Abstract
Objective:
The aim of this study was to determine whether anastomotic leakage has an independent association with overall survival and cancer-specific survival.
Summary Background Data:
There are many known prognostic indicators following surgery for colorectal cancer (CRC). However, the impact of anastomotic leakage has not been adequately assessed.
Methods:
Consecutive patients undergoing resection between 1971 and 1999 were recorded prospectively in the Concord Hospital CRC database. Total anastomotic leakage was defined as any leak, whether local, general, or radiologically diagnosed. Patients were followed until death or to December 31, 2002. The association between anastomotic leakage and both overall survival and cancer-specific survival was examined by proportional hazards regression with adjustment for other patient and tumor characteristics influencing survival. Confidence intervals (CI) were set at the 95% level.
Results:
From an initial 2980 patients, 1722 remained after exclusions. The total leak rate was 5.1% (CI 4.1–6.2%). In patients with a leak, the 5-year overall survival rate was 44.3% (CI 33.5–54.6%) compared to 64.0% (CI 61.5–66.3%) in those without leak. In proportional hazards regression–after adjustment for age, gender, urgent resection, site, size, stage, grade, venous invasion, apical node metastasis and serosal surface involvement–anastomotic leakage had an independent negative association with overall survival (hazard ratio [HR] 1.6, CI 1.2–2.0) and cancer-specific survival (HR 1.8, CI 1.2–2.6).
Conclusion:
Apart from its immediate clinical consequences, anastomotic leakage also has an independent negative association with survival.
In 1722 consecutive patients having a curative resection for colorectal cancer between 1971 and 1999, the overall 5-year survival rates were 44.3% (confidence interval 33.5–54.6%) in those with anastomotic leak and 64.0% (confidence interval 61.5–66.3%) in those without leak. This association persisted after adjustment for 10 other factors associated with survival.
Anastomotic leakage is a serious complication following restorative resection for colorectal cancer (CRC). Its reported prevalence varies as widely as from 1% to 39%, but comparisons are difficult because of a lack of standardized definition.1 Leakage may present as generalized peritonitis requiring abdominal reoperation, as a more localized collection that may discharge, or as a subclinical leak detected merely on contrast radiology. Hitherto those without peritonitis have been generally considered to be of less consequence.
Several factors have been shown to have independent prognostic significance for survival following potentially curative resection for CRC.2–7 However, there are only a few reports on the association between anastomotic leakage and long-term survival.8–11 The aim of this study was to examine the relationship between anastomotic leakage and both overall survival and cancer-specific survival in our patients.
METHODS
Since 1971, clinical, operative, pathology, adjuvant therapy, and follow-up data on consecutive patients who had a resection for CRC at Concord Hospital have been recorded prospectively in a computer database.12 From 1979, the clinical and operative data were recorded by one surgeon (P.H.C). Since the establishment of the database, 90% of all surgical specimens have been dissected and reported on by a single pathologist (R.C. Newland), who also reviewed the histology of the remaining cases and coded the pathology data.
A standard surgical technique was adopted in 1980. In addition, total anatomic dissection of the rectum was introduced in 1981 and has been performed by all members of the department since 1984. This technique has been described in detail elsewhere.13,14 Patients undergoing elective resections received a standard preoperative bowel preparation. For anterior resections, the distal rectum was lavaged with saline or water prior to division.
Histopathological examination of all surgical specimens was according to a standard protocol, and the cancers were staged using a clinicopathological system that has been validated by survival analysis.7,15 An apical lymph node was defined as the most proximal of any nodes found within 1 cm of the vessel ligation at the apex of a vascular pedicle.16 Tumor grade was assessed, taking into account the degree of differentiation and anaplasia, the nature of the tumor margin (pushing or infiltrating), and the presence of small vessel invasion. Tumors were classified as high grade or other. Venous invasion referred to involvement of thin or thick walled veins, either within or beyond the bowel wall. When doubt existed as to whether the structure involved was a vein, a negative finding was recorded.
This study was based on patients who had a resection between January 1, 1971 and December 31, 1999, and who were followed until death or at least to December 31, 2000. Patients were reviewed approximately every 3 months in the first year after resection, every 6 months in the second year, and annually thereafter. Cause of death was determined by consultation with primary and secondary clinicians and by reference to statewide and national registries of deaths.
The following patients were excluded: those with metachronous tumor, familial adenomatous polyposis coli, inflammatory bowel disease, carcinoma in situ, patients who died of causes other than colon cancer within 30 days of their operation or before discharge from hospital, those having a palliative resection, those receiving preoperative or postoperative adjuvant therapy, and those having nonrestorative resections (Fig. 1). Palliative resections were defined as those in which there was known residual tumor, either distant or local.
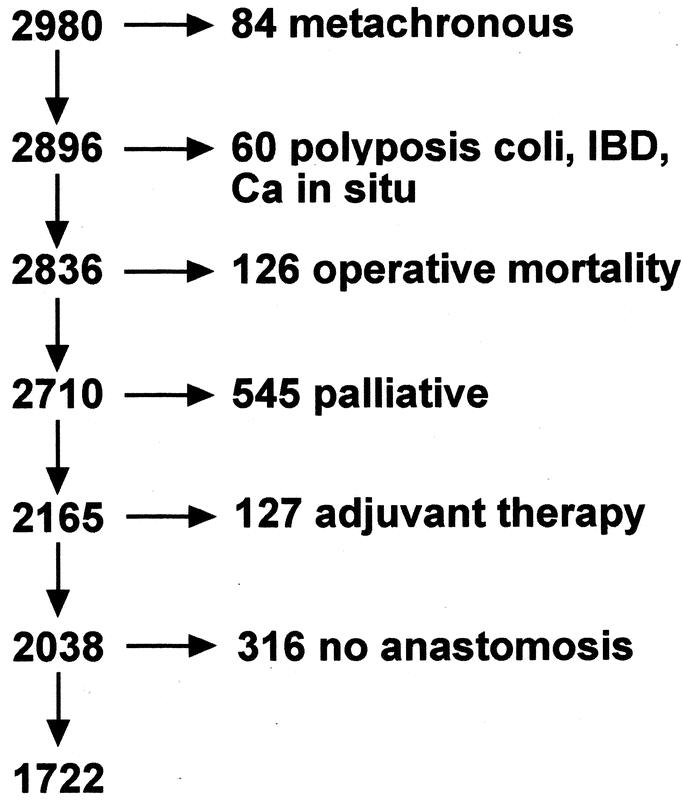
FIGURE 1. Exclusions from 2980 patients who had a resection between 1971 and 1999.
The definition of anastomotic leakage included all patients who developed any clinical or radiologic evidence of dehiscence of the anastomosis, whether or not reoperation or any other intervention was required.
Statistical Methods
Overall survival time was measured from the date of resection to the date of death due to any cause, with patients alive at December 2000 and those lost to follow-up being censored in survival analyses. Cancer-specific survival time was measured from the date of resection to the date of death due to CRC, with censoring as above but also including those who died of causes other than CRC.
The χ2 test was used to examine the significance of differences in contingency tables. Comparisons of survival time between strata of categorical variables were made with the Kaplan-Meier method and log-rank test. Multiple regression for variables showing a significant bivariate association with survival employed the Cox method. The assumption of proportional hazards was checked by examination of plots of log cumulative hazard for parallelism, and in no case was this assumption materially violated. Analyses employed SPSS for Windows, Version 11.1, SPSS Australasia Pty. Ltd, 2002, and Egret Version 1.02.11, Cytel Software Corporation, MA, 1997. The level for statistical significance was set at 0.05, and confidence intervals (CI) are at the 95% level.
RESULTS
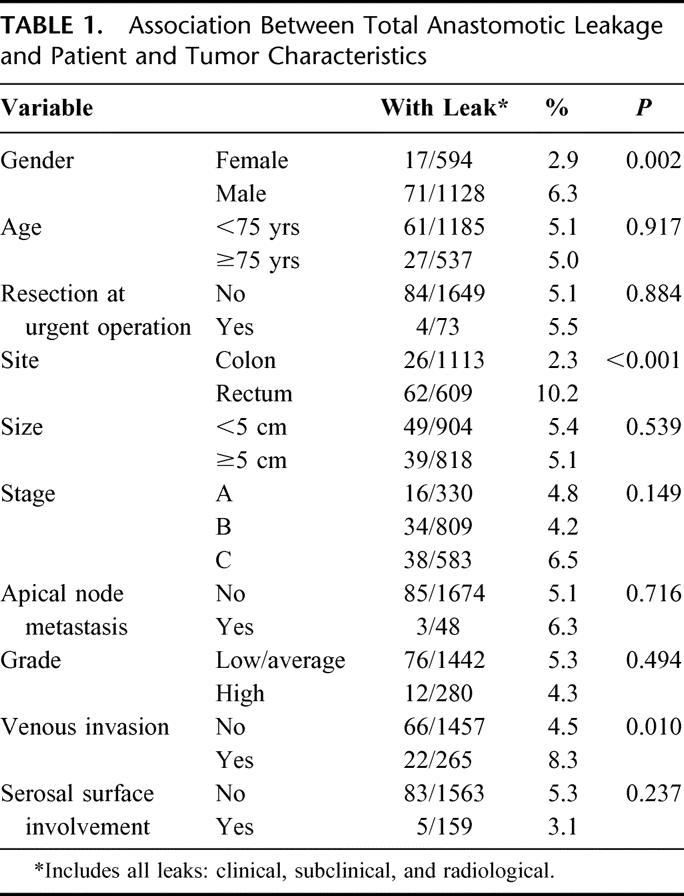
This study is based on 2980 patients who had a resection between January 1, 1971 and December 31, 1999. After exclusions (Fig. 1), 1722 remained for analysis, although 76 of these for whom the cause of death could not be identified were excluded from analyses of cancer-specific survival. Twenty-seven patients (1.6%, CI 1.0–2.3%) developed a general anastomotic leak, defined as one requiring urgent abdominal reoperation, and in a further 61 patients (3.5%, CI 2.7–4.5%), localized leakage occurred, which was treated either expectantly or by percutaneous drainage. The prevalence of leakage was significantly greater in males than in females, greater for rectal than for colonic tumors, and greater in tumors with venous invasion (Table 1). Leakage was not associated with age, urgency of operation, tumor size, stage, apical node metastasis, histologic grade, or free serosal surface involvement.
TABLE 1. Association Between Total Anastomotic Leakage and Patient and Tumor Characteristics
In patients with any anastomotic leakage, local or general, the 5-year overall survival rate was 44.3% (CI 33.5–54.6%) as compared to 64.0% (CI 61.5–66.3%) in patients without leakage (Fig. 2), and the hazard ratio (HR) for leakage was 1.6 (CI 1.3–2.1). Cancer-specific survival was also poorer in patients with leakage (HR 1.8, CI 1.3–2.6). When general leaks were excluded and local leaks examined alone, the negative association remained, both for overall survival (HR 1.7, CI 1.3–2.3) and cancer-specific survival (HR 2.2, CI 1.4–3.2). There were too few general leaks for separate analysis.
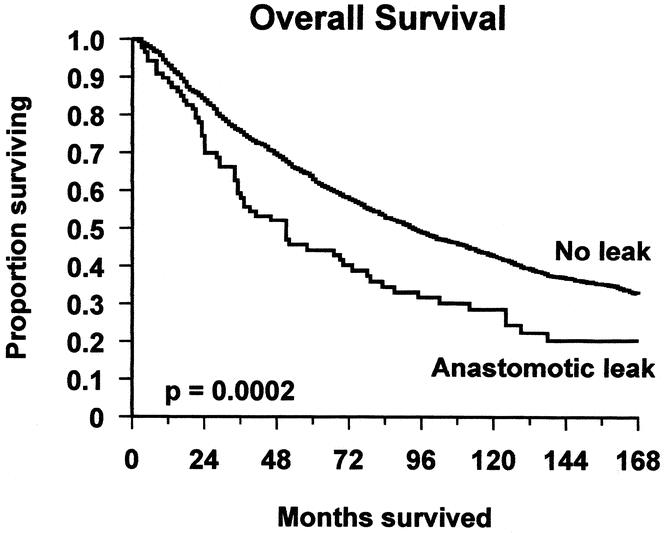
FIGURE 2. Overall survival of patients with and without anastomotic leakage.
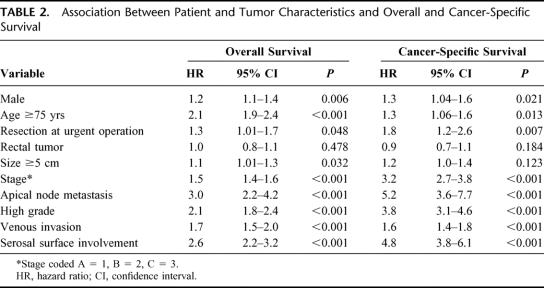
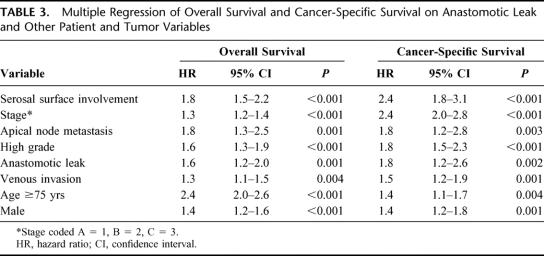
Bivariate analyses showed that both overall survival and cancer-specific survival were significantly associated with gender, age, urgency of operation, stage, grade, apical node metastasis, venous invasion, and free serosal surface involvement (Table 2). Overall survival was significantly associated with tumor size, whereas cancer-specific survival was not. Neither overall survival nor cancer-specific survival was associated with tumor site. Proportional hazards regression commencing with all of the above variables and followed by stepwise elimination of nonsignificant variables yielded final models containing the same set of variables for both overall and cancer-specific survival (Table 3). Both models showed a significant independent negative association between anastomotic leakage and survival with a hazard ratio of 1.6 for overall survival and 1.8 for cancer-specific survival. The excluded variables, size, site, and urgent resection were added separately into these models, but none had a significant effect. The initially surprising finding that leakage was associated with tumor site, whereas survival was not, arose because leakage was a rare event (total prevalence 5.1%) and therefore incapable of influencing the fundamental lack of association between site and survival.
TABLE 2. Association Between Patient and Tumor Characteristics and Overall and Cancer-Specific Survival
TABLE 3. Multiple Regression of Overall Survival and Cancer-Specific Survival on Anastomotic Leak and Other Patient and Tumor Variables
DISCUSSION
In this study, we have shown that anastomotic leakage is an independent predictor of diminished overall and cancer-specific survival. There was no relationship between tumor site and survival, and the association between leakage and survival was also independent of site. Moreover, this applied to all leaks, including those that were considered to be merely localized and were treated expectantly or by percutaneous drainage. In this study, we were unable to assess the prognostic importance of generalized anastomotic leakage separately because of insufficient numbers. However one can assume that the effect would persist in that group.
To our knowledge, this is the largest series of patients in which this subject has been examined, and our results are similar to those of the West of Scotland and Highland Anastomosis Study Group.8,9 In both of their studies, there was a significant increase in cancer-specific mortality associated with any form of anastomotic leakage. Two other retrospective studies have suggested a similar effect.10,11
The mechanism by which anastomotic leakage may adversely affect cancer-specific survival remains open to speculation. Substantial evidence exists indicating the presence of viable cancer cells in the bowel lumen of patients with CRC at the time of operation,17–21 which can be detected on suture or staple lines of anastomoses.22,23 In the event of an anastomotic leak, this may lead to extraluminal implantation of cancer cells, which, in a similar manner to involvement of a free serosal surface3 or tumor in a line of resection,4 has the effect of upstaging the disease and reducing survival. In addition, the inflammatory response to anastomotic breakdown may enhance the tumor spread and metastasis.24,25 These mechanisms may account for the association between poorer survival and both local or more generalized leakage. However, it is also possible, though perhaps unlikely, that leaks occur as a consequence of other conditions not captured in the data of this study, which are themselves precursors to poor prognosis, making leakage prognostic rather than causative.
The fact that even localized leakage is negatively associated with survival was surprising. Hitherto, little long-term clinical significance has been attributed to localized or subclinical anastomotic leakage. The results of this study contradict that long-held view.
This study emphasizes the importance of taking measures to avoid anastomotic leakage. These are known to include a tension-free anastomosis with good blood supply. In constructing colorectal anastomoses, the importance of mobilizing the splenic flexure and dividing the inferior mesenteric vein at the lower border of the pancreas to ensure a tension-free anastomosis has been emphasized previously.26 Anastomotic leak rates have been variably described in up to 39% of patients.1 Attention to surgical detail has been shown to reduce this to the more acceptable levels described in this study.
ACKNOWLEDGMENTS
Ronald C. Newland examined and reported on over 90% of all surgical specimens and reviewed the histology of the remainder.
Footnotes
Reprints: Professor E. L. Bokey, Department of Colorectal Surgery, Concord Hospital, Sydney, NSW 2139, Australia. E-mail: bokey@surgery.usyd.edu.au.
REFERENCES
- 1.Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg. 2001;88:1157–1168. [DOI] [PubMed] [Google Scholar]
- 2.Chapuis PH, Dent OF, Fisher R, et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg. 1985;72:698–702. [DOI] [PubMed] [Google Scholar]
- 3.Newland RC, Chapuis PH, Smyth EJ. The prognostic value of substaging colorectal carcinoma: a prospective study of 1117 cases with standardised pathology. Cancer. 1987;60:852–857. [DOI] [PubMed] [Google Scholar]
- 4.Newland RC, Dent OF, Chapuis PH, et al. Clinicopathologically diagnosed residual tumour after resection for colorectal cancer. A 20-year prospective study. Cancer. 1993;72:1536–1542. [DOI] [PubMed] [Google Scholar]
- 5.Newland RC, Dent OF, Lyttle MNB, et al. Pathologic determinants of survival associated with colorectal cancer with lymph node metastases. Cancer. 1994;73:2076–2082. [DOI] [PubMed] [Google Scholar]
- 6.Newland RC, Dent OF, Chapuis PH, et al. Survival after “curative” resection of lymph node negative colorectal carcinoma: a prospective study of 910 patients. Cancer. 1995;76:564–571. [DOI] [PubMed] [Google Scholar]
- 7.Fielding LP, Arsenault PA, Chapuis PH, et al. Clinicopathological staging for colorectal cancer:an international documentation system (IDS) and an international comprehensive anatomical terminology (ICAT). J Gastroenterol Hepatol. 1991;6:325–344. [DOI] [PubMed] [Google Scholar]
- 8.Akyol AM, McGregor JR, Galloway DJ, et al. Anastomotic leaks in colorectal cancer surgery: a risk factor for recurrence? Int J Colorect Dis. 1991;6:179–183. [DOI] [PubMed] [Google Scholar]
- 9.Docherty JG, McGregor JR, Akyol AM, et al. Comparison of manually constructed and stapled anastomoses in colorectal surgery. West of Scotland and Highland Anastomosis Study Group. Ann Surg. 1995;221:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita S, Teramoto T, Masahiko W, et al. Anastomotic leakage after colorectal cancer surgery: a risk factor for recurrence and poor prognosis. Jpn J Clin Oncol. 1993;23:299–302. [PubMed] [Google Scholar]
- 11.Peterson S, Freitag M, Hellmich G, et al. Anastomotic leakage: impact on local recurrence and survival in surgery of colorectal cancer. Int J Colorectal Dis. 1998;13:160–3. [DOI] [PubMed] [Google Scholar]
- 12.Chapuis PH, Dent OF, Bokey EL, et al. Prise en charge du cancer colo-rectale dans un hopital Australien. Une experience de 24 ans. Ann Chir. 1999;53:9–18. [PubMed] [Google Scholar]
- 13.Bokey EL, Öjerskog B, Chapuis PH, et al. Local recurrence after curative excision of the rectum for cancer without adjuvant therapy: role of anatomical dissection. Br J Surg. 1999;86:1164–1170. [DOI] [PubMed] [Google Scholar]
- 14.Chapuis P, Bokey L, Fahrer M, et al. Mobilization of the rectum. Anatomic concepts and the bookshelf revisited. Dis Colon Rectum. 2002;45:1–9. [DOI] [PubMed] [Google Scholar]
- 15.Newland RC, Chapuis PH, Dent OF. Clinicopathological staging. In: Williams NS, ed. Colorectal Cancer. Singapore: Churchill Livingstone; 1996:55–68. [Google Scholar]
- 16.Gabriel WB, Dukes C, Bussey HJR. Lymphatic spread in cancer of the rectum. Br J Surg. 1935;23:395–413. [Google Scholar]
- 17.Fermor B, Umpleby HC, Lever JV, et al. Proliferative and metastatic potential of exfoliated colorectal cancer cells. J Natl Cancer Inst. 1986;76:347–349. [PubMed] [Google Scholar]
- 18.Keynes WM. Implantation from the bowel lumen in cancer of the large intestine. Ann Surg. 1961;153:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg IL, Russell CW, Giles GR. Cell viability studies on the exfoliated colonic cancer cell. Br J Surg. 1978;65:188–190. [DOI] [PubMed] [Google Scholar]
- 20.Skipper D, Cooper AJ, Marston JE, et al. Exfoliated cells and in vitro growth in colorectal cancer. Br J Surg. 1987;74:1049–1052. [DOI] [PubMed] [Google Scholar]
- 21.Umpleby HC, Fermor B, Symes MO, et al. Viability of exfoliated colorectal carcinoma cells. Br J Surg. 1984;71:659–663. [DOI] [PubMed] [Google Scholar]
- 22.Gertsch P, Baer HU, Kraft R, et al. Malignant cells are collected on circular staplers. Dis Colon Rectum. 1992;35:238–241. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg IL. The aetiology of colonic suture line recurrence. Ann R Coll Surg Eng. 1979;61:251–257. [PMC free article] [PubMed] [Google Scholar]
- 24.DerHagopian RP, Sugarbaker EV, Ketcham A. Inflammatory oncotaxis. JAMA. 1978;240:374–375. [PubMed] [Google Scholar]
- 25.Shine T, Wallack MK. Inflammatory oncotaxis after testing the skin of the cancer patient. Cancer. 1981;47:1325–1328. [DOI] [PubMed] [Google Scholar]
- 26.Goligher J. Surgery of the Anus, Rectum and Colon. 5th ed. London: Bailliere Tindall; 1984. [Google Scholar]