Abstract
Objective:
To review the present status of bioartificial liver (BAL) devices and their obtained clinical results.
Background:
Acute liver failure (ALF) is a disease with a high mortality. Standard therapy at present is liver transplantation. Liver transplantation is hampered by the increasing shortage of organ donors, resulting in high incidence of patients with ALF dying on the transplantation waiting list. Among a variety of liver assist therapies, BAL therapy is marked as the most promising solution to bridge ALF patients to liver transplantation or to liver regeneration, because several BAL systems showed significant survival improvement in animal ALF studies. Until today, clinical application of 11 different BAL systems has been reported.
Methods:
A literature review was performed using MEDLINE and additional library searches. Only BAL systems that have been used in a clinical trial were included in this review.
Results:
Eleven BAL systems found clinical application. Three systems were studied in a controlled trial, showing no significant survival benefits, in part due to the insufficient number of patients included. The other systems were studied in a phase I trial or during treatment of a single patient and all showed to be safe. Most BAL therapies resulted in improvement of clinical and biochemical parameters.
Conclusions:
Bioartificial liver therapy for bridging patients with ALF to liver transplantation or liver regeneration is promising. Its clinical value awaits further improvement of BAL devices, replacement of hepatocytes of animal origin by human hepatocytes, and assessment in controlled clinical trials.
Due to shortage of donor livers, patients with acute liver failure die while waiting for liver transplantation. Bioartificial liver treatment is reviewed as a potential technique to bridge patients with acute liver failure to liver transplantation or liver regeneration.
Mortality of acute liver failure (ALF) remains high despite maximal supportive intensive care treatment. Mortality ranges from 60% to 90% depending on the cause of underlying liver disease. Survival of patients with ALF caused by acute hepatitis B is 12% to 23% in Western Europe.1 Since the 1950s, several therapies to assist the failing liver have been introduced. These therapies range from drug treatment to liver support devices and liver transplantation. At present, standard treatment of ALF is orthotopic liver transplantation (OLT). Emergency OLT is associated with a 1-year survival of 60% to 90%, depending on the cause of ALF and the selection criteria applied for OLT.1–7 However, due to the shortage of donor livers, a considerable number of patients with ALF die while on the waiting list for OLT. Despite the efforts to increase the donor liver pool by using split livers, living related donor livers, and marginal livers, the availability of donor livers is far less than the demand. In the United States at the end of 2001, 18,500 patients were waiting for OLT. In this year, 5250 out of 25,750 patients (20%) received a donor liver, whereas 1978 (7.7%) patients with hepatic failure died while waiting for OLT.7 Of the high urgency patients (category I), 14% (97 out of 695) died while waiting for a donor liver. The median waiting time for a donor liver in this group was 10 days.7
Because of these high mortality rates and the increasing waiting times for transplantation over the last years,7 there has been renewed interest in techniques for providing temporary liver support to bridge the patient with liver failure to OLT or liver regeneration. These techniques can be grossly divided into nonbiologic and biologic liver support.
NONBIOLOGIC LIVER SUPPORT
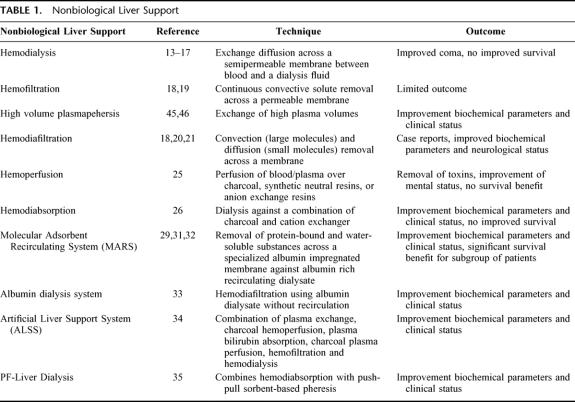
Lower and middle molecular weight toxic substances have been thought to play a crucial role in ALF. These water-soluble and protein-bound toxins cause multiple organ failure and hepatic encephalopathy, leading to coma and eventually to death. Many attempts have been made to develop nonbiologic liver support therapies based on detoxification of the patient's blood.8–12 These therapies and their effects are summarized in Table 1. In the 1950s, hemodialysis was introduced in an attempt to remove toxins; however, no improvement of survival was achieved.13–17 Hemofiltration, continuous convective solute removal across a permeable membrane, showed limited outcome.18,19 Only case reports were published concerning hemodiafiltration, convection (large molecule), and diffusion (small molecule) removal across a membrane. These case reports showed improved biochemical parameters and neurologic status.18,20,21 By hemo- and plasma perfusion, a more aggressive removal of toxic molecules that are protein bound was undertaken.22 Various types of resins have been used,23,24 especially effective in removal of lipophilic substances. Considerable experience has been obtained with activated charcoal as an adsorbent of possible toxins. However, the conclusion finally had to be drawn from controlled studies that these techniques did not improve survival.25 Hemodiabsorbtion, dialysis against a combination of charcoal and cation exchanger, showed improved biochemical parameters and clinical status, but survival did not improve.26 The nonspecific target of this technology was thought to be one of the reasons for its limited success.27,28
TABLE 1. Nonbiological Liver Support
The most promising nonbiologic support therapies combine detoxification of water-soluble and protein-bound toxins in a dialysis system, such as the Molecular Adsorbents Recirculating System (MARS),29–32 the albumin dialysis system,33 the Artificial Liver Support System (ALSS),34 and PF-Liver Dialysis.35 Beneficial effects on plasma toxin levels were observed in noncontrolled studies of the albumin dialysis system, ALSS, and PF-Liver Dialysis systems in patients with liver failure. Only MARS treatment until now showed significantly improved survival in a controlled trial of a subgroup of patients with hepatorenal syndrome. Mortality rates in the control group were 100% at day 7 compared with 63% of the MARS-treated group.31 In ALF patients, none of these systems have significantly improved survival.
In short, 1 or more nonbiologic liver support therapies may have shown benefit for short-term liver support in moderately affected patients with ALF; however, their unspecificity of removal of compounds and their lack of capacity to synthesize liver specific proteins and other hepatotrophic factors probably accounts for their limited effect. The success of OLT has demonstrated the importance not only of detoxification, but also metabolic functions in patient outcome. Because these functions can be carried out by hepatocytes, more is expected from biologic liver support systems.
BIOLOGIC LIVER SUPPORT
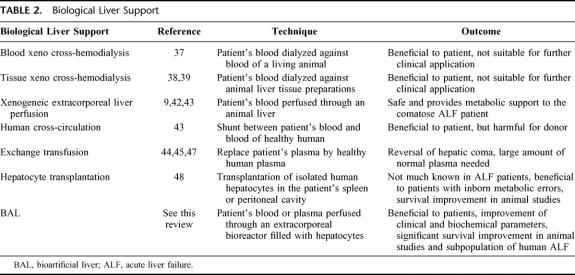
Biologic approaches rely on the functionality of livers or hepatocytes from xenogeneic or human origin that can be exploited to support the patient's liver (Table 2). These functions comprise detoxification, several metabolic functions, and synthesis of proteins and other molecules.
TABLE 2. Biological Liver Support
In 1956, it was demonstrated that fresh bovine liver homogenate could be used to metabolize salicylic and barbituric acids and keton bodies and produce urea from ammonium chloride.36 The many different biologic approaches that followed thereafter comprised xeno cross-hemodialysis, in which the patient's blood was dialyzed against blood of a living animal37 or animal liver tissue preparations.38,39 Although these techniques could be beneficial to patients with liver failure, they were not considered to be suitable for clinical application because of the complexity of the procedure or rapid loss of effectivity. Moreover, xenogeneic extracorporeal liver perfusion in humans temporarily had been shown to improve biochemical parameters and the patient's clinical neurologic condition.40,41 However, controlled clinical trials indicating survival improvement have as yet not been reported.9,42 Liver support could be provided by human cross-circulation,43 but the potential toxicity and adverse reactions in the donor severely limited this approach. Another approach, exchange transfusion was associated with reversal of hepatic coma.44–46 In combination with hemodialysis, survival increased from 18% to 50% (4 out of 8 patients) in a noncontrolled study.47 A major problem with exchange transfusion is the need of a large amount of normal plasma. Furthermore, this technique might at the same time remove essential factors, such as hepatotrophic factors.47
Isolated liver cells have been used in a variety of configurations: suspended, substrate attached, and encapsulated in semipermeable membranes. Hepatocytes used for liver support can be divided into 2 categories: implantable systems and extracorporeal systems. Several case reports and case series concerning transplantation of human hepatocytes show beneficial effects in liver failure.48 Use of xenogeneic hepatocytes for hepatocyte transplantation in patients is not yet reported. Hepatocyte transplantation in the peritoneal cavity and spleen showed prolonged survival in animals with ALF,49 but only if the transplantation occurred several days before induction of ALF.50,51 Furthermore, ongoing hepatocyte injury by viral or toxic agents may not allow donor hepatocytes to organize into normal parenchymal architecture.52
Problems with blood clotting and immune reactions in extracorporeal whole liver perfusion53 resulted in the development of BAL or hybrid liver support devices. The BAL systems are extracorporeal systems temporarily connected to the circulation of the patient. Bioartificial liver systems consist of an artificial component, i.e., the bioreactor and its equipment, and a biocomponent, i.e., hepatocytes. Although an increasing number of BAL devices have been produced or are currently under development, only 11 different BAL devices have, to date, been applied clinically. Significant prolongation of survival has been shown in animal studies with BAL systems,54–58 and, therefore, clinical application of a BAL has high expectations. Herein, we review the 11 clinically applied BAL systems and the clinical results obtained with these devices.
CLINICALLY APPLIED BIOARTIFICIAL LIVER DEVICES
In 1987, Matsumura et al59 reported the first application of a BAL support system in a patient. The principle of this BAL system was hemodialysis with a flow of 145 mL/min against a suspension of 10 × 109 functioning, cryopreserved rabbit hepatocytes. The blood of the patient was separated from the rabbit hepatocytes by a cellulose membrane, which was permeable to low and middle molecular weight molecules. The bioreactor was placed between the radial artery and basilic vein. This case report described a 45-year-old male patient in hepatic failure due to an inoperable bile duct carcinoma that involved the bifurcation of the common hepatic duct. The patient underwent 2 treatments, lasting for 5 and 4.5 hours, respectively, and he survived with no signs of adverse events.
Two years later, Margulis et al60 reported a controlled study including 126 patients in which a BAL device was used containing 40 × 106 porcine hepatocytes in a 20 mL polychlorovinyl capsule. The capsule contained a nylon filter in the outlet, which was filled with activated charcoal and granules of inorganic quartz glass. The capsule was incorporated into a forearm arteriovenous shunt. Each capsule was replaced by a fresh one every hour during a 6-hour treatment period. The blood flow through the bioreactor was 90 mL/min. Anticoagulation was obtained using heparin. Fifty-nine patients (20 hepatic coma and 39 prehepatic coma) were treated with this BAL device and were compared with a nontreated control group of 67 patients (30 hepatic coma and 37 prehepatic coma). In the control group, 27 patients (90%) died in the coma subgroup and 14 (38%) in the precoma subgroup. In the BAL-treated coma subgroup, 15 patients (75%) died, and the other patients (25%) initially regained consciousness, but died later due to progressive hepatic failure. In the BAL-treated precoma subgroup, 7 patients (18%) died, and the rest survived. Neurologic improvement was documented by clinical grading and electroencephalogram (EEG) monitoring. Overall, ammonia levels decreased with 50% compared with pretreatment levels. This BAL treatment was relatively simple and cheap.
No mention of Specified Pathogen Free (SPF) status of the animals used for hepatocyte isolation for the 2 above-mentioned systems was made. No further reports concerning patient treatment with the Matsamura et al or Margulis et al systems have been published.
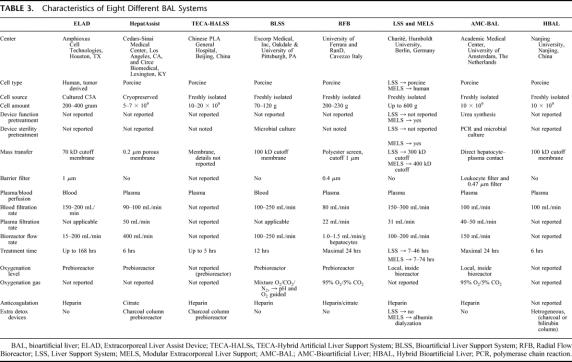
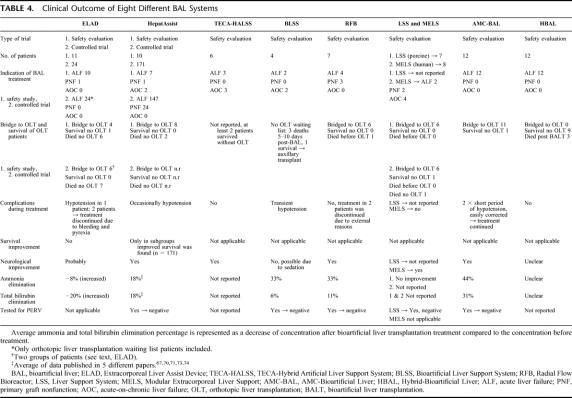
The systems that have been recently applied in the treatment of a number of patients are schematically presented in Tables 3 and 4.
TABLE 3. Characteristics of Eight Different BAL Systems
TABLE 4. Clinical Outcome of Eight Different BAL Systems
The Extracorporeal Liver Assist Device
The Extracorporeal Liver Assist Device (ELAD)29,61–65 (Houston, TX) is the only BAL device in which a human hepatocyte cell line (C3A) is used. The cell line is a clonal derivative of the hepatoblastoma cell line HepG2. The C3A cell line has been selected for use in the ELAD system because of its reduced tumorigenic potential and its high production of albumin and alpha-fetoprotein. The ELAD64 consists of a dual pump dialysis system and hollow fiber cartridges containing C3A cells. A second cartridge can be connected in series if needed. Blood flows through the cartridge, and plasma is ultrafiltrated through the cellulose acetate fibers into the extracapillary space of the cartridge, where it comes in direct contact with the C3A cells. The semipermeable membrane, which separates the C3A cells from the blood, has a molecular weight cutoff of 70 kD. Therefore, no immunoglobulins or leukocytes come into direct contact with the C3A cells. Before the ultrafiltrate is returned to the bloodstream, it is passed through a dual membrane cell filter to prevent cells and cellular debris from entering the bloodstream. A disposable membrane oxygenator is used if the patient temporarily needs to be disconnected from the ELAD. A phase I trial62 was performed in 11 patients: 10 with ALF and 1 with primary graft nonfunction (PNF). Cartridges maintained normal function during patient treatment of up to 58 hours, and their activity seemed to improve with blood perfusion. The only limitation to ELAD performance was clotting of the system. This has led to a more aggressive heparin treatment resulting in activated clotting times of 200 to 250 seconds. In this patient group, 4 patients were successfully bridged to OLT, 6 patients died before OLT, and 1 patient survived without OLT. Improvement in mental status occurred in 8 of the 11 patients. Most patients remained hemodynamically stable during ELAD treatment, and renal function was maintained in those patients who were not anuric at the start of treatment. No significant changes in vital signs, white blood cell count, or complement were noted. Several adverse events took place that were not related to ELAD treatment. However, 1 patient was noted with a short period of hypotension, which was corrected by fluid administration.
In a pilot controlled trial,61 24 patients were stratified into 2 study groups according to their predicted outcome. Group I (n = 17) comprised patients with ALF who were considered to have a substantial chance (30–50%) of spontaneous recovery. Patients in group II (n = 7) fulfilled criteria for liver transplantation at enrollment. The patients were randomized into 2 arms: in arm I, patients received standard therapy alone (control arm); in arm II, patients received ELAD support in addition to standard therapy. Six patients in group I, 3 in each arm, deteriorated and were put on the waiting list for liver transplantation. Survival in group I was 78% in the control arm and 75% in the ELAD arm. In group II, the survival rates were 25% and 33%, respectively. Eventually, 13 patients were put on the waiting list for liver transplantation. Six patients (46%) of this subgroup received a liver transplant, and 7 patients died without transplantation. Eleven patients, all in group I, survived without liver transplant. Assessment of serial changes in encephalopathy appeared to show some benefit with ELAD support. There was no significant difference in renal function between groups. Analysis of biochemical variables after ELAD treatment showed an increase of plasma ammonia (8%) and bilirubin (20%) concentrations compared with corresponding values before ELAD treatment; other variables measured were not influenced by ELAD treatment.
Few adverse events occurred in the controlled trial. Two patients were withdrawn from the study. One patient became tachypneic, tachycardic, and pyrexial. These phenomena resolved rapidly after discontinuing the hemoperfusion. The second patient developed overt bleeding because of an exacerbation of pre-existing disseminated intravascular coagulation. After stopping the perfusion and infusion of fresh-frozen plasma, bleeding ceased and the platelet count increased. No severe hypotension was observed in this controlled trial. In all, this controlled trial did not demonstrate a significant difference in survival between ELAD treated patients and controls. Furthermore, no improvement of biochemical parameters was observed.
Recently, a new clinical trial with a slightly modified version of the ELAD system has started and was preceded by a phase I trial in 5 patients66 (not listed in Tables 3 and 4). The new system uses ultrafiltrated blood instead of whole blood generated by a 120 kD cutoff membrane (instead of 70 kD). Four cartridges with approximately 100 g of C3A cells were used for each treatment. The flow rate through one cartridge is 500 mL/min instead of 150 to 200 mL/min. Oxygen and glucose consumption are frequently monitored to ensure metabolic activity of the cells in the cartridges. The 5 patients, all candidates for OLT, entered into an open-label, randomized, controlled pilot multicenter study of approximately 24 patients with clinical diagnosis of ALF or primary PNF. The treatment period ranged from 12 to 107 hours. No adverse events were observed during modified ELAD treatment. Ammonia and lactate plasma concentrations were not influenced by the ELAD treatment. Bilirubin plasma levels were not mentioned. Four patients were successfully bridged to OLT. One patient died within 2 days after OLT due to infection and deterioration of neurologic status.
The HepatAssist System
The HepatAssist BAL device67–77 developed in Los Angeles, CA, by Demetriou et al has been tested in the largest controlled clinical trial of a BAL device. The biologic component consists of 5 to 7 × 109 cryopreserved porcine hepatocytes. The microcarrier-attached cells are inoculated into the extrafiber space of a hollow fiber bioreactor. After plasma separation, plasma of the patient first passes over an activated charcoal-coated cellulose column and through an oxygenator before it is circulated through the semi-permeable fibers (Ø 0.2 μm) in the hollow fiber bioreactor. After passing the bioreactor, treated plasma and the blood cells are reconstituted and returned to the patient.
In a phase I safety evaluation study,67,76 9 adult patients and a 10-year-old boy were treated with the HepatAssist system. Nine patients had ALF, and 1 had PNF. Eight patients were successfully bridged to OLT and 2 patients died without OLT. The pediatric patient was successfully bridged to OLT. In 6 patients who had deep coma with brain edema and intracranial hypertension, a rapid normalization of intracranial pressure (ICP) was observed during treatment. Blood ammonia levels decreased in all patients by 36% on average. The mean total bilirubin concentration decreased by 11%. Treatments were well tolerated. No reactions to porcine hepatocytes were observed, and all patients remained hemodynamically stable throughout the treatment period.
In an uncontrolled follow-up study, 39 ALF patients classified in 3 groups were treated with the HepatAssist BAL.70 Group I (n = 26) patients fulfilled the criteria of ALF and were candidates for OLT. Group II (n = 3) patients had undergone OLT and had PNF. Patients in group III (n = 10) presented with acute on chronic liver disease and were not candidates for OLT. In group I, 18 patients (69%) were successfully bridged to OLT, of which 17 patients completely recovered. One patient died 7 days post-OLT due to PNF. Six patients (23%), of whom 5 had acetaminophen-induced ALF, recovered spontaneously after BAL treatment without OLT. Two patients (8%) were removed from the transplant waiting list because of initial clinical improvement during BAL treatments, but they finally died 21 and 44 days after the start of BAL treatment. All 3 patients in group II were successfully bridged to OLT and fully recovered. All patients in group III exhibited transient clinical improvement after BAL treatment; however, 8 patients (80%) died 1 to 21 days after first BAL treatment.
HepatAssist treatment was associated with improvement in neurologic status,69,70 ICP, and Glasgow Coma Scale (GCS). Biochemical parameters improved in all 3 groups. The mean total bilirubin concentration in group I decreased by 18% of the concentration at the start of treatment. Mean ammonia levels also decreased by 18% of the initial level. Data on above-mentioned biochemical parameters were obtained from 5 different publications67,70,71,73,74 for the purpose of this review. Presumably, several patients have been taken into account more than once in these publications.
In 1 patient, transient hypotension was observed after which treatment was discontinued. No other adverse events were noted. The HepatAssist system was shown to be safe, well tolerated by the patients, except for hypotension in 1 patient, and provided temporary physiologic support to patients with ALF. Patients were tested retrospectively for porcine endogenous retrovirus (PERV). There was no evidence of viral transmission from pig cells to the patients. The positive outcomes in this uncontrolled study provided the incentive to conduct a randomized, controlled clinical trial.
In this trial, 171 patients (147 ALF and 24 PNF)77 were randomized into a BAL treatment arm (n = 85) and a control arm (n = 86). The primary end point was 30-day survival; this was achieved in 71% in the BAL treatment arm and 62% in the control arm (P = 0.28). In the ALF subgroup, the 30-day survival was 73% and 59% for the BAL and control group, respectively (P = 0.10). However, a significant survival advantage of 33% (37% in the control group versus 70% in the BAL group; P = 0.018) was associated with BAL treatment of acetaminophen overdose (n = 39). Extension of this clinical trial is planned.
TECA-Hybrid Artificial Liver Support System
The TECA-Hybrid Artificial Liver Support System (TECA-HALSS)78,79 developed in Beijing, China, consists of an extracorporeal hollow fiber bioreactor loaded with 10 to 20 × 109 porcine hepatocytes. The hepatocytes circulate in suspension through the outer space of the hollow fibers in the bioreactor. Plasma is perfused through the fibers of the bioreactor. After perfusion through a charcoal filter and the bioreactor, the plasma is reconstituted with the blood cells and then returned to the patient. The treatment lasts for a maximum of 5 hours per bioreactor.
Six patients, 3 with ALF and 3 with acute-on-chronic liver failure, were treated with the TECA-HALSS system.78 During treatment, vital signs remained stable, and no thrombosis or bleeding events were noted. Neurologic improvement occurred in those patients entering with drowsiness or coma. After TECA-HALSS treatment, ammonia concentrations were substantially lower. In 1 patient, the ammonia level decreased by 31% and total bilirubin concentration decreased by 15%. Unfortunately, no mean data for the whole group are available. Neither was additional information on safety reported.
The Bioartificial Liver Support System
The Bioartificial Liver Support System (BLSS) device,80,81 developed in Pittsburgh, PA, uses 70 to 120 g of primary porcine hepatocytes. The hepatocytes, mixed with 20% collagen, are housed in an extrafiber space. Whole blood is perfused through the fibers of the bioreactor after passing through an oxygenator. Mass transfer depends on diffusion across a semipermeable fiber membrane with a 100 kD cutoff.
Four patients were treated with the BLSS in a phase I clinical trial.81 Causes of ALF in this group were acetaminophen intoxication, Wilson disease, acute alcoholic hepatitis, and chemotherapy. Survival outcome was not mentioned. Mean ammonia levels decreased by 33% compared with pretreatment levels and total bilirubin concentration decreased by 6%. Renal function and neurologic function did not seem to be influenced by BLSS perfusion. In 1 patient, transient hypotension at the start of the BLSS perfusion was observed. This adverse event was easily corrected by fluid administration. No PERV transmission was detected by examination of lymphocytes up to 12 months after BLSS treatment.
The Radial Flow Bioreactor
The Radial Flow Bioreactor (RFB),82,83 developed in Ferrara, Italy, comprises a woven-nonwoven polyester matrix sandwiched between 2 precision woven polyester screens. About 200 g of primary porcine hepatocytes are injected into the 6-mm-thick polyester matrix. The 2 polyester screens prevent hepatocytes leaking out of the bioreactor during perfusion of the plasma of the patient. Oxygenation of the cells is accomplished by perfusion of the plasma through an oxygenator before it enters the bioreactor. During RFB treatment, the function of the bioreactor is evaluated by determining its oxygen consumption. Oxygen consumption by the hepatocytes in the bioreactor decreases during treatment of a patient, indicating exhaustion of the hepatocytes.
Seven patients waiting for OLT were included in a phase I safety evaluation trial.83 The causes of ALF were viral hepatitis in 3, PNF in 3, and abdominal trauma in 1 patient. Six out of the 7 patients underwent OLT within 2 to 6 hours after completion of the RFB treatment. Five out of 6 patients survived after OLT. One patient with PNF eventually was not a candidate for retransplantation and died of multiorgan failure (MOF). Late death occurred in the trauma patient due to MOF after retransplantation. Treatment was associated with amelioration of neurologic dysfunction. Radial Flow Bioreactor treatment lowered the mean ammonia and bilirubin level by 33% and 11%, respectively. Radial Flow Bioreactor treatments were well tolerated, and patients remained hemodynamically stable throughout treatment. No adverse events were observed during or after the treatment. Porcine endogenous retrovirus transmission from the porcine cells to mononuclear cells was not detected during short-term follow-up.
The Hybrid Liver Support System and Modular Extracorporeal Liver Support
The Liver Support System (LSS) device,84–86 developed in Berlin, Germany, consists of an especially designed bioreactor aiming at improving cell oxygenation and mass exchange. The system consists of interwoven hollow fiber membranes, creating a 3-dimensional framework over which hepatocyte aggregates are distributed. Three bundles of hollow fibers are situated inside the bioreactor. Two of these bundles consist of hydrophilic fibers (300 kD cutoff) and are used for plasma perfusion. By closing 1 end of each bundle, plasma entering the bioreactor enters the extracapillary space via one fiber bundle, makes contact with the hepatocytes, and leaves the bioreactor via the second fiber bundle. The third bundle of hollow fibers is made of hydrophobic membranes and is used for gas exchange inside the bioreactor. The bioreactor contains up to 500 to 600 g of hepatocytes. The LSS is the only system that has been used in clinical studies with primary porcine hepatocytes as well as primary human hepatocytes derived from discarded donor livers.87
Seven ALF patients, with coma stage II to IV, were treated for 8 to 46 hours with the porcine cell-based LSS system.86 All patients were successfully bridged to OLT. Elevated plasma ammonia levels were not corrected by LSS treatment. No data concerning clinical parameters, total bilirubin, and adverse events have been reported. Porcine endogenous retrovirus transmission was tested negative.88
In a phase I study with primary human hepatocytes, 8 patients were treated with the Modular Extracorporeal Liver Support (MELS) system. The MELS concept combines different extracorporeal therapy units, tailored to suit the individual clinical needs of each patient.87 The MELS consists of the LSS system combined with a DetoxModule based on single-pass albumin dialysis for removing albumin-bound toxins. Human hepatocytes were harvested from donor livers that were discarded because of steatosis, cirrhosis, fibrosis, or mechanical injury. Two patients with ALF (not further specified), 2 patients with acute-on-chronic liver failure, and 2 patients with PNF were successfully bridged to OLT. The other 2 patients suffered from acute-on-chronic liver failure and were not candidates for OLT due to continuing alcohol consumption. One of these 2 patients died 3 weeks after MELS treatment. The overall MELS treatment time in this group ranged from 7 to 144 hours. The longer treatments were performed with 2 consecutive bioreactors. No adverse events were observed. In all 8 cases, neurologic status improved, and slight improvement of coagulation was observed during treatment.
The AMC-Bioartificial Liver
The AMC-Bioartificial Liver (AMC-BAL),57,58,89–96 developed in Amsterdam, the Netherlands, consists of a hollow fiber bioreactor (polysulfon housing) and a plasmapheresis system. At least 10 × 109 viable porcine hepatocytes are attached in a 3-dimensional configuration to a nonwoven hydrophilic polyester matrix. The matrix, with a thickness of 4 mm and a total surface area of 5610 cm2, is spirally wound around a massive core. In between the layers of the matrix, hollow fibers for on-site gas exchange are positioned in a longitudinal direction. The blood of the patient undergoes a plasma filtration treatment, after which the resulting plasma is perfused through the bioreactor and again reunited with the blood cells. The most noteworthy features of the AMC-BAL are the direct contact between small aggregates of hepatocytes and the plasma of the patient, resulting in optimal mass transfer, and direct oxygenation of the hepatocytes.
Seven patients were treated with the AMC-BAL in a phase I safety evaluation trial in Naples and Rome, Italy.95 All patients had hyperacute (n = 6) or acute (n = 1) liver failure according to Crepaldi et al97 and met the criteria for OLT.98,99 All patients had grade III to IV encephalopathy. The cause of ALF in 3 patients was acute hepatitis B, acute hepatitis A in 1 patient, and acute fatty liver of pregnancy in 1 patient. In 2 patients, the cause of liver failure was not determined. Duration of total AMC-BAL treatment ranged from 8 to 35 hours. Three patients received serial treatment with 2 BALs. Six of the 7 patients were successfully bridged to OLT. One patient recovered after 2 BAL treatments over an interval of 3 days without OLT. Two patients died of complications post-OLT. One patient died 1 day after OLT due to PNF of a marginal donor liver. The second patient died 14 days after OLT due to mesenteric thrombosis that resulted in massive bowel infarction. Treatment of all patients was associated with an improved neurologic state and stabilization of hemodynamics. Improved urine output was noted in patients with renal insufficiency. After AMC-BAL treatment, the average plasma ammonia and bilirubin levels decreased by 44% and 31%, respectively.95 In 2 patients, a short period of hypotension was observed after connection to the BAL system. This hypotension was easily corrected by dopamine and fluid administration. No other adverse events were observed in these patients. None of the treated patients were positive for PERV.95
Five additional patients with ALF were included in the phase I trial and treated with the AMC-BAL (unpublished data). All 5 patients were successfully bridged to OLT. One patient died 1 day after OLT due to postoperative bleeding and PNF. A second patient, with a GCS of 3, died 2 weeks after OLT due to multiorgan failure. In summary, 12 patients have been treated with the AMC-BAL in a phase I trial. Eleven patients were successfully bridged to OLT, and 1 patient survived after 2 treatments without OLT. Four patients died within a month after OLT due to disease and OLT-related problems. The 8 other patients (66%) are in good health at the moment and have post-BAL survival times ranging from 6 to 30 months.
The Bioartificial Hepatic Support System
Concerning the Bioartificial Hepatic Support (BHS)100 system, developed in Udine, Italy, only 1 case has been published. This system is not represented in the accompanying tables because of limited information about the system and the absence of a published phase I study. Fifteen billion cryopreserved porcine hepatocytes, together with 10 g hydrated collagen-coated dextran microcarriers, were loaded in the extracapillary space of a hollow fiber bioreactor with a porosity of 0.6 μm. After plasmapheresis, the plasma of the patient passed a cellulose-charcoal column, an oxygenator, and a heater before entering the bioreactor with a flow not further defined.
A 56-year-old patient with acute-on-chronic liver failure (HBV cirrhosis) and grade III portosystemic encephalopathy was treated with the BHS system.100 The patient underwent 3 treatments lasting 6 hours each, with 48-hour intervals. Pulmonary and cardiovascular functions remained stable, and the patient tolerated the procedures well. Bilirubin as well as ammonia concentrations improved during treatment. Temporary neurologic improvements were observed after each BHS treatment. The patient was not a candidate for OLT and died 13 days after the last BHS treatment.
Hybrid-Bioartificial Liver
Most recently, a Chinese group from Nanjing published a patient study with a BAL system called the Hybrid-Bioartificial Liver (HBAL).101 No earlier in vitro, animal, or patient studies with the HBAL were published. The BAL consists of a polysulfon hollow fiber bioreactor with an internal volume of about 360 mL. More than 10 × 109 porcine hepatocytes cultured overnight in cell suspension were loaded into the extrafiber compartment of the bioreactor. Plasma was perfused through the hollow fibers with a membrane cutoff of 100 kD (same material and provider as the TECA-HALLS system).
In a phase I trial, 12 patients were treated with the HBAL system.101 In some patients, the plasma was perfused through a charcoal column or through a bilirubin absorption column before entering the HBAL system, other patients received HBAL only, and in another 2 patients, plasma exchange was first performed 24 hours prior to HBAL treatment. In summary, a heterogeneous treatment regimen was described in this paper. All patients suffered from hepatitis B ALF. The ALF status or coma grade was not described in this patient group. No inclusion or exclusion criteria were mentioned. Hybrid-Bioartificial Liver treatment lasted 6 hours. Two patients received 2 consecutive treatments. No adverse effects were observed in the 12 patients; however, 3 of them died soon after the HBAL treatment. Nine patients were described as improved, but no clear data were presented in this paper.
DISCUSSION
Comparison of the clinically applied BAL devices is impaired by the variability in devices and cells, setup of the treatments, patients, and in the outcome parameters used. Nonetheless, some conclusions can be extracted from the variety of data derived from the different BAL systems, excluding the first 2 systems described by Matsumura et al59 and Margulis et al60 and the BHS system,100 of which no recent data or very limited data were reported.
Bioartificial Liver Devices
The devices described here are, except for the RFB, hollow fiber devices, which differ in the mode of oxygenation, bidirectional mass exchange, and cell type or cell treatment. These are considered crucial parameters in the design of bioreactors, but other factors, such as an extracellular matrix environment and media composition, may also have considerable impact on level and stability of hepatocyte function.
It is often overlooked that BAL systems lack a biliary system for the excretion of conjugated bilirubin. Excretion of metabolites through bile depends on the remnant damaged liver mass. Hepatocytes in the bioreactor will produce bile acids and salts, most of them being toxic protein bound substances, and further increase the concentrations in blood.102,103 Incorporation of a detoxification module into the BAL system (as in HepatAssist, TECA-HALSS, MELS, and HBAL) may lower the toxic burden for the hepatocytes in the bioreactor and for the patient. For bridging ALF patients to OLT, the combination of a BAL and an artificial detoxification module may therefore provide optimal conditions for the treatment of ALF patients, although removal of hepatotrophic factors should be prevented. The latter is especially important if the goal of BAL treatment is bridging of the patient to liver regeneration.
Bioactive Mass and Source of Hepatocytes
Bioactive mass and cell type or cell source play a key role in BAL treatment. The efficacy of the treatment depends on the bioactivity of the cells in the bioreactor. These cells should be able to take over function of the diseased or absent liver. One of the unsolved questions in BAL research is with which functions and at what level a BAL should compensate the diseased liver. It is known from partial hepatectomy studies that about 20% of healthy liver mass, which contains approximately 200 g or 20 × 109 hepatocytes, is needed to survive.104–106 Thus, if no active liver mass is left in the patient, approximately 20 × 109 well-functioning hepatocytes are theoretically needed to keep the patient alive.
A variety of cell masses are used in the different BAL systems. Some groups express cell mass in grams, whereas others use cell number by cell count, which is confusing, particularly when it is not described how these figures are obtained. Therefore, it is difficult, if not impossible, to compare the bioactive mass between systems. Cell mass used in the current, clinically applied systems ranges from 5 × 109 cells (HepatAssist) to 600 g (LSS & MELS). Most systems use about 10 × 109 to 20 × 109 hepatocytes, which is, in theory, sufficient to support a liver with limited residual function. However, the viability and function of the cells prior to loading and after culture in the bioreactor may vary considerably and influence the effective biomass. These issues are not reported for the BAL systems, except for the AMC-BAL and MELS systems in which the urea producing capacity of the cells in the bioreactor, and in case of the MELS also other liver functions, was tested preceding connection to the patient.
Different cell types are used in the BAL systems currently under clinical study, each with specific advantages and disadvantages107 (Table 5). Because of their optimal function, primary human hepatocytes are the first choice for patient treatment, but their availability is low. The main source of primary human hepatocytes for BAL use is discarded donor livers. The primary human hepatocytes derived from discarded donor livers are characterized by heterogeneity and low viability.87,108 Moreover, the logistics around discarded donor livers are complex. However, according to Gerlach et al, the number of discarded donor livers, approximately 20% to 25% of all explanted livers, corresponds to the number of patients with ALF who require bridging therapy to transplantation.88 Alternatively, primary hepatocytes from animals, most often pigs, have been used in the majority of clinically applied bioreactors. Porcine livers are available in large quantities and can be obtained on demand. However, animal hepatocytes produce xenogeneic proteins, which may cause serum sickness within a week of repetitive treatments.76,109,110 Such immunologic problems will not be expected if treatments with porcine-based artificial liver systems do not exceed 5 to 6 days. Additionally, the risk of zoonosis severely limits the clinical application of porcine-based BAL systems, because in many European countries, xenotransplantation-related treatments are prohibited. In 9 of the 11 systems reviewed, hepatocytes from xenogeneic origin were used in the treatment of about 150 patients. No clinically important zoonosis or virus transmission has been reported in these patients. An important measure to maximally reduce the risk of zoonosis is the use of SPF animals, which are bred under strict conditions and are checked for a wide range of pathogens on a frequent base. In 3 of the porcine cell-based systems (ie, TECA-HALLS, BHS, and HBAL), the SPF status of the source animals for hepatocyte isolation was not reported, which raises concerns regarding safety of these cells. On the other hand, endogenous retroviruses, like PERV, are incorporated in the pig genome and therefore are also present in SPF pigs. Therefore, the risk of zoonosis, although very limited, cannot be neglected in view of the supposed impact on public health. In 5 of the presented studies, PERV tests were included and proved negative. We would like to encourage all groups working with xenogeneic-based BAL systems to test patients as long as possible for PERV and other possible zoonosis.
TABLE 5. Characteristics of Different Cell Sources in Relation to BAL Application
Optimal preservation of primary hepatocytes is necessary to improve BAL availability and logistics. Most efforts have been put into cryopreservation of hepatocytes. Cryopreservation may, however, lead to decreased viability, cell attachment capacity, and function. These effects are most significant if porcine or human hepatocytes are cryopreserved in cell suspension111,112 (unpublished data). Hepatocytes that are cultured113–115 or attached to microcarriers68,116,117 prior to cryopreservation maintain better cell function compared to preserved cell suspensions. From the BALs reported here, the HepatAssist system makes use of cryopreserved hepatocytes that are attached to microcarriers. In contrast, the BHS system uses cryopreserved hepatocyte suspensions. After thawing, the hepatocytes were combined with hydrated collagen-coated dextran microcarriers.
Most of the presented BAL systems rely on freshly isolated hepatocytes and therefore require optimal preservation conditions during transport of the loaded bioreactor from the laboratory to a usually remote center. One possibility is to preserve the hepatocyte-loaded bioreactor at 4°C. A standard organ preservation solution is generally used, such as University of Wisconsin solution or Celsior™. Before connection to the patient, the preservation solution is washed out of the bioreactor. It should, however, be taken into account that cold preservation causes loss of hepatocyte function inside the bioreactor.118 Alternatively, the hepatocyte-loaded bioreactor can be perfused and oxygenated under (sub)normothermic conditions during transport. Under normothermic (37°C) conditions, cell function of the hepatocytes will stabilize as much as possible and will be comparable to that of a laboratory culture of the cells in the bioreactor. A normothermic transport system for BALs is therefore an attractive option and deserves further investigation.
Cell lines, most often derived from tumors, are also applied to BAL systems as an alternative for primary cells. However, the functionality and safety of these cells are a matter of discussion. The ELAD system is based on the C3A cells, which display a number of liver functions, such as albumin production, but, for example, their ammonia reducing capacity is very low. Detachment of the C3A hepatoma cells and their subsequent escape from the ELAD system to the blood stream of the patient is considered to be only a theoretical concern.64 No tumorigenic infiltration of patients by C3A cells has been described so far.
Current advances in immortalization of hepatocytes and stem cell biology may offer better prospects, but have not yet been tested in a clinical setting.119
Patient Survival
Three of the described BAL systems (ie, Margulis BAL, ELAD, HepatAssist) have been tested in a controlled clinical trial. In these studies, there was no significant effect on survival. Only a subgroup of patients with ALF due to acetaminophen overdose, showed significant improvement in survival after treatment with the HepatAssist device.77 Because BAL treatment in ALF patients is usually followed by urgent OLT, having a major influence on 30-day survival, a large number of patients is needed in a controlled trial to show efficacy and improved survival of a BAL treatment. Of the BAL systems described here, treatment with the HepatAssist,54 LSS,55 and AMC-BAL57,58,92,93 significantly improved survival in animal models. The BLSS system showed a trend to improved survival in a galactosamine-induced ALF dog model.80 The TECA-HALLS system shows beneficial outcome on survival in ALF dogs but P value for survival benefit was not reported.79 The ELAD system has been tested in a small number of anhepatic dogs. Of 3 dogs, 1 lived longer (but not statistically significant) after ELAD treatment compared to a control group (n = 3).120 No reports concerning safety and testing of efficacy in ALF animal models prior to clinical application are available of the other BAL systems (RFB, BHS, HBAL).
Apart from survival, the efficacy of the different BAL systems can also be assessed from the effects on neurologic status and blood chemistry.
Neurologic Improvement
Application of most systems was associated with neurologic improvement during and after treatment. In the LSS study, there was no report on the neurologic status of patients. However in the MELS system (primary human hepatocytes), it was reported that all patients improved in regard with neurologic status. In the BLSS study, it was not possible to measure neurologic changes due to sedation of the patient. In the other studies, neurologic improvements assessed by GCS and, in some studies, also by EEG and/or ICP measurement were associated with application of a BAL system.
Biochemical Improvement Following Bioartificial Liver Treatment
Biochemical improvement as a result of BAL treatment, as judged by elimination of ammonia and bilirubin, was seen in most clinical studies. Average ammonia and total bilirubin concentrations were not reported in the TECA-HALSS and MELS studies. No improvement in ammonia concentrations was reported in the LSS study. According to Mundt et al,86 the assessment of only biochemical variables before and after liver support treatment might fail to detect a beneficial effect, because of continuing deterioration of the patient. Ellis et al61, Hughes and Williams,121 and Colletti et al122 emphasized that any additional function provided by the device is difficult to assess because changes in blood tests may not discriminate between synthetic/detoxification functions of the liver assist device and those of the native liver. Comparing plasma samples from the inlet and outlet of the device at the same time can also be used to assess efficacy of the BAL treatment. Except for the ELAD system, which was associated with an increase in ammonia and bilirubin levels, application of other systems (HepatAssist, BLSS, RFB, AMC-BAL) was associated with more or less a biochemical improvement. Clinical application of the AMC-BAL was associated with the largest reduction in average ammonia and total bilirubin levels (Table 4). However, the variation between patients and duration of BAL treatments may have influenced this outcome.
Adverse Events
Adverse events were only reported for 4 BAL systems (i.e., ELAD, HepatAssist, BLSS, AMC-BAL). In these systems, transient hypotension was occasionally observed at the beginning of BAL treatment. After treatment with fluid expansion and/or dopamine, the hypotension associated with the BLSS and AMC-BAL systems was readily reverted and hemodynamics stabilized. In the HepatAssist trials, 1 patient with hypotension was reported. In this case, BAL treatment was immediately discontinued. This episode of hypotension might have been caused by pre-existing hypovolemia, by bradykinin release as a reaction to the extracorporeal circuit, or by xenogeneic antigens. The release of bradykinin has previously been associated with blood contacting artificial membranes or plasma filters. Along the same lines, bradykinin has been shown to cause a drop in mean arterial pressure within 10 minutes after the start of continuous renal dialysis.123 Except for this transient hypotension, no other important BAL-related adverse events have been reported. In addition, no clinically manifest adverse immunologic reactions have been observed during short-term treatment with BAL systems charged with allogeneic or xenogeneic hepatocytes.
CONCLUSIONS
The concept of BAL support has proven to be successful in animal studies. In addition, clinical application of BAL devices has proven safe. Clinical assessment of BAL treatment is severely hindered by the variation in the patient groups studied and the fact that most patients undergo subsequent OLT. However, neurologic and biochemical parameters improved after treatment with different BAL systems. To ultimately determine the effect of BAL treatment on survival, controlled, randomized clinical trials in large patient groups are required to yield statistically significant outcomes. In parallel, BAL research should focus on the replacement of hepatocytes of animal origin by hepatocytes of human origin, either primary hepatocytes or immortalized cell lines, to overcome possible immunologic reactions and zoonosis.
ACKNOWLEDGMENTS
The authors thank Dr. F. Calise, head of Centro di Biotecnologie and Liver Transplantation Unit Cardarelli Hospital, Naples, Italy, and his coworkers for their contribution to the phase I clinical trial of the AMC-BAL. The development of the AMC-BAL was partly granted by Technology Foundation STW, Utrecht, The Netherlands.
Footnotes
Reprints: Thomas M. van Gulik, Department of Surgery (Surgical Laboratory), Academic Medical Center, Meibergdreef 9, Amsterdam 1105 AZ, The Netherlands. E-mail: t.m.vangulik@amc.uva.nl.
REFERENCES
- 1.Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97–106. [DOI] [PubMed] [Google Scholar]
- 2.Adam R, Cailliez V, Majno P, et al. Normalised intrinsic mortality risk in liver transplantation: European Liver Transplant Registry study. Lancet. 2000;356:621–627. [DOI] [PubMed] [Google Scholar]
- 3.Lidofsky SD, Bass NM, Prager MC, et al. Intracranial pressure monitoring and liver transplantation for fulminant hepatic failure. Hepatology. 1992;16:1–7. [DOI] [PubMed] [Google Scholar]
- 4.de Rave S, Tilanus HW, van Der LJ, et al. The importance of orthotopic liver transplantation in acute hepatic failure. Transpl Int. 2002;15:29–33. [DOI] [PubMed] [Google Scholar]
- 5.Wall WJ, Adams PC. Liver transplantation for fulminant hepatic failure: North American experience. Liver Transpl Surg. 1995;1:178–182. [DOI] [PubMed] [Google Scholar]
- 6.Farmer DG, Anselmo DM, Ghobrial RM, et al. Liver transplantation for fulminant hepatic failure: experience with more than 200 patients over a 17-year period. Ann Surg. 2003;237:666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United Network for Organ Sharing. Liver transplantation data 2002. Available at: www.unos.org.
- 8.Rahman TM, Hodgson HJ. Review article: liver support systems in acute hepatic failure. Aliment Pharmacol Ther. 1999;13:1255–1272. [DOI] [PubMed] [Google Scholar]
- 9.Stockmann HB, Hiemstra CA, Marquet RL, et al. Extracorporeal perfusion for the treatment of acute liver failure. Ann Surg. 2000;231:460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davenport A. Artificial hepatic support. Where are we now? Blood Purif. 2001;19:1–3. [DOI] [PubMed] [Google Scholar]
- 11.Mito M. Hepatic assist: present and future. Artif Organs. 1986;10:214–218. [DOI] [PubMed] [Google Scholar]
- 12.Kjaergard LL, Liu J, Als-Nielsen B, et al. Artificial and bioartificial support systems for acute and acute-on- chronic liver failure: a systematic review. JAMA. 2003;289:217–222. [DOI] [PubMed] [Google Scholar]
- 13.Kiley JE, Gundermann KJ, Lie TS. Ammonia intoxication treated by hemodialysis. N Engl J Med. 1958;25:1156–1161. [DOI] [PubMed] [Google Scholar]
- 14.Opolon P, Rapin JR, Huguet C, et al. Hepatic failure coma (HFC) treated by polyacrylonitrile membrane (PAN) hemodialysis (HD). Trans Am Soc Artif Intern Organs. 1976;22:701–710. [PubMed] [Google Scholar]
- 15.Knell AJ, Dukes DC. Dialysis procedures in acute liver coma. Lancet. 1976;2:402–403. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara S, Okabe K, Ouchi K, et al. Continuous removal of middle molecules by hemofiltration in patients with acute liver failure. Crit Care Med. 1990;18:1331–1338. [DOI] [PubMed] [Google Scholar]
- 17.Jones RC, Strader LD, Berry WC. Peritonela dialysis in liver coma. US Armed Forces Med J. 1959;10:977–982. [PubMed] [Google Scholar]
- 18.Agarwal R, Farber MO. Is continuous veno-venous hemofiltration for acetaminophen-induced acute liver and renal failure worthwhile? Clin Nephrol. 2002;57:167–170. [DOI] [PubMed] [Google Scholar]
- 19.Bellomo R, Ronco C. Continuous hemofiltration in the intensive care unit. Crit Care. 2000;4:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadamori H, Yagi T, Inagaki M, et al. High-flow-rate haemodiafiltration as a brain-support therapy proceeding to liver transplantation for hyperacute fulminant hepatic failure. Eur J Gastroenterol Hepatol. 2002;14:435–439. [DOI] [PubMed] [Google Scholar]
- 21.Mori T, Eguchi Y, Shimizu T, et al. A case of acute hepatic insufficiency treated with novel plasmapheresis plasma diafiltration for bridge use until liver transplantation. Ther Apher. 2002;6:463–466. [DOI] [PubMed] [Google Scholar]
- 22.Schlechter DC, Nealon TF, Gibbon JH. A simple extracorporeal device for reducing elevated blood ammonia levels. Surgery. 1958;44:892–897. [PubMed] [Google Scholar]
- 23.Rozenbaum JL, Kramer MS, Raja R, et al. Resin hemoperfusion: a new treatment for acute drug intoxication. N Engl J Med. 1971;284:874–883. [DOI] [PubMed] [Google Scholar]
- 24.Juggi JS. Extracorporeal cation-exchange circuits in the treatment of hyperammonaemia of hepatic failure. Med J Aust. 1973;1:926–930. [DOI] [PubMed] [Google Scholar]
- 25.O'Grady JG, Gimson AE, O'Brien CJ, et al. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology. 1988;94:1186–1192. [DOI] [PubMed] [Google Scholar]
- 26.Ash SR. Hemodiabsorption in treatment of acute hepatic failure and chronic cirrhosis with ascites. Artif Organs. 1994;18:355–362. [DOI] [PubMed] [Google Scholar]
- 27.Yarmush ML, Dunn JC, Tompkins RG. Assessment of artificial liver support technology. Cell Transplant. 1992;1:323–341. [DOI] [PubMed] [Google Scholar]
- 28.Flendrig LM. Development of a novel bioarticial liver [thesis]. Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands; 1998. [Google Scholar]
- 29.Steiner C, Mitzner S. Experiences with MARS liver support therapy in liver failure: analysis of 176 patients of the International MARS Registry. Liver. 2002;22(suppl 2):20–25. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt LE, Sorensen VR, Svendsen LB, et al. Hemodynamic changes during a single treatment with the molecular adsorbents recirculating system in patients with acute-on-chronic liver failure. Liver Transpl. 2001;7:1034–1039. [DOI] [PubMed] [Google Scholar]
- 31.Mitzner SR, Stange J, Klammt S, et al R. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277–286. [DOI] [PubMed] [Google Scholar]
- 32.Stange J, Hassanein TI, Mehta R, et al. The molecular adsorbents recycling system as a liver support system based on albumin dialysis: a summary of preclinical investigations, prospective, randomized, controlled clinical trial, and clinical experience from 19 centers. Artif Organs. 2002;26:103–110. [DOI] [PubMed] [Google Scholar]
- 33.Seige M, Kreymann B, Jeschke B, et al. Long-term treatment of patients with acute exacerbation of chronic liver failure by albumin dialysis. Transplant Proc. 1999;31:1371–1375. [DOI] [PubMed] [Google Scholar]
- 34.Lanjuan L, Qian Y, Jianrong H, et al. Severe hepatitis treated with an artificial liver support system. Int J Artif Organs. 2001;24:297–303. [PubMed] [Google Scholar]
- 35.Ash SR. Extracorporeal blood detoxification by sorbents in treatment of hepatic encephalopathy. Adv Ren Replace Ther. 2002;9:3–18. [DOI] [PubMed] [Google Scholar]
- 36.Sorrentino F. Prime ricerche per la realizzazione di un fegato artificiale. Chir Patol Sperim. 1956;4:1401–1404. [Google Scholar]
- 37.Kimoto S. The artificial liver: experiments and clinical application. Trans Am Soc Artif Intern Organs. 1959;5:102–112. [Google Scholar]
- 38.Mikami J, Moto M, Nishimuro A, et al. Surgical treatment of acute liver failure. II: an experimental study of extracorporeal metabolism in the artificial liver using slices of canine liver. Jpn J Gastroenterol. 1959;56:1022. [Google Scholar]
- 39.Nose Y, Mikami J, Kasai N, et al. An experimental artificial liver utilizing extracorporeal metabolism with sliced or granulated canine liver. ASAIO Trans. 1963;9:358. [PubMed] [Google Scholar]
- 40.Abouna GM, Ganguly PK, Hamdy HM, et al. Extracorporeal liver perfusion system for successful hepatic support pending liver regeneration or liver transplantation: a pre-clinical controlled trial. Transplantation. 1999;67:1576–1583. [DOI] [PubMed] [Google Scholar]
- 41.Horslen SP, Hammel JM, Fristoe LW, et al. Extracorporeal liver perfusion using human and pig livers for acute liver failure. Transplantation. 2000;70:1472–1478. [DOI] [PubMed] [Google Scholar]
- 42.Eiseman B, Liem DS, Raffucci F. Heterologous liver perfusion in treatment of hepatic failure. Ann Surg. 1965;162:329–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnell JM, Dawborn JK, Epstein RB, et al. Acute hepatic coma treated by cross-circulation or exchange perfusion. N Engl J Med. 1967;276:935–943. [DOI] [PubMed] [Google Scholar]
- 44.Ymazaki K, Kamai F, Ieszuki Y, et al. Extracorporeal methods of liver failure treatment. Biomater Artif Cells Artif Organs. 1987;15:667–675. [PubMed] [Google Scholar]
- 45.Kondrup J, Almdal T, Vilstrup H, et al. High volume plasma exchange in fulminant hepatic failure. Int J Artif Organs. 1992;15:669–676. [PubMed] [Google Scholar]
- 46.Clemmesen JO, Kondrup J, Nielsen LB, et al. Effects of high-volume plasmapheresis on ammonia, urea, and amino acids in patients with acute liver failure. Am J Gastroenterol. 2001;96:1217–1223. [DOI] [PubMed] [Google Scholar]
- 47.Brunner G, Losgen H. Benefits and dangers of plasma with fulminant hepatic failure. In: Oda T, Shiokawa Y, Inoue N, eds. Therapeutic Plasmapheresis VI. Cleveland, OH: ISAO Press; 1987:187–191. [Google Scholar]
- 48.Strom S, Fisher R. Hepatocyte transplantation: new possibilities for therapy. Gastroenterology. 2003;124:568–571. [DOI] [PubMed] [Google Scholar]
- 49.Nagata H, Ito M, Cai J, et al. Treatment of cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology. 2003;124:422–431. [DOI] [PubMed] [Google Scholar]
- 50.Demetriou AA, Reisner A, Sanchez J, et al. Transplantation of microcarrier-attached hepatocytes into 90% partially hepatectomized rats. Hepatology. 1988;8:1006–1009. [DOI] [PubMed] [Google Scholar]
- 51.Vogels BA, Maas MA, Bosma A, et al. Significant improvement of survival by intrasplenic hepatocyte transplantation in totally hepatectomized rats. Cell Transplant. 1996;5:369–378. [DOI] [PubMed] [Google Scholar]
- 52.Braun KM, Degen JL, Sandgren EP. Hepatocyte transplantation in a model of toxin-induced liver disease: variable therapeutic effect during replacement of damaged parenchyma by donor cells. Nat Med. 2000;6:320–326. [DOI] [PubMed] [Google Scholar]
- 53.McLaughlin BE, Tosone CM, Custer LM, et al. Overview of extracorporeal liver support systems and clinical results. Ann N Y Acad Sci. 1999;875:310–325. [DOI] [PubMed] [Google Scholar]
- 54.Suh KS, Lilja H, Kamohara Y, et al. Bioartificial liver treatment in rats with fulminant hepatic failure: effect on DNA-binding activity of liver-enriched and growth-associated transcription factors. J Surg Res. 1999;85:243–250. [DOI] [PubMed] [Google Scholar]
- 55.Gerlach JC, Botsch M, Kardassis D, et al. Experimental evaluation of a cell module for hybrid liver support. Int J Artif Organs. 2001;24:793–798. [PubMed] [Google Scholar]
- 56.Berry MN, Phillips JW. The isolated hepatocyte preparation: 30 years on. Biochem Soc Trans. 2000;28:131–135. [DOI] [PubMed] [Google Scholar]
- 57.Flendrig LM, Calise F, Di Florio E, et al. Significantly improved survival time in pigs with complete liver ischemia treated with a novel bioartificial liver. Int J Artif Organs. 1999;22:701–709. [PubMed] [Google Scholar]
- 58.Sosef MN, Abrahamse LS, van de Kerkhove MP, et al. Assessment of the AMC-bioartificial liver in the anhepatic pig. Transplantation. 2002;73:204–209. [DOI] [PubMed] [Google Scholar]
- 59.Matsumura KN, Guevara GR, Huston H, et al. Hybrid bioartificial liver in hepatic failure: preliminary clinical report. Surgery. 1987;101:99–103. [PubMed] [Google Scholar]
- 60.Margulis MS, Erukhimov EA, Andreiman LA, et al. Temporary organ substitution by hemoperfusion through suspension of active donor hepatocytes in a total complex of intensive therapy in patients with acute hepatic insufficiency. Resuscitation. 1989;18:85–94. [DOI] [PubMed] [Google Scholar]
- 61.Ellis AJ, Hughes RD, Wendon JA, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24:1446–1451. [DOI] [PubMed] [Google Scholar]
- 62.Sussman NL, Gislason GT, Conlin CA, et al. The Hepatix extracorporeal liver assist device: initial clinical experience. Artif Organs. 1994;18:390–396. [DOI] [PubMed] [Google Scholar]
- 63.Sussman NL, Gislason GT, Kelly JH. Extracorporeal liver support. Application to fulminant hepatic failure. J Clin Gastroenterol. 1994;18:320–324. [PubMed] [Google Scholar]
- 64.Gislason GT, Lobdell DD, Kelly JH, et al. A treatment system for implementing an extracorporeal liver assist device. Artif Organs. 1994;18:385–389. [DOI] [PubMed] [Google Scholar]
- 65.Sussman NL, Kelly JH. Improved liver function following treatment with an extracorporeal liver assist device. Artif Organs. 1993;17:27–30. [DOI] [PubMed] [Google Scholar]
- 66.Millis JM, Cronin DC, Johnson R, et al. Initial experience with the modified extracorporeal liver-assist device for patients with fulminant hepatic failure: system modifications and clinical impact. Transplantation. 2002;74:1735–1746. [DOI] [PubMed] [Google Scholar]
- 67.Demetriou AA, Rozga J, Podesta L, et al. Early clinical experience with a hybrid bioartificial liver. Scand J Gastroenterol Suppl. 1995;208:111–117. [DOI] [PubMed] [Google Scholar]
- 68.Rozga J, Holzman MD, Ro MS, et al. Development of a hybrid bioartificial liver. Ann Surg. 1993;217:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samuel D, Ichai P, Feray C, et al. Neurological improvement during bioartificial liver sessions in patients with acute liver failure awaiting transplantation. Transplantation. 2002;73:257–264. [DOI] [PubMed] [Google Scholar]
- 70.Hui T, Rozga J, Demetriou AA. Bioartificial liver support. J Hepatobiliary Pancreat Surg. 2001;8:1–15. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe FD, Mullon CJ, Hewitt WR, et al. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann Surg. 1997;225:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen SC, Hewitt WR, Watanabe FD, et al. Clinical experience with a porcine hepatocyte-based liver support system. Int J Artif Organs. 1996;19:664–669. [PubMed] [Google Scholar]
- 73.Demetriou AA, Watanabe F, Rozga J. Artificial hepatic support systems. Prog Liver Dis. 1995;13:331–348. [PubMed] [Google Scholar]
- 74.Arkadopoulos N, Detry O, Rozga J, et al. Liver assist systems: state of the art. Int J Artif Organs. 1998;21:781–787. [PubMed] [Google Scholar]
- 75.Neuzil DF, Rozga J, Moscioni AD, et al. Use of a novel bioartificial liver in a patient with acute liver insufficiency. Surgery. 1993;113:340–343. [PubMed] [Google Scholar]
- 76.Rozga J, Podesta L, Lepage E, et al. A bioartificial liver to treat severe acute liver failure. Ann Surg. 1994;219:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stevens C, Demetriou AA. An interim analysis of a phase II/III prospective randomized, multicenter, controlled trial of the HepatAssist bioartificial liver support system for the treatment of fulminant hepatic failure. Hepatology. 2001;34:299a. [Google Scholar]
- 78.Xue YL, Zhao SF, Luo Y, et al. TECA hybrid artificial liver support system in treatment of acute liver failure. World J Gastroenterol. 2001;7:826–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen XP, Xue YL, Li XJ, et al. Experimental research on TECA-I bioartificial liver support system to treat canines with acute liver failure. World J Gastroenterol. 2001;7:706–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patzer JF, Mazariegos GV, Lopez R, et al. Novel bioartificial liver support system: preclinical evaluation. Ann N Y Acad Sci. 1999;875:340–352. [DOI] [PubMed] [Google Scholar]
- 81.Mazariegos GV, Kramer DJ, Lopez RC, et al. Safety observations in phase I clinical evaluation of the Excorp Medical Bioartificial Liver Support System after the first four patients. ASAIO J. 2001;47:471–475. [DOI] [PubMed] [Google Scholar]
- 82.Morsiani E, Brogli M, Galavotti D, et al. Long-term expression of highly differentiated functions by isolated porcine hepatocytes perfused in a radial-flow bioreactor. Artif Organs. 2001;25:740–748. [DOI] [PubMed] [Google Scholar]
- 83.Morsiani E, Pazzi P, Puviani AC, et al. Early experiences with a porcine hepatocyte-based bioartificial liver in acute hepatic failure patients. Int J Artif Organs. 2002;25:192–202. [DOI] [PubMed] [Google Scholar]
- 84.Gerlach JC. Development of a hybrid liver support system: a review. Int J Artif Organs. 1996;19:645–654. [PubMed] [Google Scholar]
- 85.Sauer IM, Obermeyer N, Kardassis D, et al. Development of a hybrid liver support system. Ann N Y Acad Sci. 2001;944:308–319. [DOI] [PubMed] [Google Scholar]
- 86.Mundt A, Puhl G, Muller A, et al. A method to assess biochemical activity of liver cells during clinical application of extracorporeal hybrid liver support. Int J Artif Organs. 2002;25:542–548. [DOI] [PubMed] [Google Scholar]
- 87.Sauer IM, Zeilinger K, Obermayer N, et al. Primary human liver cells as source for modular extracorporeal liver support-a preliminary report. Int J Artif Organs. 2002;25:1001–1005. [DOI] [PubMed] [Google Scholar]
- 88.Gerlach JC, Zeilinger K, Sauer IM, et al. Extracorporeal liver support: porcine or human cell based systems? Int J Artif Organs. 2002;25:1013–1018. [DOI] [PubMed] [Google Scholar]
- 89.Flendrig LM, te Velde AA, Chamuleau RA. Semipermeable hollow fiber membranes in hepatocyte bioreactors: a prerequisite for a successful bioartificial liver? Artif Organs. 1997;21:1177–1181. [DOI] [PubMed] [Google Scholar]
- 90.Flendrig LM, la Soe JW, Jorning GG, et al. In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound nonwoven polyester matrix for hepatocyte culture as small aggregates. J Hepatol. 1997;26:1379–1392. [DOI] [PubMed] [Google Scholar]
- 91.Flendrig LM, Sommeijer D, Ladiges NC, et al. Commercially available media for flushing extracorporeal bioartificial liver systems prior to connection to the patient's circulation: an in vitro comparative study in two and three dimensional porcine hepatocyte cultures. Int J Artif Organs. 1998;21:467–472. [PubMed] [Google Scholar]
- 92.Flendrig LM, Maas MA, Daalhuisen J, et al. Does the extend of the culture time of primary hepatocytes in a bioreactor affect the treatment efficacy of a bioartificial liver? Int J Artif Organs. 1998;21:542–547. [PubMed] [Google Scholar]
- 93.Flendrig LM, Chamuleau RA, Maas MA, et al. Evaluation of a novel bioartificial liver in rats with complete liver ischemia: treatment efficacy and species-specific alpha-GST detection to monitor hepatocyte viability. J Hepatol. 1999;30:311–320. [DOI] [PubMed] [Google Scholar]
- 94.Sosef MN, van de Kerkhove MP, Abrahamse LS, et al. Blood Coagulation in Anhepatic Pigs: Effects of Treatment with the AMC Bioartificial Liver. J Thromb Haemost. 2003;1:511–515. [DOI] [PubMed] [Google Scholar]
- 95.van de Kerkhove MP, Di Florio E, Scuderi V, et al. Phase I clinical trial with the AMC-bioartificial liver. Academic Medical Center. Int J Artif Organs. 2002;25:950–959. [DOI] [PubMed] [Google Scholar]
- 96.Abrahamse SL, van de Kerkhove MP, Sosef MN, et al. Treatment of acute liver failure in pigs reduces hepatocyte function in a bioartificial liver support system. Int J Artif Organs. 2002;25:966–974. [DOI] [PubMed] [Google Scholar]
- 97.Crepaldi G, Demetriou AA, Muraca M. Experience in design of controlled clinical trials in acute liver failure. In: Riordan SM, Williams R, eds. Bioartificial Liver Support Systems. Rome: CIC Edizioni Internazionali; 1997:104–115. [Google Scholar]
- 98.O'Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. [DOI] [PubMed] [Google Scholar]
- 99.Bernuau J, Goudeau A, Poynard T, et al. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology. 1986;6:648–651. [DOI] [PubMed] [Google Scholar]
- 100.Donini A, Baccarani U, Risaliti A, et al. Temporary neurological improvement in a patient with acute or chronic liver failure treated with a bioartificial liver device. Am J Gastroenterol. 2000;95:1102–1104. [DOI] [PubMed] [Google Scholar]
- 101.Ding YT, Qiu YD, Chen Z, et al. The development of a new bioartificial liver and its application in 12 acute liver failure patients. World J Gastroenterol. 2003;9:829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hughes RD. Review of methods to remove protein-bound substances in liver failure. Int J Artif Organs. 2002;25:911–917. [DOI] [PubMed] [Google Scholar]
- 103.Hughes RD, Cochrane AM, Thomson AD, et al. The cytotoxicity of plasma from patients with acute hepatic failure to isolated rabbit hepatocytes. Br J Exp Pathol. 1976;57:348–353. [PMC free article] [PubMed] [Google Scholar]
- 104.DeMatteo RP, Fong Y, Blumgart LH. Surgical treatment of malignant liver tumours. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:557–574. [DOI] [PubMed] [Google Scholar]
- 105.McCarter MD, Fong Y. Metastatic liver tumors. Semin Surg Oncol. 2000;19:177–188. [DOI] [PubMed] [Google Scholar]
- 106.van de Kerkhove MP, De Jong KP, Rijken AM, et al. MARS treatment in posthepatectomy liver failure. Liver Int. 2003;23(suppl 3):44–51. [DOI] [PubMed] [Google Scholar]
- 107.Allen JW, Hassanein T, Bhatia SN. Advances in bioartificial liver devices. Hepatology. 2001;34:447–455. [DOI] [PubMed] [Google Scholar]
- 108.Mitry RR, Hughes RD, Aw MM, et al. Human hepatocyte isolation and relationship of cell viability to early graft function. Cell Transplant. 2003;12:69–74. [DOI] [PubMed] [Google Scholar]
- 109.Lawley TJ, Bielory L, Gascon P, et al. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med. 1984;311:1407–1413. [DOI] [PubMed] [Google Scholar]
- 110.te Velde AA, Flendrig LM, Ladiges NC, et al. Immunological consequences of the use of xenogeneic hepatocytes in a bioartificial liver for acute liver failure. Int J Artif Organs. 1997;20:229–233. [PubMed] [Google Scholar]
- 111.Koebe HG, Dahnhardt C, Muller-Hocker J, et al. Cryopreservation of porcine hepatocyte cultures. Cryobiology. 1996;33:127–141. [DOI] [PubMed] [Google Scholar]
- 112.Morsiani E, Brogli M, Galavotti D, et al. Biologic liver support: optimal cell source and mass. Int J Artif Organs. 2002;25:985–993. [DOI] [PubMed] [Google Scholar]
- 113.Darr TB, Hubel A. Postthaw viability of precultured hepatocytes. Cryobiology. 2001;42:11–20. [DOI] [PubMed] [Google Scholar]
- 114.Hubel A, Conroy M, Darr TB. Influence of preculture on the prefreeze and postthaw characteristics of hepatocytes. Biotechnol Bioeng. 2000;71:173–183. [DOI] [PubMed] [Google Scholar]
- 115.Chen Z, Ding Y, Zhang H. Cryopreservation of suckling pig hepatocytes. Ann Clin Lab Sci. 2001;31:391–398. [PubMed] [Google Scholar]
- 116.Demetriou AA, Whiting J, Levenson SM, et al. New method of hepatocyte transplantation and extracorporeal liver support. Ann Surg. 1986;204:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morsiani E, Rozga J, Scott HC, et al. Automated liver cell processing facilitates large scale isolation and purification of porcine hepatocytes. ASAIO J. 1995;41:155–161. [PubMed] [Google Scholar]
- 118.Calise F, Mancini A, Amoroso P, et al. Functional evaluation of the AMC-BAL to be employed in a multicentric clinical trial for acute liver failure. Transplant Proc. 2001;33:647–649. [DOI] [PubMed] [Google Scholar]
- 119.Hoekstra R, Chamuleau RA. Recent developments on human cell lines for the bioartificial liver. Int J Artif Organs. 2002;25:182–191. [DOI] [PubMed] [Google Scholar]
- 120.Kelly JH, Koussayer T, He D, et al. Assessment of an extracorporeal liver assist device in anhepatic dogs. Artif Organs. 1992;16:418–422. [DOI] [PubMed] [Google Scholar]
- 121.Hughes RD, Williams R. Evaluation of extracorporeal bioartificial liver devices. Liver Transpl Surg. 1995;1:200–206. [DOI] [PubMed] [Google Scholar]
- 122.Colletti LM, Johnson KJ, Kunkel RG, et al. Mechanisms of hyperacute rejection in porcine liver transplantation. Antibody-mediated endothelial injury. Transplantation. 1994;57:1357–1363. [DOI] [PubMed] [Google Scholar]
- 123.Stoves J, Goode NP, Visvanathan R, et al. The bradykinin response and early hypotension at the introduction of continuous renal replacement therapy in the intensive care unit. Artif Organs. 2001;25:1009–1013. [DOI] [PubMed] [Google Scholar]