI] [PubMed] [Google Scholar]
- 29.Busuttil RW, Shaked A, Millis JM, et al. One thousand liver transplants. The lessons learned. Ann Surg. 1994;219:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards EB, Roberts JP, McBride MA, et al. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med. 1999;341:2049–2053. [DOI] [PubMed] [Google Scholar]
- 31.Yoo HY, Thuluvath PJ. Outcome of living donor liver transplantation (LDLT) in adults: A case controlled study using UNOS data (abstract). Hepatology. 2002;36:305A:568.