Abstract
Objective:
We sought to reduce the high incidence of abdominal wall incisional hernias using sustained release growth factor therapy.
Summary Background Data:
Incisional hernias complicate 11% of abdominal wall closures, resulting in 200,000 incisional hernia repairs in the United States each year. Mechanical improvements alone in mesh, suture material, and surgical technique have failed to reduce the high rate of fascial wound failure.
Methods:
Sprague-Dawley rats underwent midline celiotomies that were closed with fast-absorbing suture to induce early biomechanical wound failure and incisional hernia formation. In primary wounds, fascial incisions were closed adjacent to a continuous release polygalactone polymer rod containing basic fibroblast growth factor (bFGF), no growth factor (control-rod), or without rods. In a second group, incisional hernias were repaired with either bFGF or control-rod therapy. Breaking strength was measured on postoperative day (POD) 7, and the incidence of incisional hernia formation was determined on POD 28.
Results:
Treatment with bFGF rods significantly increased fascial wound breaking strength. In the “hernia-prevention” experiments, incisional hernias developed in 90% of untreated incisions, 60% of control-rod incisions, and only 30% of bFGF-rod incisions (P < 0.05). In the “hernia-treatment” experiments, recurrent incisional hernias developed in 86% of control-rod incisions compared with only 23% of bFGF-rod treated incisions (P < 0.05). Immunohistochemistry demonstrated increased angiogenesis and collagen protein production in bFGF treated incisions.
Conclusion:
The treatment of abdominal fascial incisions with a sustained-release bFGF polymer significantly lowered the incidence of incisional hernias and the recurrence rate after repair.
Fascial incisions were repaired adjacent to a delayed release source of basic fibroblast growth factor (bFGF) in a rat model of abdominal wall wound failure. bFGF therapy significantly reduced both primary and recurrent incisional hernia formation. bFGF induced increased angiogenesis, collagen production, and breaking strength within the abdominal wall fascial wounds.
Prospective, well-controlled studies find that incisional hernias complicate 11% of all abdominal wall closures after celiotomy.1,2 In as many as 1 in 3 abdominal wall closures, the fascial layer of the wound will fail to heal reliably in patients operated upon for aortic aneurysm disease, during periods of hemodynamic instability or in those with gross contamination of the deep wound space, especially in malnourished patients.3 As a result, approximately 200,000 incisional hernia repairs are performed each year in the United States alone at a financial cost of nearly 2.5 billion dollars.4,5 The cost in terms of prolonged recovery times and patient disability is more difficult to measure.6 With nearly 4 million abdominal and pelvic operations performed each year in the United States, it is estimated that another 200,000 incisional hernias may be going unrecognized or untreated.
Incisional hernias occur as the result of combined biomechanical failure in an acute fascial wound when considering the clinically relevant impediments to acute tissue repair together with the normal function of the abdominal wall to support increasing loads during the postoperative recovery period.7 Most studies now support the theory that acute fascial separation occurs early in the postoperative period, leading to the delayed clinical development of abdominal wall incisional hernias.8 This phenomena occurs early in the trajectory of acute wound healing at a time when wound tensile strength is very low or absent (postoperative days 0–30).9 It is during the earliest period of acute wound healing that the wound depends entirely on suture integrity to maintain abdominal wall closure. Simultaneously, most patients are recovering from their procedures and returning to increased levels of activity and placing increasing loads across the acute wound during its weakest phase. The most frequently identified clinical risk factors for fascial wound failure and primary incisional hernia formation include a suboptimal closure technique, deep wound infections, malnutrition, perioperative hypotension, steroid use, and aortic aneurysm disease.3,10,11
The fundamental biologic mechanism of fascial wound failure leading to incisional hernia formation is unknown. Normally, an acute fascial wound needs to pass through a complex series of well-orchestrated molecular and cellular events beginning with hemostasis and inflammation and leading through angiogenesis and fibroplasia until a provisional matrix is formed that is capable of resisting the distractive forces of the abdominal wall.12 The end point of acute wound healing therefore is the nearest approximation of normal uninjured tissue structure and function. In the case of the abdominal wall, this means the timely reestablishment of an efficient load-bearing scar at the myofascial layer.
Abnormal progression of the acute wound-healing trajectory impairs the recovery of wound tensile strength.9 A biologic intervention at the host:wound level designed to optimize fascial healing may prevent and/or treat incisional hernias.13 To date, systematic studies have been difficult owing to the lack of a reproducible animal model of incisional hernias. The mechanism of incisional hernia formation is most often attributed to early mechanical wound failure as a result of either to the pulling through of suture passed through adjacent wound tissue, too loose or too-tight suture placement, or suture failure all occurring at a time when wound tensile strength is essentially zero.7 A model of incisional hernia formation was therefore designed with the intentional early failure of the fascial suture.7 In this rodent model, there is progressive midline abdominal wall wound healing failure that reliably results in acute fascial separation early in the postoperative period. The incisional hernias that develop have a well-defined hernia ring, hernia sacs, and visceral adhesions, all characteristic of the incisional hernias that clinically develop in humans.14
The improved understanding of the cellular and molecular mechanisms activated during acute tissue repair now allows the application of biologic approaches to the problem of incisional hernia formation. Biologically augmenting growth factor levels during the lag period of acute fascial repair may prevent or treat incisional hernias. Endogenous tissue growth factors are an important class of repair signaling peptides up-regulated during the inflammatory phase of acute wound healing. Growth factors recruit and activate the cellular and molecular components necessary for the fibroproliferative burst that characterizes the log phase of acute wound healing. Acute wound therapy with proliferative growth factors is known to accelerate the appearance of fibroblasts and collagen into the wound thereby shortening the natural lag-phase for gain in injured tissue tensile strength.13,15 Several reports have demonstrated the fibroproliferative and prominent angiogenesis-stimulating ability of bFGF in the dermis.16 The current study was designed to determine whether bFGF accelerates fascial incisional wound healing thereby reducing the incidence of incisional hernia formation in a model of progressive midline fascial wound failure.
MATERIALS AND METHODS
Incisional Hernia Model
The hernia model is illustrated in Fig. 1A. After intraperitoneal injection of ketamine (87 mg/kg) and xylazine (13 mg/kg) for anesthesia, the ventral abdominal wall hair was shaved with electric clippers and the field prepped with alcohol and Betadine solution. A 6- × 3-cm full-thickness dermal incision was placed 2 cm lateral to the ventral midline and a rectangular skin flap raised through the avascular prefascial plane, thereby separating the skin incision from the underlying fascia, exposing the linea alba. A 1:2 ratio of flap length to width was maintained to prevent dermal flap ischemia. The design of this ventral abdominal wall dermal flap model allows acute fascial healing to occur isolated from the overlying dermis. A 5-cm full-thickness myofascial incision was then placed in the midline and closed with 5-O plain catgut suture placed across the wound with 0.5-cm bites. Previous experience with this model predicts an 80% incidence of fascial incisional hernias after 28 days.7 The skin flap was then replaced and the dermal incisions closed with skin clips placed 1 cm apart. After 30 minutes of recovery under heat lamps, the rats were returned to individual cages.
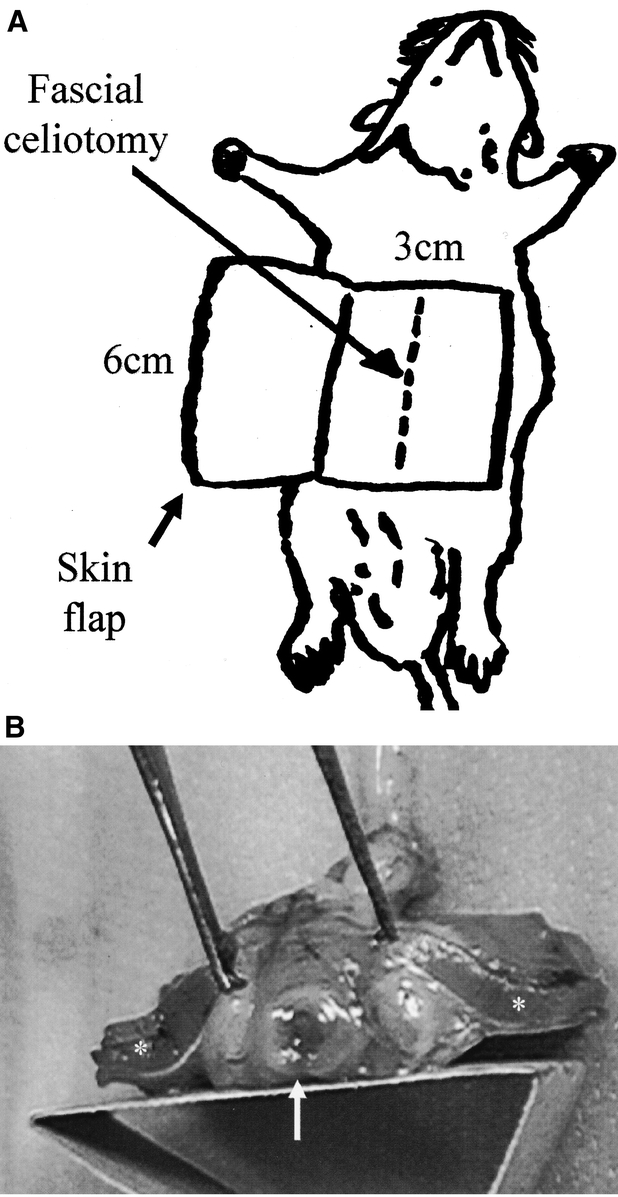
FIGURE 1. A, The ventral abdominal wall incisional hernia model. A 5-cm celiotomy incision is placed through the fascia of the midline linea alba and it is repaired with a single, 5-O plain catgut suture. In the untreated rat, rapid absorption of the catgut suture results in intentional acute fascial wound failure and herniation. Previous experience with this model predicts an 80% incidence of fascial incisional hernias after 28 days without biologic intervention. B, In the polygalactone rod-treated groups, the polymer was incorporated to the ventral edge of the fascial wound closure. The overlying 6-cm wide by 3-cm long skin flap is known to be viable.14 It functions to isolate the fascial wound from the dermal wounds and to secure abdominal contents into a hernia sac.
Study Design
Using the rat incisional hernia model, 70 male Sprague-Dawley rats weighing 275–300 g (Harlan Laboratories, Indianapolis, IN) were acclimated to laboratory conditions for 7 days and fed a standard rat chow. All procedures were performed with the prior approval of the Ann Arbor Veterans Affairs Medical Center Institutional Animal Care and Use Committee.
The first set of experiments was designed to test the activity of basic fibroblast growth factor (bFGF) in preventing the development of incisional hernias. Animals were randomly assigned into 1 of 3 groups. In the first “hernia prevention” group (n = 20), a pliable, delayed-release polygalactone polymer-rod formulation of basic fibroblast growth factor (bFGF-rod; 10 μg, Scios Inc, Sunnyvale, CA) was sewn to the ventral edge the primary fascial wound closure (Fig. 1B). Each rod was 5 cm in length and 1 mm in diameter. Previous experience in our laboratory established 10 μg as the optimal dose to stimulate the angiogenic and fibroplastic properties of bFGF in vivo (M. Franz, unpublished data). A growth factor delivery control group (n = 20) had rods without bFGF incorporated into the fascial closure (empty rod control). In the hernia model control group (n = 30), no rods were placed (hernia model control). The incidence of primary incisional hernia was determined during necropsies performed on postoperative day (POD) 28 on 10 animals each in the bFGF rod treatment and empty rod control groups.
The recovery of fascial wound mechanical strength was measured on POD 7 using 10 rats each from the bFGF-rod treatment and empty-rod control groups. The rats were killed with an overdose of an intraperitoneal injection of nembutal (100 mg/kg) and the entire ventral abdominal wall was excised. After separation of the skin, the musculofascial layer was divided into 3 1-cm wide by 4-cm long strips cut perpendicular to the fascial wound for biomechanical analysis. Breaking strength analysis was performed on the fascial wound strips using an Instron pneumatic tensiometer (Model MN44, Instron Corporation, Canton, MA) set at a crosshead speed of 10 mm/min. The breaking strength was defined as the force in Newton's required to rupture the resultant incisional scar. Blood samples were obtained at necropsy and centrifuged for ELISA immunoassay analysis of serum bFGF levels (R&D Systems Inc. Minneapolis, MN).
The second set of experiments was designed to test whether bFGF augments the acute fascial repair of mature incisional hernias. Twenty-seven incisional hernias developed in the hernia model control group of 30 rats. The hernias were reduced and fascial defects repaired. The rats were then randomized to receive either bFGF rod “hernia treatment” (n = 13) or control empty-rod therapy (n = 14). The incidence of recurrent incision hernias was determined during necropsies performed 28 days after hernia repair.
All hernia repair rats were killed on POD 28 by intraperitoneal nembutal overdose (100 mg/kg). The entire ventral abdominal wall was then excised and the skin was separated from the musculofascial layer. The peritoneal and subcutaneous surfaces of each abdominal wall were carefully inspected for the presence of herniated abdominal contents. A minimum of 2-mm fascial separation was required for a wound to be scored as containing an incisional hernia. Sagittal fascial wound and/or hernia sections were then cut and immediately fixed in 10% neutral-buffered formalin in preparation for histologic analysis. Several 2-mm biopsies were taken of the fascial: fascial interface or hernia ring were snap frozen in liquid nitrogen for gene expression analysis.
Histology and Immunohistochemistry
POD 28 normal and herniated abdominal wall wound sections were fixed in formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin, trichrome, or antibodies specific for rat collagen type I (Chemicon International, Inc., Temecula, CA).
Inflammatory cell infiltrate and wound angiogenesis were measured using hematoxylin and eosin and trichrome staining. The intensity of wound collagen type 1 deposition was measured by quantifying the digital intensity of staining over 3 HPF using computer-aided image analysis software (Scion Image, Scion Corp., Bethesda, MD).
Collagen mRNA Expression
Total RNA was extracted from homogenized fascial specimens using TRIZOL reagent. After extraction with chloroform and ethanol precipitation, the RNA pellet was resuspended in 20 μL of diethypyrocarbonate (DEPC)-treated water. The RNA solution was incubated for 10 minutes at 60°C and 2 μL of RNAse inhibitor (10 μ/μL) was added into each tube. One microliter of RNA solution was taken and diluted into 100 μL with DEPC water for spectrophotometric verification of RNA presence.
All reverse transcript (RT) reactions were performed simultaneously using a master mix to eliminate variability and ensure fidelity of RT efficiency. cDNA was synthesized in 50-μL volumes, which contains 2 μg of total RNA, 0.5 μg 0ligo (dT),12–18 0.01 M DTT, 200 μM of each dNTP, and 200 U/μg of RNA Molony murine leukemia virus reverse transcription. The reaction was incubated at 25°C for 10 minutes, 37°C for 60 minutes, and 92°C for 5 minutes. Collagen I and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were designed using conserved sequences from published GenBank complete and partial mRNA sequences of various species. PCR amplification was conducted in a 50-μL reaction volume containing 5 μL of RT reaction, 25 μL of the 2X Taq ReadyMix (Sigma, St. Louis, MO), and 50 pmol of each 3′ and 5′ PCR primer. Amplification reactions were conducted in 35 sequential cycles of 94°C for 1.5 minutes, 58°C for 2 minutes, and 72°C for 3 minutes in a GeneAmp PCR system 96 (Perkin Elmer, Norwalk, CT). PCR products were separated on 2% agarose gel containing 0.1 μg/μL ethidium bromide and photographed using Polaroid type 667 black and white film (Sigma, St. Louis, MO). Photographs were scanned using a Hewlett-Packard flat scanner and stored as BMP files using Microsoft Editor. Scion Image (Scion Corporation, Frederick, MD) was used to determine the ratio of intensity of each band relative to GAPDH. RT-PCR was quantified in the linear range of PCR products for each molecule.
Statistical Analysis
Data were analyzed using SigmaStat software (Jandel Scientific, Corte Madera, CA). The Fisher exact test was used to determine differences in the incidence of incisional hernias. Student t test or analysis of variance was used to determine differences in cell count, collagen staining, angiogenesis, and collagen mRNA band intensity after RT-PCR. P values of < 0.05 were considered significant.
RESULTS
bFGF Delivery
Sustained delivery of bFGF was documented 7 days after surgical implantation. Analysis of POD 7 serum revealed a significant increase in systemic bFGF delivery compared with controls (0.033u ± 0.017 bFGF rod treatment, 0.012u ± 0.0097 empty rod control, and 0.010u ± 0.008 hernia model control, P < 0.05).
Breaking Strength
All wounds mechanically disrupted at the fascial: fascial interface. Wounds treated with bFGF-impregnated rods developed increased breaking strength in the early postoperative fascial incision. The bFGF rod-treated animals breaking strength was 12.29N ± 2.98 versus 8.61N ± 3.17 in the empty rod control animals (P < 0.05; Fig. 2).
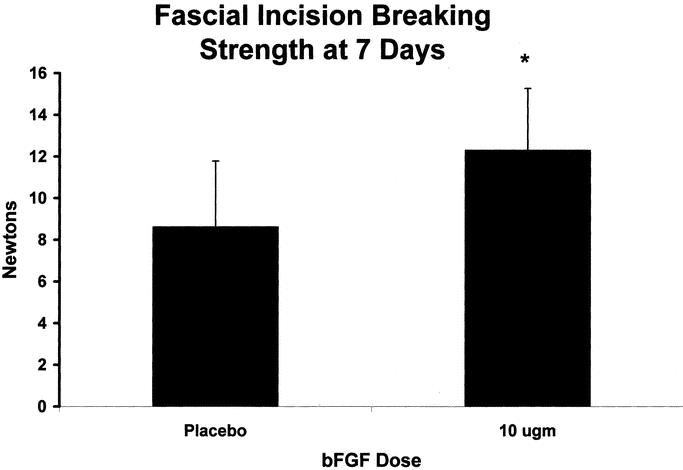
FIGURE 2. Fascial breaking strength was measured with an Instron Tensiometer on POD 7. Values are the mean ± SEM of 10 wound biopsies each from the vehicle control and the bFGF-treated groups.
Prevention of Incisional Hernia Development With bFGF
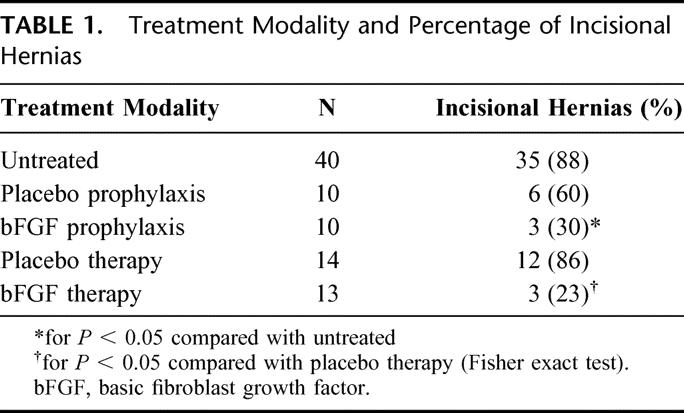
Incisional hernia formation was significantly reduced with bFGF rod therapy. Incisional hernias developed in 90% (27/30) of control incisions compared with 60% (6/10) in empty rod control-treated incisions and only 30% (3/10) of the bFGF-impregnated rod-treated incisions after 28 days (P < 0.05; Table 1). Full-thickness fascial sectioning perpendicular to the wound revealed the polymer rod incorporated into the provisional matrix. There were no deaths during the study period and no evidence of evisceration, intestinal incarceration, obstruction, or strangulation. There were no obvious systemic effects of the bFGF observed in the postoperative period or at necropsy.
TABLE 1. Treatment Modality and Percentage of Incisional Hernias
Treatment of Mature Incisional Hernias With bFGF
Incisional hernia recurrence was significantly reduced with bFGF rod therapy. The 27 incisional hernias that developed in the hernia model control group were operatively repaired 28 days after the initial procedure. Before repair, the rats were randomized to receive the 10 μg of bFGF polymer or placebo polymer incorporated into the fascial closure. After another 28 days, 86% (12/14) of the empty rod control-treated hernia repairs developed recurrent hernias compared with only 23% (3/13) of the bFGF-treated group (P < 0.05; Table 1).
Inflammatory Infiltrate
Basic histology demonstrated rods were still present, although approximately 50% smaller than when initially applied to the wound demonstrating a slow absorption pattern of the polygalactone rod polymer. There was no difference in size between the bFGF rod and empty rod absorption or incorporation into the wound. There was a modest nongranulomatous inflammatory infiltrate surrounding the bFGF and empty rod polymers. Hematoxylin and eosin staining suggested a gradient effect for polymorphonuclear leukocytes and macrophages in the bFGF-treated incisions.
Angiogenesis
Grossly, there was a visible increase in granulation tissue deposition adjacent to the bFGF rods, whereas the control animal fascial incisions were pale in comparison. Dense capillary and provisional matrix deposition was observed in bFGF-treated incisions, resulting in macroscopic nodules similar to small hemangiomas. Microscopic neovascularization was most pronounced adjacent to the bFGF rods and decreased as a function of distance from the rod (Fig. 3).
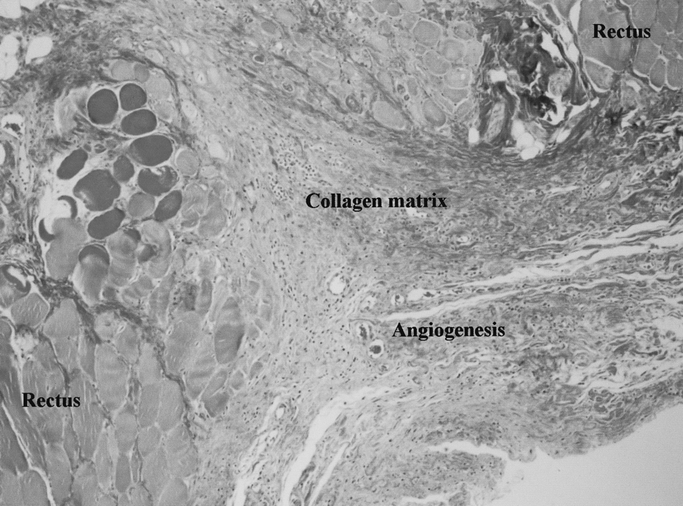
FIGURE 3. Prominent angiogenesis in the bFGF-treated healing fascial incision demonstrated on this trichrome-stained photomicrograph taken adjacent to a bFGF-impregnated polyglactone rod.
Collagen Production
Basic FGF had no significant effect on wound collagen mRNA levels by POD 28 (Fig. 4). Histology and immunohistochemistry, however, demonstrated a gradient effect for fibroblast chemotaxis and collagen protein production in bFGF-treated incisions. Collagen I staining was significantly increased adjacent to the polymer (103.72u ± 1.70 vs. 24.42u ± 6.56, P < 0.01; Fig. 5). This intense collagen staining decreased as a function of distance from the rod suggesting either down-regulation of collagen gene expression by POD 28 or enhanced posttranscriptional collagen synthesis stimulated by bFGF.
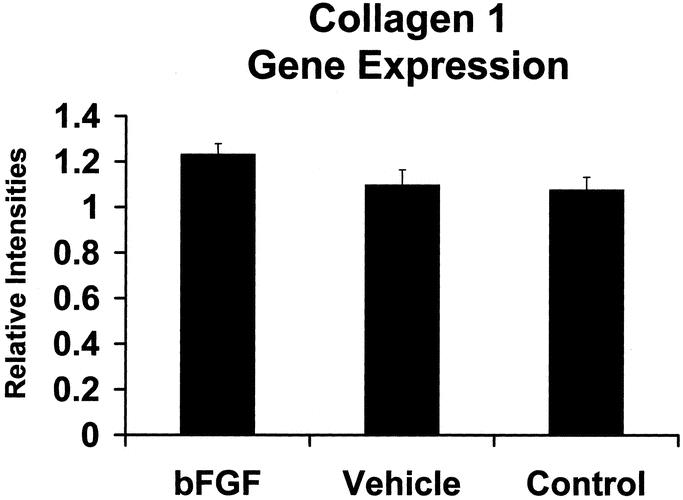
FIGURE 4. Relative expression of type I collagen mRNA in bFGF-treated, empty rod control, and hernia model control fascial incisions. Values expressed in arbitrary units, P > 0.05).
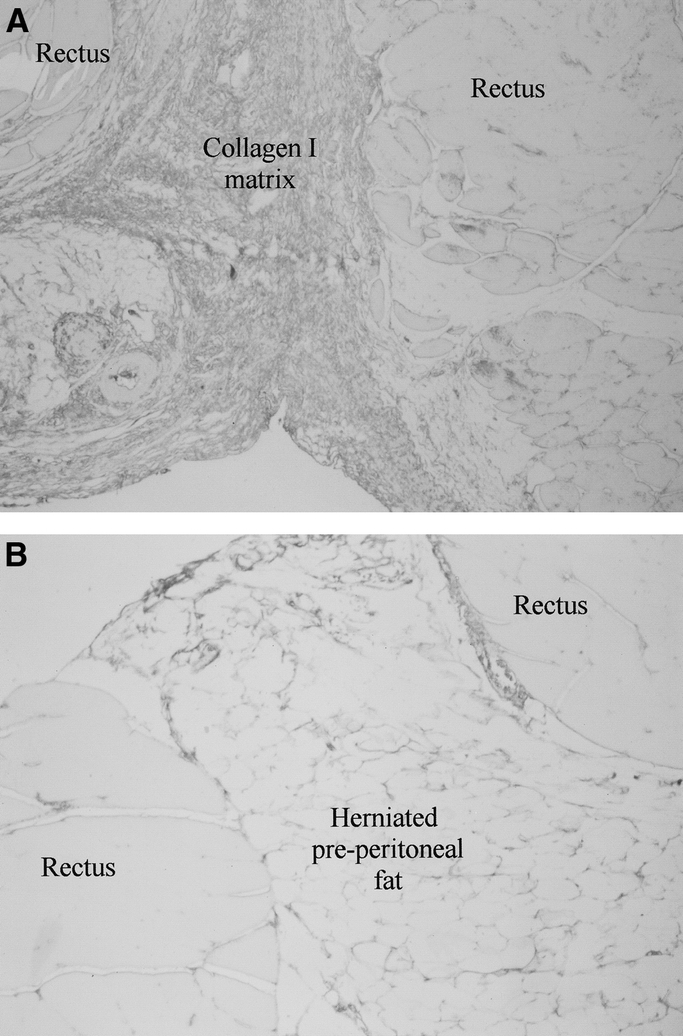
FIGURE 5. A, Immunostaining for type I collagen was significantly increased in POD 28 fascial incisions treated with bFGF 100X. B, Control wounds stained weakly for type I collagen and contained more herniated preperitoneal fat.
DISCUSSION
New approaches to the problem of abdominal wall wound failure are needed because the 11% incidence of primary and 24–58% incidence of recurrent incisional hernias remain unchanged over 50 years.1,2,17 Mechanical approaches alone, including optimized suture length to wound length ratios, incision location and orientation, layered versus mass closure and even mesh herniorrhaphy, have all failed to significantly impact this very common surgical complication.1 Mesh herniorrhaphy is the only surgical intervention that has decreased recurrent incisional hernia formation.17 However, the incidence of recurrent incisional hernias remains unacceptably high even after mesh repair (34–48%).17 There are also unique morbidities associated with this type of repair. Mesh is usually permanent, can stiffen, and can become uncomfortable over time. There is also a slightly increased risk of wound infection with mesh as well as increased risk of enteric fistulization, adhesion formation, and erosion after mesh incisional hernia repair.19,20 It is unlikely, therefore, that mesh would ever be used prophylactically during the approximately 4 million primary abdominal wall closures performed annually in the United States. The cloning of fibroproliferative tissue growth factors along with our improved understanding of the cellular and molecular mechanism of normal acute wound healing now make the biologic augmentation of acute tissue repair a possible clinical alternative.21
We previously reported the development of a new rat model of incisional hernia formation to better study and define the molecular mechanisms responsible for acute fascial wound failure.7 The model is based on intentional early abdominal wall mechanical wound failure during the lag-phase of acute healing when wound tensile strength is essentially zero and on prevailing theories that most incisional hernias develop soon after acute wound closure.7,8 After celiotomy, the midline fascial incisions are closed with a single rapidly absorbed suture. This results in an 80% incisional hernia rate after 28 days. In our previous report, incisional hernia formation was completely eliminated by priming the linea alba with aqueous TGF-β2 prior to midline celiotomy.18 The amelioration of early acute wound failure was associated with enhanced macrophage and fibroblast chemotaxis in addition to increased collagen I and III production in TGF-β2-treated incisions. However, no differences were observed in neovascularization between control and TGF-β2-treated incisions, a process fundamental to acute wound repair. The fascia anatomically is less vascular than the dermis, which may be a rate-limiting function of acute fascial wound tensile strength recovery.
The current study was designed to test the clinically available fibroproliferative tissue growth factor, bFGF, and a novel delayed release polygalactone rod polymer delivery method. Although the fibroproliferative properties are reported to be inferior to TGF-β2,22,23 bFGF is a potent stimulator of angiogenesis16 when compared with TGF-β2, which is reported to possess antiangiogenic properties.24 Our hypothesis was that the combined fibroproliferative and angiogenic properties of bFGF would accelerate acute fascial wound repair. Shifting the acute fascial wound healing trajectory to the left will theoretically reduce the opportunity for incisional wound failures (hernias) to occur.9 To test this hypothesis, a bFGF impregnated polymer was incorporated into primary fascial closures and incisional hernia repairs. Early postoperative fascial incisions recovered enhanced breaking strength at POD 7 in the bFGF therapy animals compared with the empty rod controls demonstrating accelerated early postoperative fascial wound repair.
Biologically augmenting fascial repair after celiotomy lowered the incidence of fascial separation in the rat model of intentional abdominal wall wound failure. Prophylactic therapy for the celiotomy wound site with a delayed release polymer formulation of bFGF resulted in a significant decrease in incisional hernia formation. Control animals had a 90% incidence of incisional hernia formation whereas bFGF-treated incisional hernia rate was only 30%. In addition to preventing incisional hernia formation, treatment of mature incisional hernias with bFGF polymer resulted in a decreased recurrent incisional hernia rate. This distinction is important because a “mature” incisional hernia physiologically shares many characteristics of a chronic wound as opposed to the first set of experiments where an acute wound was biologically modified. Primary apposition of fascial edges with incorporation of “empty rods” in the controls resulted in an 86% recurrent incisional hernia rate, whereas bFGF-impregnated rod therapy decreased the recurrent incisional hernia rate to 23%. This is one of the first reports of therapeutically treating a mature incisional hernia with growth factors and significantly impacting recurrence rate.
Basic FGF treatment induced pronounced angiogenesis, earlier fibroplasia, and enhanced collagen production within the acute fascial wound. In vivo, bFGF appears in high initial peak concentration in the early acute wound fluid (within 48 hours) and then tapers off to baseline levels within a few days.25 This pharmacokinetic pattern is characteristic of insulin-like growth factor, epidermal growth factor, platelet-derived growth factor AB, and transforming growth factor β-1.26 The early response growth factors are known to recruit the cellular and molecular events necessary for the transition to the fibroproliferative stage. Specifically, bFGF stimulates endothelial cell proliferation and induces the production of proteases, required for neovessel penetration thought the extracellular matrix.16 Basic FGF also has modest fibroproliferative-stimulating properties.22 These biologic functions theoretically make bFGF the ideal growth factor for penetrating the organized mature dense scar that characterizes incisional hernia rings with proteases and stimulating neovascularization to support primary apposition of the fascial edges. Several other advantageous clinical applications for bFGF have been demonstrated in experimental animal models of acute wound healing in tissues with relatively poor blood supply. Incorporation of tracheal autographs,27 devascularized sternal wound healing,28 fractured bone healing,29 and tendon repair30 all have been demonstrated to have enhanced wound healing with the application of bFGF to the acute wound. bFGF also improves healing parameters in patients with dysfunctional wound repair such as those with diabetes mellitus.31
Growth factor delivery remains a difficult problem in models of acute wound healing.32 Previous techniques all have shortcomings. Applying the growth factors as an aqueous preparation to an open incision makes containment within the wound difficult and equal distribution throughout the wound nearly impossible. Carboxymethylcellulose suspensions improve the handling and delivery of growth factors for open wound models but are cumbersome and unreliable for closed primary wounds. Fibrin based carriers can also improve growth factor delivery but may act as mechanical barriers to incision healing at specific concentrations.32 We previously reported an aqueous TGF-β2 preparation injected into the prospective wound site prior to incision using a small diameter needle system.18 This “priming” of the tissue prior to injury allows ease of handling but had the shortcomings of a single dose delivery and difficult application in the treatment of developed incisional hernias with separated fascial edges.
Semirigid polygalactone polymers aid in the delivery and handling of growth factor preparations. The delayed release formulation greatly improves sustained tissue infiltration resulting in a better chemotactic gradient within the tissue when compared with wound surface application alone. It is well known that a decreasing wound-tissue growth factor gradient is one of the important mechanisms for fibroblast and macrophage recruitment.33 This study documents sustained delivery of bFGF at 7 days after incorporation into the fascial incision, well beyond the time point when the physiologic “postinjury” bFGF levels return to baseline. The main proposed drawback of semi-rigid rods is that unless the carrier polymer rapidly absorbs and is pliant, it may also act as a mechanical barrier to acute tissue approximation and repair. However, empty-rod therapy in this study resulted in a slightly decreased trend in the incidence of primary incisional hernia formation compared with the hernia model control animals (60%, or 6/10, vs. 90%, or 27/30). We observed a modest inflammatory reaction adjacent to the polymer, which in itself stimulates angiogenesis and may account for the decreased incisional hernia trend observed with empty rod controls. Alternatively, the polymer rod may mechanically stent the fascial defect resulting in this trend. In contrast to the hernia prevention studies, empty rod therapy resulted in an 86% incidence of recurrent incisional hernia formation in the treatment of mature incisional hernias, similar to the control rate of 90% suggesting no therapeutic benefit. Based upon this data, the mechanical presence of the polymer itself at least did not function to impede acute fascial wound healing and may have a mild therapeutic effect on primary wounds.
The mechanism for the majority of incisional hernias likely involves early acute mechanical wound failure occurring at a time when acute fascial wound strength is less than 10% of normal tissue tensile strength.1,7 During this 4- to 6-week period, a celiotomy wound relies almost exclusively on the integrity of the suture closure. If a suture fails, pulls through the fascia or loosens as the patient recovers and increases activity, the natural load placed across the early acute wound will frequently lead to mechanical wound failure, occult fascial dehiscence, and clinical incisional hernia formation. Enhanced delivery of bFGF to the early postoperative wound functionally shortens the lag phase of tissue repair thereby reducing the period of total mechanical reliance. Primary incisional hernia formation was decreased with bFGF, as was the incidence of recurrent incisional hernia development after repair. Not surprisingly, the mechanism includes enhanced fibroblast and macrophage recruitment into the region of the fascial incision as well as enhanced collagen and extracellular matrix synthesis, including markedly increased neovascularization. In the future, a combined biomechanical approach similar the one reported here may be applied clinically, especially in cases where there is a high risk for acute fascial wound failure. These would include, for example, recurrent incisional herniorrhaphy, acute wounds in patients older than 60, those closed during hemodynamic instability or in the setting of abdominal sepsis, severely malnourished patients, and those wounds associated with abdominal aortic aneurysm or morbid obesity surgery. Acute wound therapy with proliferative growth factors such as bFGF offers a promising new strategy for reducing the incidence of this common surgical complication.
Footnotes
Supported by a Department of Veterans Affairs Merit Review Grant (MGF).
Reprints: Michael G. Franz, MD, Section of General Surgery, University of Michigan Health System, 1500 E. Medical Center Drive, 2922 H Taubman Center, Ann Arbor, MI 48109-0331. E-mail: mfranz@umich.edu.
REFERENCES
- 1.Santora TA, Rosylyn JJ. Incisional hernia: hernia surgery. Surg Clin North Am. 1993;73:557–570. [DOI] [PubMed] [Google Scholar]
- 2.Mudge M, Hughers LE. Incisional hernia: a 10 year prospective study. Br J Surg. 1985;72:70–71. [DOI] [PubMed] [Google Scholar]
- 3.Carlson MA. Acute wound failure. Wound healing. Surg Clin North Am. 2001;77:607–635. [DOI] [PubMed] [Google Scholar]
- 4.National Health Statistics Center. Detailed diagnose & procedures. National hospital discharge survey 1995;12(122).
- 5.Wedbush Morgan Securities. Biotechnology in wound care 2001;4:1–82.
- 6.Bay-Nielson M, Perkins F, Kehlet H. Pain and functional impairment one year after inguinal herniorrhaphy: a nationwide questionnaire study. Ann Surg. 2001;233:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franz MG, Kuhn MA, Nguyen K, et al. Early biomechanical wound failure: a mechanism for ventral incisional hernia formation. Wound Rep Reg. 2001;9:140–141. [Google Scholar]
- 8.Pollock AV, Evans M. Early prediction of late incisional hernias. Br J Surg. 1989;76:953–954. [DOI] [PubMed] [Google Scholar]
- 9.Franz MG, Robson MC. The use of the wound healing trajectory as an outcome determinant for acute wound healing. Wound Repair Regen. 2001;8:511–516. [DOI] [PubMed] [Google Scholar]
- 10.Viidik A, Gottrup F. Mechanics of healing soft tissue wounds. In Schmidt-Schonbein G, Woo S, Zweifach B, eds. Frontiers in Biomechanics. New York: Springer; 1986:263–279. [Google Scholar]
- 11.Anthony T, Bergen PC, Kim LT, et al. Factors affecting recurrence following incisional herniorrhaphy. World J Surg. 2000;24:95–101. [DOI] [PubMed] [Google Scholar]
- 12.DuBay DA, Franz MG. The biology of acute wound healing failure. Surg Clin North Am. 2002; Dec. [DOI] [PubMed]
- 13.Franz MG, Kuhn MA, Nguyen K, et al. A biological approach to prevention and treatment of incisional hernias. Surg Forum. 2000;51:585–587. [Google Scholar]
- 14.Franz MG, Smith PD, Wright TE, et al. Fascial healing exceeds skin: a novel model of abdominal wall repair. Surgery. 2001;129:203–208. [DOI] [PubMed] [Google Scholar]
- 15.Mustoe TA, Pierce GF, Thomason A, et al. Accelerated healing of incisional wounds in rats induced by transforming growth factor-β. Science. 1987;237:1333–1336. [DOI] [PubMed] [Google Scholar]
- 16.Montesano R, Vassalli J-D, Gaird A, et al. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci USA. 1986;83:7297–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luijendijik RW, Hop WCJ, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343:392–398. [DOI] [PubMed] [Google Scholar]
- 18.Franz MG, Kuhn MA, Nguyen K, et al. Transforming growth factor beta-2 lowers the incidence of incisional hernias. J Surg Res. 2001;97:109–116. [DOI] [PubMed] [Google Scholar]
- 19.Leber GE, Garb JL, Alexander AI, et al. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133:378–82. [DOI] [PubMed] [Google Scholar]
- 20.Morris-Stiff GJ, Hughes LE. The outcomes of nonabsorbable mesh placed within the abdominal cavity: Literature review and clinical experience. J Am Coll Surg. 1986;186:352–67. [DOI] [PubMed] [Google Scholar]
- 21.Hudson-Goodman P, Girard N, Jones MB. Wound repair and the potential use of growth factors. Heart Lung. 1990;19:379–84. [PubMed] [Google Scholar]
- 22.McGee GS, Davidson JM, Buckley A, et al. Recombinant basic fibroblast growth factor accelerates wound healing. J Surg Res. 1988;45:145–153. [DOI] [PubMed] [Google Scholar]
- 23.Davidson JM, Klagsbrun M, Hill KE, et al. Accelerated wound repair, cell proliferation, and collagen accumulation are produced by a cartilage-derived growth factor. J Cell Biol. 1985;100:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sporn MB, Roberts AB, Wakefield LM, et al. Some recent advances in the chemistry and biology of transforming growth factor beta. J Cell Biol. 1987;105:1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brem H, Shing Y, Wantanabe H, et al. Temporal expression of basic fibroblast growth factor during wound healing. Surg Forum. 1992;43:664. [Google Scholar]
- 26.Vogt PM. Lehnhardt M, Wagner D, et al. Determination of endogenous growth factors in human wound fluid: Temporal presence and profiles of secretion. Plast Reconstr Surg. 1998;102:117–23. [DOI] [PubMed] [Google Scholar]
- 27.Nakanishi R, Hashimoto M, Yasumoto K. Improved airway healing using basic fibroblast growth factor in a canine autotransplantation model. Ann Surg. 1998;227:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwakura A, Tabata Y, Nishimura K, et al. Basic fibroblast growth factor may improve devascularized sternal healing. Ann Thorac Surg. 2000;70:824–828. [DOI] [PubMed] [Google Scholar]
- 29.Radomsky ML, Thompson AY, Spiro RC, et al. Potential role of fibroblast growth factor in enhancement of fracture healing. Clin Orthop Rel Res. 1998;355(Suppl):S283–S293. [DOI] [PubMed] [Google Scholar]
- 30.Chan BP, Chan KM, Maffulle N, et al. Effect of basic fibroblast growth factor. An in vitro study of tendon healing. Clin Orthop Rel Res. 1997;342:239–247. [PubMed] [Google Scholar]
- 31.Greenhalgh DG, Sprugel KH, Murray MJ, et al. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 32.Wright TE, Hill DP, Ko F, et al. The effect of TGF-B2 in various vehicles on incisional wound healing. Int J Surg Invest 2000;2:133. [PubMed] [Google Scholar]
- 33.Banda MJ, Dwyer KS, Beckmann A. Wound fluid angiogenesis factor stimulates the directed migration of capillary endothelial cells. J Cell Biochem. 1985;29:183. [DOI] [PubMed] [Google Scholar]


