Abstract
Objective:
To summarize the clinical experience with a new open repair for pectus excavatum (PE), with minimal cartilage resection.
Summary Background Data:
A wide variety of modified techniques of the Ravitch repair for PE have been used over the past 5 decades, with the complications and results being inconsistent. Extensive subperiosteal costal cartilage resection and perichondrial sheath detachment from the sternum may not be necessary for optimal repair.
Methods:
During a 12-month period, 75 consecutive patients with symptomatic PE underwent open repair using a new less invasive technique. After exposing the deformed costal cartilages, a short chip was resected medially adjacent to the sternum and laterally at the level where the chest had a near normal contour, allowing the cartilage to be elevated to the desired level with minimal force. A transverse anterior sternal osteotomy was used on most patients. A substernal support strut was used for 66 patients; the strut was placed anterior to the sternum in 9 patients under age 12 and over age 40 years. The strut was routinely removed within 6 months.
Results:
With a mean follow-up of 8.2 months, all but 1 patient regarded the results as very good or excellent. Mean operating time was 174 minutes; mean hospitalization was 2.7 days. There were no major complications or deaths.
Conclusions:
The open repair using minimal cartilage resection is effective for all variations of PE in patients of all ages, uses short operating time, provides a stable early postoperative chest wall, causes only mild postoperative pain, and produces good physiologic and cosmetic results.
A new less invasive open repair with resection of small chips of deformed costal cartilages medially and laterally was performed on 75 consecutive pectus excavatum patients. The repair is effective for all variations of deformities, has few complications, has mild pain, produces an early stable chest, and has good physiologic and cosmetic results.
Pectus chest deformities are among the most common major congenital anomalies, occurring in approximately 1 in every 400 births.1 Pectus excavatum (PE) is commonly recognized during the first year of life, although frequently asymptomatic until adolescent skeletal growth occurs, and the deformity becomes much more severe.2 Increasing evidence indicates that PE deformities cause physiologic impairment and limitations, as well as adverse cosmetic and psychologic effects.3 Patients with PE commonly try to keep up with their peers in physical activities using wider diaphragmatic excursions with tachypnea to compensate for diminished chest wall excursions during respiration and tachycardia to compensate for reduced cardiac output. The majority of patients, however, experience a worsening of their symptoms until full skeletal growth has been achieved. The great majority of PE deformities will remain with the same severity throughout adult life.
Until the past few years, most surgeons performed only a small number of corrective operations for PE each year, using a variety of open surgical techniques based on the reports by Ravitch,4 Welch,5 and others. The reported results have been inconsistent, which has often caused reluctance among referring physicians, as well as patients, to recommend correction of the deformity. During the past 7 years, with the new concept of minimally invasive PE repair (MIRPE) reported by Nuss et al,6 and the availability of several informative pectus web sites on the Internet, many patients of all ages have become aware that their deformities can be corrected with a higher degree of success and lower risk for morbidity than in previous years.
The present report summarizes our clinical experience with a new highly modified and less extensive open repair for PE deformities in 75 consecutive patients. Consent for the study was granted by the UCLA Medical Center Institutional Review Board.
METHODS
During the period from September 2002 to September 2003, 75 consecutive patients underwent repair of PE deformities at UCLA Medical Center. There were 58 males and 17 females, ranging in age from 7 to 51 years (mean 18.4 years). Children under age 10 years commonly have only mild symptoms and are infrequently advised to undergo repair for primarily cosmetic benefits. The deformity was asymmetric in 34 of the patients (45%). Six patients were referred for repair of recurrent PE deformities. The most frequent symptoms common to all of the patients were dyspnea with mild exercise, reduced endurance, tachycardia, and anterior chest discomfort. A more detailed review of symptoms has been presented previously.7 All 75 of the patients had varying degrees of displacement of the heart into the left chest on radiographs or computed tomography (CT) scans. The pectus severity index (width of chest divided by the distance between the sternum and spine) ranged from 3.7 to 14.2 (mean 4.9). The mean severity index for both children and adults with a normal chest is 2.5.8
Operative Technique
The operative technique was an extensive modification of that described by Ravitch,9,10 Welch5 and others with minimal cartilage resection, and includes the following major features. General endotracheal anesthesia was used. An orogastric tube was placed in all patients, and a Foley bladder catheter was for all patients over 12 years of age. Intravenous cefazolin was given preoperatively. A chevron inframammary incision was made with a short midline extension superiorly in all patients who had more than 4 deformed costal cartilages (Fig. 1). In females, the incision was placed in the submammary sulcus. Short skin flaps were elevated superiorly and inferiorly using needle-tip electrocautery. The pectoralis muscles were reflected laterally, and the abdominal muscles were mobilized inferiorly just sufficient to expose the deformed costal cartilages. Short cautery incisions (5–7 mm) were made through the perichondrium of the deformed cartilages adjacent to the sternum; a second 8 mm incision was made laterally near or beyond the costochondral junction, where the chest wall was at the highest level. Short segments of cartilage (3–8 mm) were resected medially and laterally from each of the deformed ribs using Freer and Haight elevators, carefully preserving the perichondrium. For patients with asymmetric deformities, it was occasionally necessary to resect a short segment of bony rib lateral to the costochondral junction for 2 or 3 ribs on the right side to obtain optimal contour. For patients with concave lower costal cartilages and/or outward protrusion of the lowermost cartilages over the abdomen, it has been helpful to remove 1 or more chips from the mid and far lateral portions of the cartilage. The xiphoid and the lower 2 perichondrial sheaths were detached from the lower sternum for 66 patients (Fig. 2). The remaining perichondrial sheaths were left attached to the sternum. The retrosternal space for these 66 patients was mobilized from 4 to 5 cm with cautery. The right pleural space was opened widely for drainage, and a small chest tube was inserted. A transverse wedge osteotomy was made across the anterior table of the sternum at the level where the sternum angled to depress posteriorly. The posterior table of the sternum was gently fractured at the osteotomy without detachment; the lower end of the sternum was then twisted, when indicated, and elevated to the desired position. Seven patients who had 6 deformed costal cartilages reconstructed did not require a sternal osteotomy.
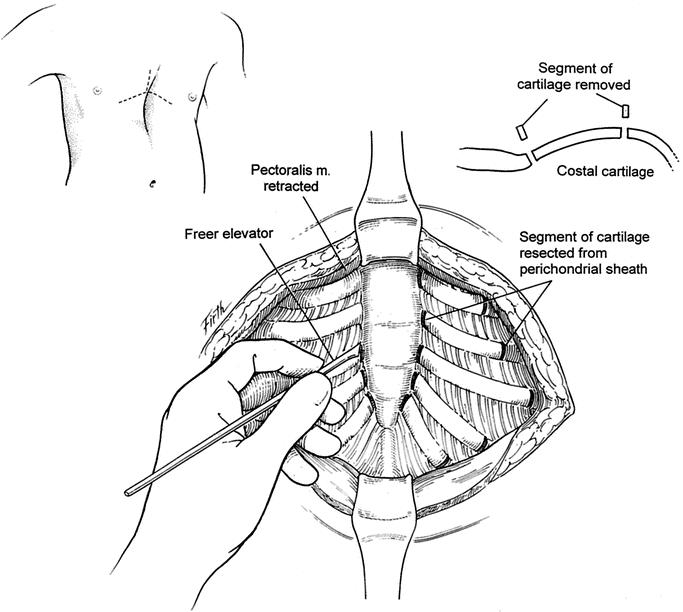
FIGURE 1. A chevron inframammary incision with a short vertical extension superiorly is commonly used. Cutaneous, as well as pectoralis and abdominal muscle flaps, are elevated just sufficient to expose the deformed costal cartilages. Short (4–8 mm) incisions are made through the perichondrium on the medial and lateral ends of the deformed cartilages, and 3–8 mm chips of cartilage are removed using a Freer elevator.
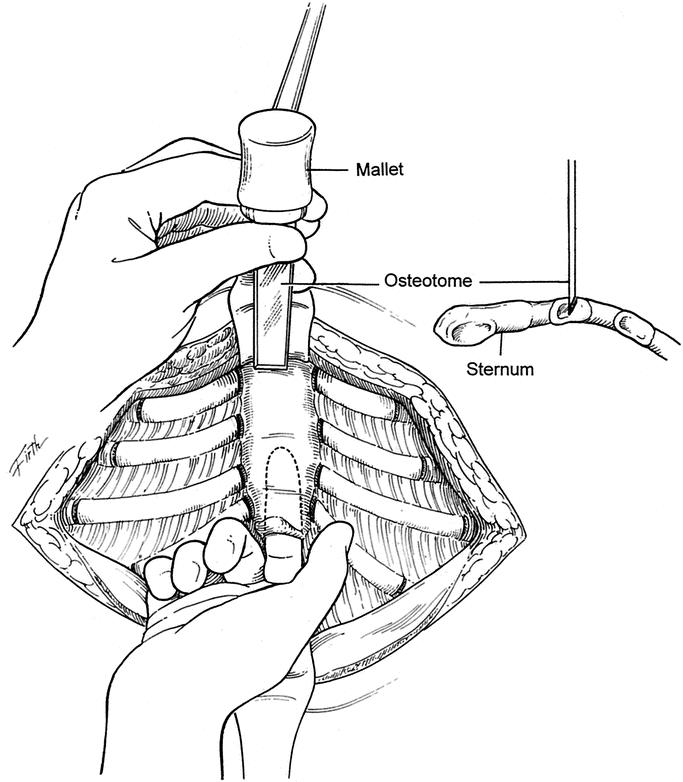
FIGURE 2. The xiphoid and the lowermost 2 perichondrial sheaths are detached from the lower sternum. The retrosternal space is mobilized over a distance of 4 to 5 cm. A small chest tube is placed into the right pleural space for drainage in most patients. A transverse wedge osteotomy is made across the anterior table of the sternum at the desired level. The posterior table of the sternum is fractured but not detached, and the lower sternum is elevated to the desired level.
A thin stainless steel Adkins strut11 (Baxter Health Care Corp. Operating Room Division, McGraw Park, IL) was placed across the lower anterior chest posterior to both the sternum and the costal cartilages on each side to elevate the sternum as well as the anterolateral chest to the desired level (Fig. 3). For patients with 5 or more deformed cartilages, maximum support for the midsternum is provided when the strut extends obliquely upward on the right side. The strut was attached to the anterior surface of the appropriate rib on each side with fine wire after resecting a 1 cm segment of costal cartilage laterally and making a small opening in the perichondrial sheath for the bar to exit. For 9 patients under age 12 years, or over the age of 40 years, minimal retrosternal dissection was performed, and the strut was placed external to the cartilaginous repair with several large absorbable sutures placed through the anterior table of the sternum and attached to the strut for stability. For 2 of the patients with recurrent PE deformities, 2 anterior struts were placed in each patient. The xiphoid was sutured back to the sternum for all patients. The medial end of each of the deformed cartilages was reattached to the side of the sternum with nonabsorbable sutures; the lateral end was attached to the appropriate rib with sutures or fine wire. The costal cartilages in adults are somewhat soft and brittle, making it necessary to avoid tension with sutures. The periosteum is sutured over the wedge sternal osteotomy to enhance hemostasis.
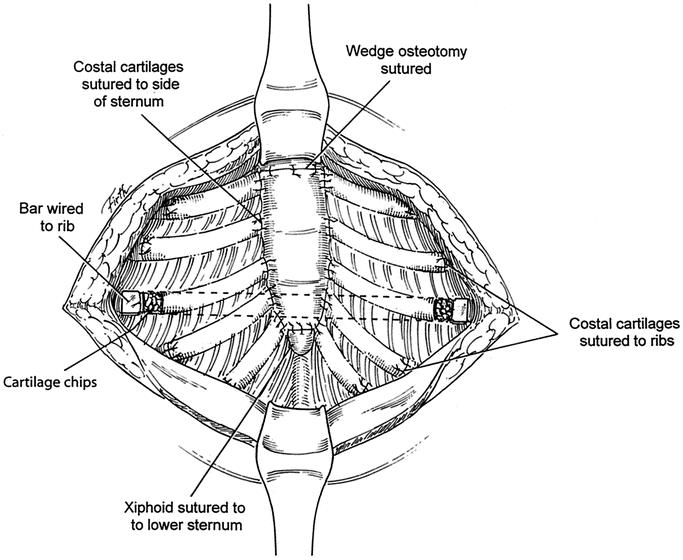
FIGURE 3. An Adkins strut is placed across the lower anterior chest, posterior to the sternum and costal cartilages, with the tip emerging on each side through a small incision in the perichondrial sheath. The strut is attached to the anterior surface of the appropriate rib on each side with fine wire. The xiphoid and each of the deformed costal cartilages are reattached to the sternum with nonabsorbable sutures. The lateral ends of the costal cartilages are sutured back to the ribs. The periosteum is sutured over the wedge osteotomy. Finely minced segments of the cartilage chips are placed in all sites where the costal cartilage is reattached and around the sites where the strut emerges from the chest.
Finely minced segments of the cartilage chips that had been removed earlier were then placed in spaces where the costal cartilage did not make complete contact with the sternum or ribs and around the sites where the strut was wired to the rib to enhance cartilage regeneration. The pectoralis muscles were approximated in the midline, and the abdominal muscles were attached to the pectoralis across the lower chest with absorbable sutures. For the 9 patients with a presternal support bar, a small suction drainage catheter was placed between the muscle layer and the cartilaginous repair. Thorough hemostasis was achieved with electrocautery, and the wound was copiously irrigated with cephazolin solution throughout the operation. The skin was closed with absorbable subcuticular sutures, and Steri-Strips.
The endotracheal tube was removed in the operating room, and the orogastric tube was removed in the recovery room for all patients. No patients were placed in an intensive care unit. Postoperative care adhered to an established clinical pathway program. Only 1 chest x-ray was obtained during the postoperative period. The chest tubes and Foley catheters were routinely removed within 24 hours. The wound drains (9 patients) were removed 5 days postoperation in the office. Intravenous cefazolin was given for 3 days, and oral cephalexin was given for 4 additional days. Postoperative pain was mild for almost all patients and was managed with intravenous patient-controlled analgesics for the first 48 hours and by oral nonnarcotic medications thereafter. Few patients took any analgesic medications after 6 days. Epidural analgesics were not used for any patients.
The mean duration of the operation was 174 minutes (± 26) and 133 minutes (± 19) for patients undergoing repair of recurrent deformities. The mean blood loss was 88 mL. None of the patients were hospitalized for more than 3 days (mean 2.7 days). All but 2 patients returned to school or work within 12 days. Two or less (mean 1.6) postoperative office visits were made during the first 6 months following repair. The sternal support bar was removed through a 2 cm incision on one side of the chest on an outpatient basis under light general anesthesia approximately 6 months after repair (52 patients); the mean operating time was 18 minutes (± 6). There were no complications following bar removal.
RESULTS
Communication was maintained with each of the 75 patients by direct office visits, telephone, or e-mail after the operation (mean follow-up 8.2 months). All but 1 of the patients indicated that they considered the result following repair to be very good or excellent. The chest wall was stable in almost all patients immediately following repair, with minimal paradox in respiratory movements. All patients noted improvement in exercise tolerance, with less dyspnea and increased stamina and endurance as well as reduction of pain or discomfort in the anterior chest within 4 months. Improvement in other symptoms were similar to that noted in previous reports from our hospital.12
Postoperative complications included transient pleural effusion in the right chest in 3 patients, small left pneumothorax in 1 patient, and slight movement of the support bar in 2 patients; no specific postoperative therapy was necessary for any of these patients. Mild to moderate hypertrophy of the cutaneous scar occurred in 9 patients. It is our current practice to have the patient apply Med Derma gel on the scar twice daily for 6 weeks postoperation. Triamcinolone solution (10 mg/mL) is injected into any hypertrophic scar when identified and into the majority of scars at the time of sternal bar removal. There were no perioperative deaths.
DISCUSSION
The operative technique for correction of PE deformities has varied considerably during the 5 decades since Ravitch,4,9 Welch,5 Baronofsky13 and others developed the major concepts of: 1) resection of deformed costal cartilages with preservation of the perichondrial sheaths to permit regeneration of new cartilage or bone in the desired position; 2) transverse posterior or wedge anterior sternal osteotomy with elevation of the lower sternum to the desired level; and 3) some type of internal or external fixation to support the sternum. Many surgeons have detached all the lower perichondrial sheaths and intercostal muscle bundles from the side of the sternum to provide wide exposure with subsequent reattachment to the sternum. For many patients, the suturing of the perichondrial sheaths back to the sternum has resulted in incompletely regenerated cartilage attachment to the sternum, allowing movement at this junction, which commonly produces pain. In patients with PE, the xiphoid is commonly attached to the posterior surface of the lower sternum, which worsens the pectus depression. Resection of the xiphoid has also been common practice; however, xiphoid removal should be avoided because it adds stability to the anterior chest inferior to the sternum and assists in attaching the rectus muscles to the sternum and pectoralis muscle. When the xiphoid angles anteriorly or posteriorly, reattachment to the desired level is desirable.
A tripod fixation technique using the lowermost normal cartilage attached to the sternum to provide support has been used by a few surgeons.14 A variety of struts placed posterior or anterior to the sternum and attached to the ribs on each side have been used to provide stability following repair. Ribs, perichondrial sheaths, metallic or bioabsorbable struts, mesh, vascular grafts, sternal plates, and other prostheses have been used in recent years by various surgeons. The thin metallic strut developed by Adkins and Blades,11 however, has been used by many surgeons, including the present study, because of the effectiveness and the ease of placement and removal. The costal cartilages as well as the sternum should be elevated by the strut to obtain optimal chest contour and reattachment of the costal cartilages to the sternum. Maintenance of the elevated sternum in the corrected position by external traction using harnesses or other cumbersome devices has almost universally been abandoned during the past several years in favor of various methods of internal fixation.
Because such a wide variation of techniques for open repair of pectus deformities, generally referred to as the Ravitch repair, has been used by different surgeons, even in recent years, and because the majority of pediatric and thoracic surgeons perform only a small number of operations each year, it is not surprising that the results have been inconsistent, with recurrence being moderately frequent.15 The recent development of the MIRPE by Nuss et al,6 which avoids an anterior chest incision, cartilage resection, and sternal osteotomy, has been used by many pediatric surgeons in the past few years because the operative technique is less invasive and more standard than the many variations of the Ravitch technique. The MIRPE is primarily applicable for younger patients and those with symmetric PE deformities. Postoperative pain and complications have been reported to be somewhat higher with the MIRPE than with the modified Ravitch repair.16 With the recent increase in awareness of the large number of patients with symptomatic pectus deformities who might benefit from repair, a reassessment of the details of the open technique of PE repair appears justified.
The present report describes a much less extensive repair than the previously reported modifications of the Ravitch technique. When the deformed costal cartilages are removed with preservation of the perichondrial sheaths, the sheaths are often damaged, and the regenerated cartilage is often thin, irregular, and commonly rigid with varying amounts of bone and calcification. Regeneration of the costal cartilages in adult patients is often irregular and incomplete, occasionally producing a somewhat unstable chest. If the regenerated cartilage is rigid, the chest essentially becomes a cylinder, with respiratory motions being largely dependent on diaphragmatic excursions, which limits the depth of lung expansion and requires more effort than normal respiration. Because normal respiratory motions are largely dependent on the flexibility of the costal cartilages, in contrast to the rigid bony ribs, reconstruction of PE deformities should attempt to maintain the cartilage flexibility. Furthermore, some authors have cautioned that removing large segments of costal cartilage in children may interfere with rib growth plates and produce a narrow chest.17 When a small chip of costal cartilage is removed both medially and laterally, the remaining cartilage can be elevated or depressed similar to having a small hinge at either end. Although 7 cartilages attach directly to the sternum, many surgeons have been reluctant to correct more than the lower 4 deformed costal cartilages, which may leave a persistent depression of the upper anterior chest following repair. More than 76% of patients in the present study required repair of 5 or 6 of the costal cartilages to obtain an optimal chest contour.
When the sternal deformity is corrected after the transverse wedge osteotomy, and supported with an Adkins strut, the costal cartilages are sewn back to the sternum medially, and the ribs laterally to provide immediate stability to the chest. The firm suture reattachment of the costal cartilages to the sternum and ribs minimizes the likelihood that there will be movement of the costal cartilages causing pain and instability during the postoperative period. Placement of small chips of autologous cartilage at the sites where the costal cartilages are reattached to the sternum or rib enhances firm attachment, similar to what occurs when autologous bone chips are used during spinal fusion. The intercostal muscles and all but the 2 lowest perichondrial sheaths are not detached from the sternum during the repair. After the metal strut is removed in 6 months, the cartilages appear to regain normal flexibility with respiration.
When the sternum is mobilized with the anterior transverse osteotomy and the costal cartilages are released from their attachment to the sternum medially and ribs laterally, the force necessary to elevate the sternum and anterior chest wall to the desired level is rarely more than 2 pounds.18 Postoperative pain is remarkably mild when little force is necessary to elevate the chest wall and the sternum and anterior chest wall are stabilized with a metal strut. For young patients with a relatively short sternum, and for occasional adult patients over age 40 years who required minimal force to elevate the sternum to the desired level, the Adkins strut has been placed anterior to the sternum and costal cartilages to avoid entering the pleural cavity. The sternum is secured to the strut with several large absorbable sutures. The results in these patients have been similar to those observed in patients with a retrosternal strut. For patients with pectus carinatum, the support strut has routinely been placed anterior to the sternum for many years.
Patients recover more quickly from the less extensive open repair with minimal cartilage resection than with the commonly used variations of the modified Ravitch repair as noted by the short operating time, the stable postoperative chest, the relatively mild postoperative pain, the mean hospital stay of 2.7 days, and the early return to school or work, compared to our previous experience.7,12 The less extensive repair has been very helpful in correcting long and asymmetric deformities and permits construction of a rounded contour for patients with a very narrow anterior to posterior diameter of the chest. Variations of the less extensive open repair have been successful in correcting 6 patients referred for correction of recurrent PE deformities. There appears to be no advantage to the modifications of the more extensive Ravitch repair compared with the presently described technique. Longer follow-up will be necessary to determine if the results observed in this initial study are maintained for many years.
Footnotes
Reprints: Eric W. Fonkalsrud, MD, Department of Surgery, UCLA Medical Center, Los Angeles, CA 90095. E-mail: efonkalsrud@mednet.ucla.edu.
REFERENCES
- 1.Molik KA, Engum SA, Rescorla FJ, et al. Pectus excavatum repair: experience with standard and minimal invasive techniques. J Pediatr Surg. 2001;36:324–328. [DOI] [PubMed] [Google Scholar]
- 2.Shamberger RC. Congenital chest wall deformities. Curr Prob Surg. 1996;33:469–552. [DOI] [PubMed] [Google Scholar]
- 3.Shamberger RC, Welch KJ. Cardiopulmonary function in pectus excavatum. Surg Gynecol Obstet. 1988;166:383–391. [PubMed] [Google Scholar]
- 4.Ravitch MM. Operative technique of pectus excavatum repair. Ann Surg. 1949;129:429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch KJ. Satisfactory surgical correction of pectus excavatum deformity in childhood: a limited opportunity. J Thorac Surg. 1958;36:697–713. [PubMed] [Google Scholar]
- 6.Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545–552. [DOI] [PubMed] [Google Scholar]
- 7.Fonkalsrud EW, DeUgarte D, Choi E. Repair of pectus excavatum and carinatum deformities in 116 adults. Ann Surg. 2002;236:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haller JA Jr., Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg. 1987;22:904–906. [DOI] [PubMed] [Google Scholar]
- 9.Ravitch MM. Operative treatment of congenital deformities of the chest. Am J Surg. 1961;19:588–597. [DOI] [PubMed] [Google Scholar]
- 10.Ravitch MM. Technical problems in the operative correction of pectus. Ann Surg. 1965;162:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adkins PC, Blades B. A stainless steel strut for correction of pectus excavatum. Surg Gynecol Obstet. 1961;113:111–113. [PubMed] [Google Scholar]
- 12.Fonkalsrud EW, Dunn JCY, Atkinson JB. Repair of pectus excavatum deformities: 30 years experience with 375 patients. Ann Surg. 2000;231:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baronofsky ID. Technique for the correction of pectus excavatum. Surgery. 1957;42:884–890. [PubMed] [Google Scholar]
- 14.Haller JA, Peter GN, Mazur D, White JJ. Pectus excavatum: a 20 year surgical experience. J Thorac Cardiovasc Surg. 1970;60:375–383. [PubMed] [Google Scholar]
- 15.DeUgarte D, Choi E, Fonkalsrud EW. Repair of recurrent pectus deformities. Ann Surg. 2002;68:1075–1079. [PubMed] [Google Scholar]
- 16.Fonkalsrud EW, Beanes S, Hebra A, et al. Comparison of minimally invasive and modified Ravitch pectus excavatum repair. J Pediatr Surg. 2002;37:413–417. [DOI] [PubMed] [Google Scholar]
- 17.Martinez D, Juame J, Stein T, et al. The effect of costal cartilage resection on chest wall development. Pediatr Surg Int. 1990;5:170–173. [Google Scholar]
- 18.Fonkalsrud EW, Reemtsen B. Force required to elevate the sternum of pectus excavatum patients. J Am Col Surg. 2002;195:575–577. [DOI] [PubMed] [Google Scholar]


