Abstract
Objective:
Evaluate the safety and efficacy of bariatric surgery in older patients.
Background:
Because of an increased morbidity in older patients who may not be as active as younger individuals, there remain concerns that they may not tolerate the operation well or lose adequate amounts of weight.
Methods:
The database of patients who had undergone bariatric surgery since 1980 and National Death Index were queried for patients <60 and ≥ 60 years of age. GBP was the procedure of choice after 1985. Data evaluated at 1 and 5 years included weight lost, % weight lost (%WL), % excess weight loss (%EWL), % ideal body weight (%IBW), mortality, complications, and obesity comorbidity.
Results:
Eighty patients underwent bariatric surgery: age 63 ± 3 years, 78% women, 68 white, 132 ± 22 kg, BMI 49 ± 7 kg/m2, 217 ± 32%IBW. Preoperative comorbidity, was greater (P < 0.001) in patients ≥ 60 years. There were no operative deaths but 11 late deaths. Complications: 4 major wound infections, 2 anastomotic leaks, 10 symptomatic marginal ulcers, 5 stomal stenoses, 3 bowel obstructions, 26 incisional hernias (nonlaparoscopic), and 1 pulmonary embolism. At 1 year after surgery (94% follow-up), patients lost 38 ± 11 kg, 57%EWL, 30%WL, BMI 34.5 ± 7 kg/m2, %IBW 153 ± 31. Comorbidities decreased (P < 0.001); however, %WL and %EWL and improvement in hypertension and orthopedic problems, although significant, were greater in younger patients. At 5 years after surgery (58% follow-up), they had lost 31 ± 18 kg, 50%EWL, 26%WL, BMI 35 ± 8 kg/m2, and %IBW 156 ± 36.
Conclusions:
Bariatric surgery was effective for older patients with a low morbidity and mortality. Older patients had more pre- and post-operative comorbidities and lost less weight than younger patients. However the weight loss and improvement in comorbidities in older patients were clinically significant.
Eighty patients 60 years of age or older underwent bariatric surgery with excellent weight loss and correction of obesity comorbidity. Although their weight loss and comorbidity improvement were not as favorable as those younger than 60 years, their clinical improvement was still significant and warrants bariatric surgery in older patients.
Although several papers have been published regarding the efficacy of bariatric surgery in older patients,1,2 there remain concerns regarding the operative morbidity and mortality and the adequacy of weight loss due to the relative immobility of older patients.3–5 We have performed bariatric surgery on a number of older patients and were interested in comparing their results to our younger patients.
METHODS
After obtaining Institutional Review Board approval, we queried our database on patients < and ≥ 60 years of age with regards to age, race, gender, preoperative weight (kg), body mass index (BMI) in kg/m2, and ideal body weight (IBW) based on medium bone density according to the 1959 Metropolitan Life Insurance Company,6 preoperative comorbidity as well as postoperative kilograms lost, percent decrease in BMI > 25 kg/m2, percent IBW achieved, percent weight loss, percent excess weight loss with excess weight defined as weight in kilograms above IBW, and resolution of comorbidities at 1 and 5 years after surgery.
The diagnosis of type 2 diabetes mellitus (DM) required an elevated fasting blood sugar (≥ 150 mg/dL) and either a “diabetic diet” recommended by their primary care physician, oral hypoglycemic medications, or insulin treatment. The diagnosis of systemic hypertension (HTN) required a sitting blood pressure at the time of their initial visit of ≥ 150 mm Hg systolic and/or ≥90 mm Hg diastolic (using a wide blood pressure cuff taken with an automatic sphygmomanometer) or use of antihypertensive medications. Resolution of DM was defined as a fasting blood sugar ≤ 120 mg/dL and absence of diabetic medications while resolution of HTN required no use of antihypertensive medications, a systolic blood pressure ≤ 135 mm Hg, and diastolic blood pressure ≤ 85 mm Hg. Obesity hypoventilation syndrome (OHS) was defined as a PaO2 ≤ 55 mm Hg and/or PaCO2 ≥ 65 mm Hg, sleep apnea syndrome (SAS) as a respiratory disturbance index ≥ 10 hypopneic and/or apneic episodes/hr of sleep, degenerative joint/back disease (DJBD) as self-reported complaints of pain in the weight-bearing joints or back, gastroesophageal reflux disease (GERD) as “heart burn” symptoms requiring drug therapy, chronic venous stasis disease (CVSD) as the presence of pretibial venous stasis ulcers or bronze edema, and urinary incontinence (UI) as a history of difficulty controlling their urine with a need in women to wear a perineal pad.
Procedures performed during this time in the older patients included horizontal gastroplasty7 (HGP) in 1981, vertical-banded gastroplasty8 (VBG) from 1982 to 1985. As a result of our randomized and selective studies9,10 confirmed by others,11–16 proximal gastric bypass (P-GBP) was the procedure of choice after 1985. Long-limb gastric bypass (LL-GBP) with a 150-cm Roux (alimentary limb) was the procedure of choice for patients with a BMI ≥ 50 kg/m2 after 1991.17 Hand-assisted laparoscopic GBP (HAL-GBP) was performed in 1998 and totally laparoscopic GBP (L-GBP) in most patients after 1999.18,19 A few patients < 60 years had a malabsorptive distal gastric bypass (D-GBP).20 All GBP patients were asked to take lifetime supplemental vitamin B12 (500 mcg/d po or 1 mg/mo IM), and calcium (500 mg/d). Menstruating women were told to take ferrous sulfate (325 mg bid). Laboratory data obtained at the annual follow-up visit included standard hemoglobin and complete metabolic profiles, vitamin B12, serum magnesium, and iron levels. Patients were contacted by letter if deficiencies were noted for additional supplementation. Major efforts were made to contact patients who had not returned for follow-up visits. The National Death Index was queried for deaths that may have been unknown. Data are expressed as mean ± sd. Frequency data were analyzed using Fisher exact test, Kruskal-Wallis test, Wilcoxon scores, and Satterthwaite test as appropriate; means were analyzed using ANOVA.
RESULTS
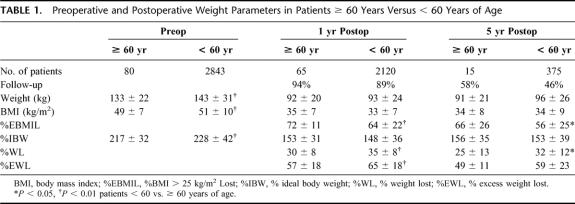
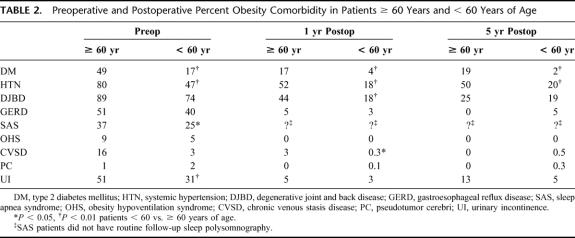
There were 80 patients ≥ 60 years who underwent a primary bariatric surgery procedure between 1981 and January 2003 (age 63 ± 3 years; range, 60.1–74.5 years). There were 62 (78%) women, 68 (85%) whites, 11 blacks, and 1 Hispanic. Procedures included 1 HGP, 3 VBG, 28 P-GBP, 24 LL-GBP, 2 HAL-GBP, and 22 L-GBP. They weighed 133 ± 22 kg, BMI 49 ± 7 kg/m2, and 217 ± 32%IBW. During this same time interval, 2843 patients < 60 years had undergone bariatric surgery at VCU: 81% women, 72% white. Thus, the older group represents only 2.7% of our total bariatric surgical population. There has been a marked increase (P < 0.0001) in the frequency of GBP in both older and younger patients since 1999. The patients ≥ 60 years weighed somewhat less than our younger patients (Table 1) but had a much higher frequency of obesity comorbidities (3.8 ± 1.5 comorbidities/patient ≥ 60 years vs. 2.4 ± 1.5 in patients < 60 years, P < 0.0001, Table 2). Of those patients ≥ 60 years versus < 60 years diagnosed as having type 2 DM, 42% versus 31% required insulin for control and 20% versus 45% required an oral agent (both, P < 0.001). There were no deaths within 30 days of a primary bariatric operation. One patient died postoperatively after conversion to P-GBP 4 years after a HGP secondary to an obstructed tracheostomy that had been placed prior to her operation for severe obstructive sleep apnea. There were 10 other late deaths, 18 months to 10 years (4.5 ± 2.7 years) after bariatric surgery in the older patients. Unfortunately, we were unable to determine the cause of death in these patients, as we could not obtain access to their death certificates and this information was not provided with our query of the National Death Index. One patient underwent reversal of the gastric bypass in another city for unknown reasons, and 1 patient with an inadequate weight loss was converted to a malabsorptive distal GBP.20 One patient who had lost only 9% of his excess weight remained hypertensive and suffered a hemiparetic stroke 6 years after his GBP. Early complications within 30 days of surgery in the patients ≥ 60 years included 4 major wound infections, 2 anastomotic leaks, and 1 pulmonary embolus. Late complications included 10 symptomatic marginal ulcers (all treated effectively with acid suppression medications), 5 stomal stenoses (all treated successfully with endoscopic dilatation), 3 bowel obstructions, and 26 incisional hernias (none in the totally laparoscopic cases).
TABLE 1. Preoperative and Postoperative Weight Parameters in Patients ≥ 60 Years Versus < 60 Years of Age
TABLE 2. Preoperative and Postoperative Percent Obesity Comorbidity in Patients ≥ 60 Years and < 60 Years of Age
At 1 year after surgery (94% follow-up of 69 available patients ≥ 60 years), these patients lost a significant amount of weight (Table 1); 85% of this weight loss had occurred within 6 months after surgery. There were also clinically significant improvements in all obesity comorbidities (Table 2). However, the %WL and %EWL, although significant in amount, was less (P < 0.001) and the improvement in hypertension and orthopedic problems less (P < 0.001) than that seen in our younger patients, while there were no differences in the improvements in the other obesity comorbidities (Tables 1, 2). At 5 years after surgery with 57% follow-up in 26 surviving older patients, there continued to be significant weight loss and improvement in their obesity comorbidity (Tables 1, 2). At 1 year after surgery, their hemoglobin was 12.7 ± 2 g/dL, B12 627 ± 496 ìg/mL, calcium 9 ± 0.5 mg/dL, magnesium 2 ± 0.2 mg/dL, and albumin 3.8 ± 2 g/dL. Equivalent data were noted at 5 years after surgery. Only 5 of 13 patients available for follow-up were seen at 10 years postoperatively; they had lost 22 ± 11 kg for 21 ± 12%WL, 44 ± 25%EWL with a 34 ± 4 kg/m2 BMI, which was 49 ± 25%EBMIL for a 151 ± 26%IBW achieved.
DISCUSSION
In this and other reports,1,2 bariatric surgery in older patients was safe and effective for weight loss and, most importantly, for improvement in obesity comorbidities. Almost all of the weight loss was seen within 1 year of surgery, and 85% of this occurred within the first 6 months. Wheelchair-bound patients were often fully ambulatory within months of surgery. There has been a concern that the impaired ambulation and physical activity in older patients would preclude an adequate response to bariatric surgery. Although the weight loss and improvement in obesity comorbidities were less in our older compared with our younger patients, they were clinically significant. If total joint replacement and coronary artery bypass grafts are considered appropriate for octogenarians and nanogenarians,21,22 it certainly seems reasonable to offer bariatric surgery for patients who live into their 60s and 70s and are impaired by their severe obesity.
The effects of current weight loss medications have not been published in patients ≥ 60 years of age. In young, responding patients, they lose about 10% of their weight at 1 year when combined with a behavior modification and exercise program. Weight loss after gastric bypass in our older patients was 30% (or about 60% of excess weight). Multiple randomized, prospective, and retrospective trials have documented the superiority of gastric bypass compared with gastroplasty for weight loss in severe obesity.9–16 The adjustable silicone gastric band (LapBand) was approved for use in the United States in 2001 by the Food and Drug Administration. This device provided the loss of only 38% of excess weight at 3 years after surgery in our series of younger patients and 11% of excess weight in our black patients.23 Many of these patients have had the LapBand removed for either inadequate weight loss, dysphagia, esophageal dilatation, or band slippage. In the Swedish Obese Subjects (SOS) trial initiated in 1987, involving patients less than 57 years of age, it was planned that 1000 patients would undergo bariatric surgery and 10,000 treated in their primary health care clinics.24 The surgically and medically treated patients were then matched according to age, sex, BMI, and comorbidity. Of the surgical group, 94% underwent gastroplasty or laparoscopic placement of an adjustable silastic gastric band, and these patients lost significantly less weight than the 6% of patients who underwent a gastric bypass. There was an overall decrease in the use of sick leave and disability pension in the surgically treated patients than those in the nonsurgical arm of the SOS trial.24 The control of type 2 diabetes mellitus was 47% and hypertension 42%; however, there was loss of the improvement in hypertension at 3 to 5 years after surgery.25 With subset analysis, there were still significant decreases in both systolic and diastolic blood pressures at ≥ 5 years in the small percentage of patients who had undergone gastric bypass.26 In other studies, gastric bypass was associated with resolution of type 2 diabetes mellitus in 79% to 85%27–29 and hypertension in 60% to 75% of patients.30,31 We have also noted that resolution of hypertension and diabetes is proportional to weight loss.32 One of our older patients lost only 9% of his weight, remained hypertensive, and suffered a hemiparetic stroke 9 years after gastric bypass.
Bariatric surgery also has been shown to be effective for other obesity comorbidity problems, including obesity hypoventilation,33,34 sleep apnea,34,35 pseudotumor cerebri,36,37 GERD,38 urinary incontinence,39 and chronic venous stasis disease.40 We have noted that the average age of patients without diabetes or hypertension was 35 years, 5 years younger than patients with either diabetes or hypertension and 10 years younger than those with both diabetes and hypertension.32 In the current study, the frequency of both diabetes and hypertension in patients ≥ 60 years were twice that in younger patients with a significantly greater need for insulin for adequate glucose control. Others have noted that the longer a patient is diabetic, the less likely will be their correction of diabetes after gastric bypass.27–29 We have had several patients referred for gastric bypass who have had complications of their diabetes, including pedal gangrene, neuropathy, renal dysfunction, and retinopathy. These factors suggest an earlier aggressive approach to the morbidly obese diabetic patient.
There are several weaknesses in this study. The inability to obtain 100% follow-up has always been a problem in the United States, where insurance preauthorization is required, the cost of laboratory studies are at least partially borne by the patients, it is not permissible to contact patients through their social security numbers without prior consent, and patients move frequently throughout the country. Major efforts were made to have our patients return for their follow-up visits, including letters and phone calls with the primary goal of monitoring their care and laboratory data. They were seen at each visit by a registered dietitian who offered behavioral modification suggestions if they were regaining weight. The absence of objective data with regards to several of the patients’ comorbidity problems are also a concern, such as preoperative and postoperative glucose tolerance tests or hemoglobin A1c levels for diabetes, 24-hour pH studies for GERD, or sleep polysomnography for sleep apnea. However, in the absence of symptoms, insurance companies would not authorize these postoperative tests, and a research grant to cover 25 years of patient follow-up would not have been feasible. Nevertheless, the patients’ symptoms seem to be a reasonable estimate of their response to weight reduction surgery.
CONCLUSION
Until better medical management becomes available for the severely obese older patient, bariatric surgery appears to be a reasonable and effective treatment. Gastric bypass has been shown to be more effective than gastroplasty. Now that it is possible to perform the gastric bypass laparoscopically with a lower risk of wound complications, including major wound infections and incisional hernias,18,19 we think that it is the procedure of choice in older patients. However, it is extremely important that surgeons performing this procedure have both adequate training and a commitment to the long-term care of these patients. Class II evidence regarding the use of laparoscopic gastric bypass compared with laparoscopic adjustable gastric banding for the treatment of severely obese patients will require long-term randomized, prospective trials. These can only be performed in Veterans Administration Hospitals in the United States, as most insurance companies currently do not support clinical research trials involving surgical procedures.
Footnotes
Reprints: Eric J. DeMaria, MD, Department of Surgery, Box 980519, Richmond, VA 23298.
REFERENCES
- 1.Macgregor AM, Rand CS. Gastric surgery in morbid obesity: outcome in patients aged 55 years and over. Arch Surg. 1993;128:1153–1157. [DOI] [PubMed] [Google Scholar]
- 2.Murr MM, Siadati MR, Sarr MG. Results of bariatric surgery for morbid obesity in patients older than 50 years. Obes Surg. 1995;5:399–402. [DOI] [PubMed] [Google Scholar]
- 3.Printen KJ, Mason EE. Gastric bypass for morbid obesity in patients more than fifty years of age. Surg Gynecol Obstet. 1977;144:192–194. [PubMed] [Google Scholar]
- 4.Bobbioni-Harsch E, Huber O, Morel P, et al. Factors influencing energy intake and weight loss after gastric bypass. Eur J Clin Nutr. 2002;56:551–556. [DOI] [PubMed] [Google Scholar]
- 5.Livingston EH, Huerta S, Arthur D, et al. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg. 2002;236:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metropolitan Life Insurance Co. New weight standards for men and women. Stat Bull Metropol Life Insur Co. 1959;40:1–4. [Google Scholar]
- 7.Sugerman HJ, Wolper JC. Failed gastroplasty for morbid obesity: revised gastroplasty vs. roux-en-y gastric bypass. Am J Surg. 1984;148:331–336. [DOI] [PubMed] [Google Scholar]
- 8.Mason EE. Vertical banded gastroplasty for obesity. Arch Surg. 1982;117:701–706. [DOI] [PubMed] [Google Scholar]
- 9.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg. 1987;205:613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugerman HJ, Londrey GL, Kellum JM, et al. Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg. 1989;157:93–102. [DOI] [PubMed] [Google Scholar]
- 11.MacLean LD, Rhode BM, Sampalis J, et al. Results of the surgical treatment of obesity. Am J Surg. 1993;165:155–162. [DOI] [PubMed] [Google Scholar]
- 12.Hall JC, Watts JM, O'Brien PE, et al. Gastric surgery for morbid obesity: the Adelaide study. Ann Surg. 1990;211:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yale CE. Gastric surgery for morbid obesity: complications and long-term weight control. Arch Surg. 1989;124:941–946. [DOI] [PubMed] [Google Scholar]
- 14.Howard L, Malone M, Michalek A, et al. Gastric bypass and vertical banded gastroplasty: a prospective randomized comparison and 5-year follow-up. Obes Surg. 1995;5:55–60. [DOI] [PubMed] [Google Scholar]
- 15.Brolin RL, Robertson LB, Kenler HA, et al. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg. 1994;220:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capella JF, Capella RF. The weight reduction operation of choice: vertical banded gastroplasty or gastric bypass? Am J Surg. 1996;171:74–79. [DOI] [PubMed] [Google Scholar]
- 17.Brolin RE, Kenler HA, Gorman JH, et al. Long-limb gastric bypass in the superobese: a prospective randomized study. Ann Surg. 1992;215:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen NT, Goldman C, Rosenquist J, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality-of-life, and costs. Ann Surg. 2001;234:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeMaria EJ, Sugerman HJ, Kellum JM, et al. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg. 2002;235:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugerman HJ, Kellum JM, DeMaria EJ. Conversion of proximal to distal gastric bypass for failed gastric bypass for superobesity. J Gastrointest Surg. 1997;1:517–525. [DOI] [PubMed] [Google Scholar]
- 21.Brander VA, Malhotra S, Jet J, et al. Outcome of hip and knee arthroplasty in persons aged 80 years and older. Clin Orthop. 1997;345:67–78. [PubMed] [Google Scholar]
- 22.Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients > or = 80 years: results form the National Cardiovascular Network. J Am Coll Cardiol. 2000;35:731–738. [DOI] [PubMed] [Google Scholar]
- 23.DeMaria EJ, Sugerman HJ, Meador JG. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narbro K, Agren G, Jonsson E, et al. Sick leave and disability pension before and after treatment for obesity: a report from the Swedish Obesity Subjects (SOS) study. Int J Obes Relat Metab Disord. 1999;23:619–624. [DOI] [PubMed] [Google Scholar]
- 25.Sjostrom CD, Peltonen M, Wedel H, et al. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000;36:20–25. [DOI] [PubMed] [Google Scholar]
- 26.Sjostrom CD, Peltonen M, Sjostrom L. Blood pressure and pulse pressure during long-term weight loss in the obese: the Swedish Obese Subjects (SOS) intervention study. Obes Res. 2001;9:188–195. [DOI] [PubMed] [Google Scholar]
- 27.Pories WJ, MacDonald KG Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr 1992;55(suppl):582–585. [DOI] [PubMed] [Google Scholar]
- 28.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald KG Jr, Long SD, Swanson MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg. 1997;1:213–220. [DOI] [PubMed] [Google Scholar]
- 30.Foley EF, Benotti PN, Borlase BC, et al. Impact of gastric restrictive surgery on hypertension in the morbidly obese. Am J Surg. 1992;163:294–297. [DOI] [PubMed] [Google Scholar]
- 31.Carson JL, Ruddy ME, Duff AE, et al. The effect of gastric bypass surgery on hypertension in morbidly obese patients. Ann Intern Med. 1994;154:193–200. [PubMed] [Google Scholar]
- 32.Sugerman HJ, Wolfe LG, Sica DA, et al. Diabetes and hypertension in severe obesity and effects of gastric bypass induced weight loss. Ann Surg. 2003;237:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugerman HJ, Baron PL, Fairman RP, et al. Hemodynamic dysfunction in obesity hypoventilation syndrome and the effects of treatment with surgically induced weight loss. Ann Surg. 1988;207:604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugerman HJ, Fairman RP, Sood RK, et al. Long-term effects of gastric surgery for treating respiratory insufficiency of obesity. Am J Clin Nutr 1992;55(suppl):597–601. [DOI] [PubMed] [Google Scholar]
- 35.Charuzi I, Lavie P, Peiser J, et al. Bariatric surgery in morbidly obese sleep-apnea patients: short- and long-term follow-up. Am J Clin Nutr 1992;55(suppl):594–596. [DOI] [PubMed] [Google Scholar]
- 36.Sugerman HJ, Felton WL III, Sismanis A, et al. Gastric surgery for pseudotumor cerebri associated with severe obesity. Ann Surg. 1999;229:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugerman HJ, Felton WL, Sismanis A, et al. Effects of surgically induced weight loss on pseudotumor cerebri in morbid obesity. Neurology. 1995;45:1655–1659. [DOI] [PubMed] [Google Scholar]
- 38.Smith SC, Edwards CB, Goodman GN. Symptomatic and clinical improvement in morbidly obese patients with gastroesophageal reflux disease following Roux-en-Y gastric bypass. Obes Surg. 1997;7:479–484. [DOI] [PubMed] [Google Scholar]
- 39.Bump RC, Sugerman HJ, Fantl JA, et al. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol. 1992;167:392–399. [DOI] [PubMed] [Google Scholar]
- 40.Sugerman HJ, Sugerman EL, Wolfe L, et al. Risks/benefits of gastric bypass in morbidly obese patients with severe venous stasis disease. Ann Surg. 2001;234:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]