Abstract
Objective:
Using acute appendicitis as a model, we tested the hypothesis that polymorphisms in genes involved in host defense can be associated with the severity of local infection-inflammation in humans.
Summary Background Data:
Innate immunity is the body's front-line system for antimicrobial host defense. Local inflammation is a major innate immune mechanism for containing and destroying microbes, but it may also contribute to tissue injury.
Methods:
We studied 134 patients with acute appendicitis treated at an urban hospital. We looked for associations between the severity of appendicitis (uncomplicated vs. perforated or gangrenous), plasma and peritoneal cytokine concentrations, and single nucleotide polymorphisms in genes involved in recognizing bacterial molecules [CD14 (−159 C→T); TLR4 (896 A→G)] and in mounting an inflammatory response [IL-6 (−174 G→C), TNF-α (−308 G→A), IL-1β (−31 C → T)].
Results:
Ninety-one patients (68%) had uncomplicated appendicitis and 43 (32%) had complicated disease. The SNPs in the CD14, TLR4, IL-1β, and TNF-α genes were not associated with the severity of appendicitis. A strong association was found between C-allele carriage at −174 in the IL-6 gene and decreased risk of complicated disease (adjusted odds ratio = 0.24, 95% CI = 0.07–0.76). Lower plasma and peritoneal fluid IL-6 concentrations in the IL-6 −174 C-carriers than in the GG homozygotes suggest that this polymorphism contributes to decreased IL-6 production in vivo.
Conclusions:
Polymorphism in the IL-6 gene was associated with the severity of appendicitis, even after adjustment for duration of symptoms. The risk for developing appendiceal perforation or gangrene may be determined, in part, by variation in the IL-6 gene.
To study the influence of genetic polymorphism on the severity of local infection-inflammation in humans, we obtained plasma, peritoneal fluid, and DNA from patients with acute appendicitis. We found that the G→C transition at −174 in the interleukin-6 promoter was associated both with the pathologic severity of appendicitis and with regional and systemic interleukin-6 concentrations. Our findings suggest that the severity of acute appendicitis is influenced, at least in part, by genetic differences in the innate immune response.
The innate immune response has been highly conserved during evolution. It involves an orchestrated polyphony of local and systemic responses that rapidly destroy invading microbes.1 Key innate immune mechanisms include those that recognize microbial molecular patterns and initiate the local response that attracts and activates leukocytes, increases vascular permeability, elicits pain, and enhances blood flow to the infected tissue.2
Many features of innate immunity are characteristic of acute appendicitis, a common form of moderately severe, yet localized, bacterial infection.3 Although the pathophysiology of acute appendicitis is not entirely understood, obstruction of the appendiceal lumen appears to be the initiating event in most cases.4 Bacterial overgrowth and invasion occur within the distended, obstructed appendix. The bacteriology is complex, involving both aerobic and anaerobic bacterial constituents of the normal gastrointestinal flora.5,6 Pathologically, neutrophil infiltration of the muscularis is a necessary microscopic feature; in complicated disease, it is often accompanied by bacterial invasion. The likelihood of postoperative complications, which are primarily infectious, is strongly related to the severity of inflammation.3,7 In addition, the severity of disease, and thus the risk for developing postoperative complications, has been tied to patient- and surgeon-related delays in surgery and to the lack of health care insurance, factors of considerable importance in the delivery of health services.8,9
Furthermore, appendicitis is also a useful human model of local inflammation, since appendiceal infection elicits both regional and systemic responses and because standard management permits measurement of regional (peritoneal) and systemic (blood) cytokine concentrations. Since the disease is treated, in most cases, by appendectomy and as pathologic examination of the inflamed tissue is always performed, the inflammatory response can be measured and characterized. Additional advantages of acute appendicitis for the study of local inflammation are that many patients are relatively young and healthy prior to developing the disease and the duration of symptoms prior to study is generally short.
We hypothesized that the severity of local inflammation, as it occurs in acute appendicitis, can be influenced by polymorphisms in genes involved in innate immunity. To test this hypothesis, we studied patients with acute appendicitis for the occurrence of single nucleotide polymorphisms (SNPs) in genes that encode participants in two general stages of the innate response, microbial recognition and local inflammation. CD14 initiates the response to Gram-negative organisms by binding to bacterial lipopolysacharide (LPS), peptidoglycan, and other bacterial molecules,10 physical contact between CD14-bound LPS, MD-2, and Toll-like receptor 4 (TLR4) initiates LPS signal transduction within macrophages, with the TLR4 molecule being the critical transmembrane component of the signaling pathway for LPS.11,12 Since SNPs in the CD14 and TLR4 genes (CD14 −159 C→T, TLR4 896 A→G) have been associated with altered responses to LPS,13,14 we hypothesized that these SNPs might be present in individuals with complicated appendicitis. In addition to these SNPs in CD14 and TLR4, we also studied polymorphisms in the genes for three proteins that figure prominently in local inflammation, regardless of the inciting factor(s). Tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) link microbial recognition, as well as many noninfectious stimuli, to acute inflammatory responses. Polymorphisms in the regulatory regions of each of these genes have been associated with altered cytokine responses and outcomes in a variety of experimental and clinical conditions.15–17
MATERIALS AND METHODS
Appendicitis Patient Recruitment, Sampling, and Clinical Diagnosis
From August 10, 2000 to September 8, 2001, we recruited patients admitted to the Emergency Department at Parkland Memorial Hospital who had a clinical diagnosis of appendicitis and included those for whom the diagnosis was confirmed by pathologic examination of the excised appendix. All study subjects provided informed consent to blood sampling, genotyping, and inclusion in the study. The Institutional Review Board at the University of Texas Southwestern Medical Center approved the study protocol. Fifty-six of these patients are the subject of a previous report concerning the severity of appendicitis and local and systemic inflammation.18
We obtained detailed clinical data including anti-inflammatory drug and antibiotic use, the presence of preexisting medical illnesses and determined the duration of symptoms derived from the time the patient first felt ill to the start of surgery. Blood anticoagulated with EDTA was obtained preoperatively and peritoneal fluid was sampled intraoperatively, following a standardized procedure. After the peritoneum was incised, 20 mL of 0.9% NaCl was instilled into the peritoneal cavity adjacent to the appendix, allowed to dwell for 1 minute, and then aspirated. Blood and peritoneal aspirates were centrifuged (1000g, 20 minutes, 4°C) and the plasma and supernatants were stored at −80°C until analyzed. A pathologist (G.L.), blinded to clinical data and to the patient genotypes, confirmed the diagnosis and assigned the severity of appendicitis. Complicated appendicitis required microscopic confirmation, which included evidence of gangrene, necrosis, or perforation of the appendix. Patients were followed in hospital and in clinic for postoperative infectious complications. A surgical wound infection was defined as purulent drainage from the wound, wound cellulitis that was treated with antibiotics or opening of a previously closed wound. Intraabdominal abscess was defined as an intraabdominal collection of purulent material that was drained surgically or under radiologic guidance. The skin and subcutaneous tissue were closed at the discretion of the operating surgeon. In general, delayed closure was limited to patients with evidence of complicated appendicitis at the time of surgery.
DNA Extraction, Polymerase Chain Reaction, Pyrosequencing, and ELISA
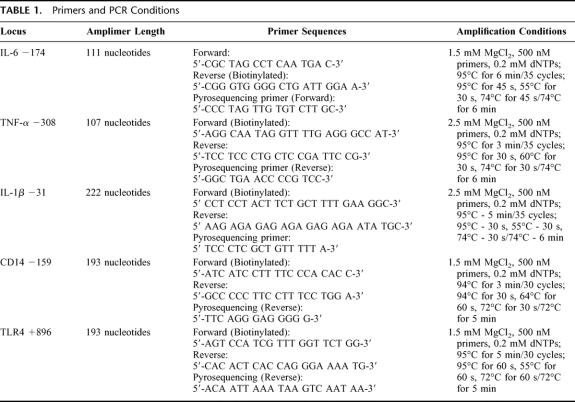
Genomic DNA was extracted from buffy coats using the QIAamp DNA Blood Midi Kit (Qiagen; Valencia, CA) according to manufacturer's instructions and DNA was stored at −20°C until amplified. Fragments containing each of the SNPs were individually amplified from genomic DNA by polymerase chain reaction using TaqDNA polymerase (Roche Diagnostics; Indianapolis, IN). All amplifications were carried out in a PTC 200 thermal cycler (MJResearch; Watertown, MA,) using a thermal profile, reaction conditions, and primer sequences specific for each SNP (Table 1).
TABLE 1. Primers and PCR Conditions
All genotypes were determined by pyrosequence analysis on a PSQ 96 Pyrosequencer (Pyrosequencing AB, Westborough, MA) and genotypes were resolved using PSQ 96 SNP Software, v 1.2 AQ. Each SNP was assayed with a specific primer sequence (Table 1), which enabled the scoring of heterozygotes and alternate homozygotes with equal reliability.19 All genotypes were confirmed by repeat pyrosequencing. We have determined the accuracy of pyrosequencing in comparison to dye terminator sequencing for TNFα −308 and for IL-6 −174. For the other SNPs, repeat pyrosequencing agreed in all cases. An individual blinded to the clinical and biochemical data recorded genotype. The clinical and DNA databases were then linked electronically using the assigned study number, and no genetic information was ever directly linked to patient identifiers.
Peritoneal fluid samples were available from 105 patients and plasma samples were available from 115 patients. IL-1β, IL-6, and TNF-α concentrations in plasma and peritoneal fluid were determined by ELISA, using OptEIA Sets (BD-Pharmingen, San Diego, CA) according to the manufacturer's instructions. The lower detection limit for IL-6 and IL-1β was 7.8 pg/mL, whereas that for TNF-α was 5.0 pg/mL. Peritoneal fluid was not diluted for TNF-α and IL-1β analysis and was diluted 40-fold to measure IL-6. Plasma samples were analyzed undiluted. TNF-α was infrequently detectable in the plasma of the initial 56 subjects; therefore, we did not measure this cytokine in the plasma of the remaining patients. C-reactive protein was measured in the plasma of the first 104 patients. In a pilot study, IL-1β, IL-6, and TNF-α were not detectable in the plasma or peritoneal fluid of 18 patients undergoing elective abdominal (laparotomy or laparoscopy) surgery.20 Plasma C-reactive protein was measured by a high-sensitivity assay as previously described.21
Data Presentation and Statistical Analyses
Comparisons of categorical data were made using χ2 analysis while continuous data were compared with the Student t test. Exact P values and/or 95% confidence intervals are reported for all analyses. Initial comparisons were followed by multivariate logistic regression analysis, which tested potential clinical and genetic risk factors. We also evaluated potential confounders. Variables remained in the final model as risk factors (included and considered to be significant if P ≤ 0.05) or as confounders (observed to alter the effects of other risk factors). Plasma and peritoneal cytokine concentrations were compared using Student t test and analysis of variance.
RESULTS
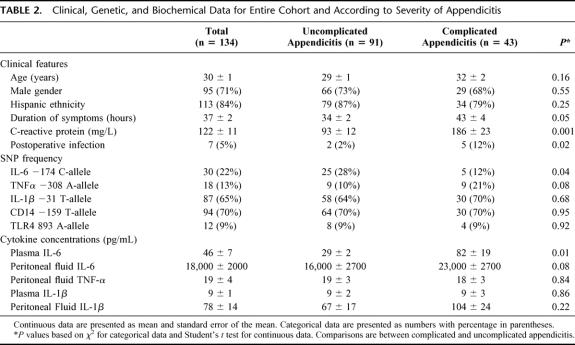
Of 209 patients who underwent emergency appendectomy, 14 were found not to have acute appendicitis and 61 did not provide informed consent (they either declined or were not approached). The clinical features of the 134 included patients are shown in Table 2. Patients with complicated appendicitis were slightly older, had symptoms for a longer time prior to surgery, and were more likely to develop a postoperative infectious complication. As expected, plasma C-reactive protein concentrations were higher in patients with complicated appendicitis than in patients with uncomplicated disease. All but 7 patients met one or more SIRS criteria at the time of evaluation in the emergency department, and 66% met 2 or more criteria. By self-report, most of our subjects (86%) were of Hispanic ancestry.
TABLE 2. Clinical, Genetic, and Biochemical Data for Entire Cohort and According to Severity of Appendicitis
To determine whether these SNPs were risk factors for the development of appendicitis, we compared the allele frequencies in the patients with acute appendicitis to those in a control group (n = 150) of similar age, gender, and ethnic background. No statistically significant differences were noted (data not shown). We then focused our attention on the patients with acute appendicitis. Carriage of the C-allele at IL-6 −174 was associated with a lower risk of complicated appendicitis (unadjusted relative risk = 0.42, 95% CI = 0.17–0.98, P = 0.04). None of the other SNPs was associated with the risk for complicated appendicitis. Although the risk for having complicated appendicitis was somewhat higher in TNF-α −308 A-allele carriers, the association was not statistically significant (adjusted odds ratio = 2.7, 95% CI = 0.9–7.9, P = 0.07).
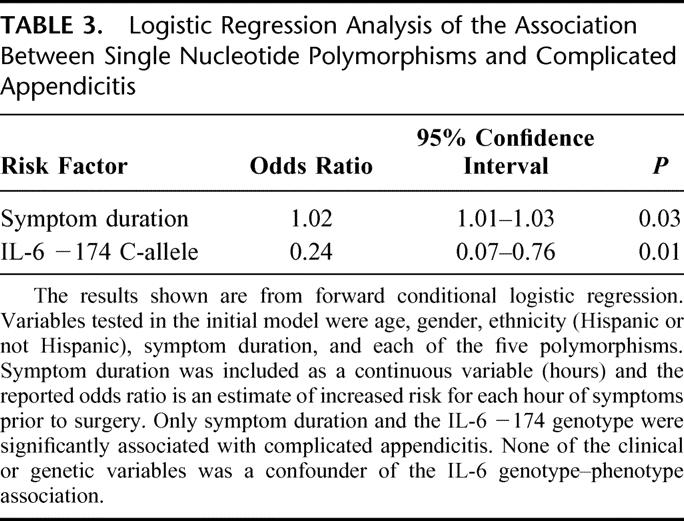
For multivariate analysis, we included the clinical parameters (age, gender, and duration of symptoms) that have been reported by others to be associated with complicated appendicitis.22 As the frequency of the SNPs studied here differs among racial/ethnic groups, we also included this variable in our model. Race (black, white, or Asian) and ethnicity (Hispanic or non-Hispanic) assignment were based upon self-report by the patient. Because the largest ethnic group in our study was Hispanic, we analyzed our data according to whether the patient was of Hispanic or non-Hispanic ethnicity. In our multivariate analysis, we found that age, gender, and ethnicity were not associated with severity of disease and did not influence the relationship between genotype and disease severity. Two variables were significantly associated with risk of complicated appendicitis: IL-6 −174 C-allele carriers had a significantly lower risk of complicated appendicitis, whereas the risk for complicated appendicitis increased with increasing duration of symptoms (Table 3). Only 2 patients were homozygous for the C-allele at the −174 position, so it was not possible to analyze these individuals separately from the heterozygotes. The TNF-α −308 A-allele was associated with a modestly increased risk for having complicated appendicitis (adjusted odds ratio = 2.7, 95% CI = 0.9–7.9, P = 0.07) and was therefore not included in the final regression model.
TABLE 3. Logistic Regression Analysis of the Association Between Single Nucleotide Polymorphisms and Complicated Appendicitis
We also conducted a secondary analysis that included only Hispanic individuals. In this subgroup, the unadjusted relative risk for complicated appendicitis associated with the TNF-α −308 A-allele was 2.2 (95% CI = 1.2–3.8, P = 0.02). Adjusting for the IL-6 −174 SNP and for duration of symptoms, the odds ratio for complicated appendicitis associated with the TNFα −308 A-allele was 4.0 (95% CI = 1.2–13.3, P = 0.02). Adjusting for duration of symptoms and the TNF-α −308 SNP, IL-6 −174 C-carriers had an odds ratio of 0.23 (95% CI = 0.06–0.9, P = 0.03) for complicated appendicitis. Thus, both the TNF-α −308 A-allele (increased risk) and the IL-6 −174 C-allele (decreased risk) were associated with complicated appendicitis in the Hispanic individuals.
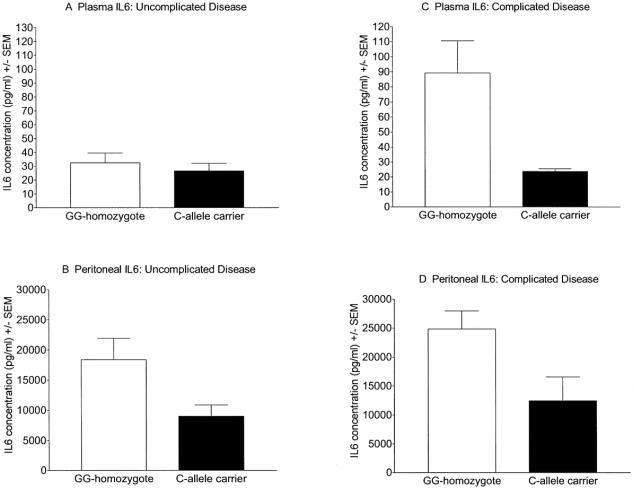
Plasma IL-6 concentrations were lower in C-allele carriers (mean 21.3 ± 3.5 pg/mL, n = 26) than in GG-homozygotes (mean 53 ± 9.2 pg/mL, n = 89, P = 0.001). Similarly, peritoneal fluid aspirate IL-6 concentrations were also lower in C-allele carriers (9900 ± 1600 pg/mL, n = 21) than in GG-homozygotes (20,700 ± 2400 pg/mL, n = 84, P < 0.001). As there was an imbalance in the distribution of genotypes by disease severity (few IL-6 −174 C-carriers [n = 5] had complicated disease), we also compared the relationship between genotype and cytokine concentrations separately for complicated and uncomplicated appendicitis. In patients with uncomplicated disease, plasma IL-6 concentrations were only slightly lower in C-allele carriers (Fig. 1A), whereas peritoneal IL-6 levels were significantly lower in the C-allele carriers than in the GG-homozygotes (Fig. 1B). Among those with complicated disease, both plasma (Fig. 1C) and peritoneal (Fig. 1D) IL-6 concentrations were significantly lower in C-allele carriers than in GG-homozygotes. TNF-α was detected in the peritoneal fluid from the majority of patients (76 of 105; 72%). The peritoneal TNF-α concentration was not related to the severity of appendicitis or to TNFα −308 genotype, however. Plasma and peritoneal IL-1β concentrations were not associated with IL-1β −31 genotype or with disease severity (TNF-α and IL-1β data not shown).
FIGURE 1. Plasma and peritoneal interleukin-6 concentrations according to −174 promoter genotype and severity of appendicitis. In patients with uncomplicated appendicitis, plasma (1A) IL-6 concentrations were similar in GG-homozygotes and C-allele carriers (32.2 ± 6.0 pg/mL vs. 20.5 ± 4.1 pg/mL, respectively, P = 0.11). Peritoneal fluid aspirate (1B) IL-6 levels were higher in GG-homozygotes than C-allele carriers (18,400 ± 3500 pg/mL vs. 9000 ± 1900 pg/mL, respectively, P = 0.02). In patients with complicated disease, plasma (1C) IL-6 concentrations were higher in GG-homozygotes than in C-allele carriers (89.3 ± 21.4 pg/mL vs. 23.7 ± 1.7 pg/mL, respectively, P = 0.005). Peritoneal fluid aspirate IL-6 concentrations (1D) were also higher in GG-homozygotes than in C-allele carriers (24,000 ± 3000 pg/mL vs. 13, 000 ± 3200 pg/mL, respectively, P = 0.02). All statistical comparisons were by ANOVA or Student t test.
Postoperative complications included 4 wound infections and 3 intra-abdominal abscesses. Infectious complications occurred more frequently in patients with complicated disease (Table 2). No association was found between polymorphisms at any of the SNPs examined and postoperative infectious complications.
DISCUSSION
The important elements of innate immunity are “hard-wired” in the genome, shaped during evolution to respond to microbial signals without further gene modification.1 Although allelic polymorphisms in various innate immunity genes have been associated with increased risk of acquiring certain infectious diseases,23 and in some cases with increased disease severity,15,24 the role that genetic differences might play in determining the severity of localized infection with commensal bacteria has not previously been studied in humans.
Recognizing that appendicitis is a complex disease that involves mechanical obstruction of the lumen, ischemia, thrombosis, and bacterial overgrowth, we looked for severity-associated polymorphisms in genes that contribute to microbial recognition or local inflammation. It is important to note that the gene polymorphisms studied here were not risk factors for the development of appendicitis; rather, they may modify the pathologic severity of disease and the associated cytokine responses once appendicitis occurs. CD14 and TLR4 are central to the recognition and initiation of the response to LPS, the predominant “microbe-associated molecular pattern” on Gram-negative bacteria. While the C→T exchange at −159 alters Sp1 nuclear protein binding to the CD14 promoter, the phenotypic impact of this polymorphism is uncertain;25–27 one study identified an association between this SNP and risk for myocardial infarction, but a second study did not.26,28 The findings have also been conflicting in patients with severe sepsis, with one group observing no association and another finding a relationship between the T-allele and death from sepsis.27,29 Our observations suggest that the severity of appendicitis is unrelated to this polymorphism. TLR4 is a transmembrane protein that initiates the signaling cascade that produces an inflammatory response to LPS.30 An A→G transition at nucleotide 896 of the human TLR4 gene results in the substitution of glycine for aspartic acid at amino acid 299 and reduces LPS responsiveness to inhaled LPS in humans.13 This polymorphism has been weakly associated with the development of, and decreased survival from, septic shock but does not appear to be associated with the severity of acute appendicitis.31
In this study, we also looked for associations between disease severity and polymorphisms in the genes for TNF-α, IL-6, and IL-1β, 3 key mediators of inflammation. The G →C SNP at the −174 position in the IL-6 gene has been reported to alter gene transcription in transient transfection experiments and to be associated with a number of chronic conditions in which inflammation is considered to play a role.32–34 Our results suggest that this SNP can be a useful marker for IL-6 responses that correlate with the severity of local inflammation.35 Our most striking observation was the statistically significant association between the IL-6 −174 C-allele and a reduced risk of complicated appendicitis, both by unadjusted and adjusted (logistic regression) analyses. It is necessary to note that complicated appendicitis is rather common (32% in our cohort); therefore, the adjusted odds ratio overestimates the relative risk. According to the estimates of Zhang and Yu,36 the adjusted relative risk for complicated appendicitis associated with the IL-6 −174 C-allele is approximately 0.3 (rather than 0.24 as estimated by the adjusted odds ratio). The statistical significance of this association remains unchanged.
Although observations in IL-6-deficient mice indicate that this cytokine has important systemic anti-inflammatory actions, it also contributes to the severity of local inflammation in antigen-induced arthritis in mice, and homozygosity for the −174 G-allele has been associated with an increased risk for severe juvenile arthritis in humans.32,37,38 The correlations observed here between peritoneal and plasma IL-6 concentrations, IL-6 −174 alleles and the severity of appendiceal inflammation suggest that the IL-6 −174 SNP influences the production of IL-6, which in turn may contribute to the severity of local inflammation. Thought to be the major pro-coagulant cytokine, IL-6 induces tissue factor (TF) mRNA expression and increases monocyte surface TF protein.39 It is thus possible that IL-6 production contributes to local thrombosis and, as a consequence, to perforation and gangrene. In addition, IL-6 appears to delay neutrophil apoptosis and to promote neutrophil degranulation and elastase release, both of which may contribute to increased inflammation.40,41
The −308 SNP in the TNF-α promoter influences gene transcription, TNF-α production, and disease severity, and carriage of the rare A-allele has been associated with an increased risk for developing severe sepsis and septic shock and with death from septic shock.15,24,42,43 TNF-α has activities that might influence the severity of inflammation in appendicitis; it has been shown to increase procoagulant activity (increased TF expression and decreased tissue factor pathway inhibitor expression) that could contribute to local microvascular thrombosis, tissue necrosis, and gangrene.44 In addition, TNF-α induces the expression of various leukocyte adhesion molecules and suppresses leukocyte apoptosis.45,46 The absence of a strong association of TNF-α genotype with peritoneal TNF-α concentrations and severity of appendicitis may reflect an insufficient sample size but may also indicate a true lack of association.
There is less information regarding the effect of the IL-1β −31 C→T SNP on acute inflammation. Although it seems that the T-allele is associated with reduced nuclear binding in vitro, the identity of the bound nuclear protein is unknown and it is uncertain whether this leads to altered gene expression in vivo.17 Despite a prominent role for IL-1β in acute inflammation, a diminished ability to produce this cytokine did not impair overall responses to endotoxin.47 We did not observe any differences in appendicitis severity or in systemic and regional IL-1β concentrations related to the IL-1β–31 C→T SNP.
This study had a number of limitations. First, we studied a multiracial cohort, across which baseline genetic heterogeneity certainly exists. Although we found no evidence for racial differences in the association between the IL-6 −174 SNP and complicated appendicitis, IL-6 haplotypes differ among ethnic groups, and these differences may have affected our results in unappreciated ways.35 We did find that the TNF-α −308 A-allele was more strongly associated with complicated appendicitis in the patients of Hispanic origin than in the total population of Hispanics and non-Hispanics. Second, complicated acute appendicitis likely has multiple causes. How genetic and nongenetic factors interact is unknown. For instance, does a “low-risk” genotype protect against the development of complicated disease, despite delays in seeking or receiving treatment? The interplay of these genetic and environmental (including treatment) risk factors is of great interest, with implications both for understanding the biology of appendicitis and for knowing how medical interventions influence disease outcomes. Our study is underpowered to detect small to modest genotype–phenotype associations. It will certainly be necessary to study much larger cohorts to determine whether these and other genetic differences influence the risk of postoperative complications. It is also possible that, had we enrolled more patients, significant associations between SNPs in CD14, IL-1β, TNF-α, or TLR4 and complicated appendicitis would have been detected. On the other hand, it is also possible that we have identified a false-positive association between the IL-6 SNP and complicated appendicitis, since we did not adjust for multiple comparisons in our initial analyses. Setting highly conservative significance levels would limit falsely positive associations, but it has recently been observed that, while false-positive studies are not rare, most initial reports of genotype–phenotype associations are confirmed by subsequent reports.48 Conversely, overly conservative significance thresholds may result in the failure to identify modest but important associations. Replication by other investigators is likely the best way to ensure that observed associations are real.48,49
CONCLUSION
These results suggest that the human response to a local infection, such as acute appendicitis, is influenced by inherited differences in innate immunity genes, such as IL-6. Furthermore, delays in diagnosis and treatment are not the only reasons for developing complicated appendicitis.50
Footnotes
R.S.M. is supported by NIH grants AI18188 and AI38596 and the Jan and Henri Bromberg Chair in Internal Medicine. G.E.O'K. is supported by NIH grants 5P50GM021681-370013 and 1R01GM066946.
Reprints: Grant O'Keefe, MD, University of Washington, Department of Surgery, Box 359796, Harborview Medical Center, 325 Ninth Avenue, Seattle, WA 98104-2499. E-mail: gokeefe@u.washington.edu.
REFERENCES
- 1.Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000;343:338–344. [DOI] [PubMed] [Google Scholar]
- 2.Munford RS, Pugin J. The crucial role of systemic responses in the innate (non-adaptive) host defense. J Endotoxin Res. 2001;7:327–332. [PubMed] [Google Scholar]
- 3.Blomqvist PG, Andersson RE, Granath F, et al. Mortality after appendectomy in Sweden, 1987–1996. Ann Surg. 2001;233:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones BA, Demetriades D, Segal I, et al. The prevalence of appendiceal fecaliths in patients with and without appendicitis: a comparative study from Canada and South Africa. Ann Surg. 1985;202:80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soffer D, Zait S, Klausner J, et al. Peritoneal cultures and antibiotic treatment in patients with perforated appendicitis. Eur J Surg. 2001;167:214–216. [DOI] [PubMed] [Google Scholar]
- 6.Maltezou HC, Nikolaidis P, Lebesii E, et al. Piperacillin/tazobactam versus cefotaxime plus metronidazole for treatment of children with intra-abdominal infections requiring surgery. Eur J Clin Microbiol Infect Dis. 2001;20:643–646. [DOI] [PubMed] [Google Scholar]
- 7.Korner H, Sondenaa K, Soreide JA. Perforated and non-perforated acute appendicitis: one disease or two entities? Eur J Surg. 2001;167:525–530. [DOI] [PubMed] [Google Scholar]
- 8.Temple CL, Huchcroft SA, Temple WJ. The natural history of appendicitis in adults: a prospective study. Ann Surg. 1995;221:278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braveman P, Schaaf VM, Egerter S, et al. Insurance-related differences in the risk of ruptured appendix. N Engl J Med. 1994;331:444–449. [DOI] [PubMed] [Google Scholar]
- 10.Kitchens RL, Thompson PA, Viriyakosol S, et al. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest. 2001;108:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poltorak A, Ricciardi-Castagnoli P, Citterio S, et al. Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA. 2000;97:2163–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 13.Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. [DOI] [PubMed] [Google Scholar]
- 14.Vercelli D, Baldini M, Stern D, et al. CD14: a bridge between innate immunity and adaptive IgE responses. J Endotoxin Res. 2001;7:45–48. [PubMed] [Google Scholar]
- 15.Mira JP, Cariou A, Grall F, et al. Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA. 1999;282:561–568. [DOI] [PubMed] [Google Scholar]
- 16.Schluter B, Raufhake C, Erren M, et al. Effect of the interleukin-6 promoter polymorphism (-174 G/C) on the incidence and outcome of sepsis. Crit Care Med. 2002;30:32–37. [DOI] [PubMed] [Google Scholar]
- 17.El Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. [DOI] [PubMed] [Google Scholar]
- 18.Rivera-Chavez FA, Wheeler H, Lindberg G, et al. Regional and systemic cytokine responses to acute inflammation of the vermiform appendix. Ann Surg. 2003;237:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alderborn A, Kristofferson A, Hammerling U. Determination of single-nucleotide polymorphisms by real-time pyrophosphate DNA sequencing. Genome Res. 2000;10:1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera-Chavez F, Munford RS, O'Keefe GE. Characterization of local and systemic cytokine responses during acute inflammation in humans. Shock. 2002;13:65. [Google Scholar]
- 21.Jialal I, Stein D, Balis D, et al. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–1935. [DOI] [PubMed] [Google Scholar]
- 22.Andersson RE, Hugander AP, Ghazi SH, et al. Diagnostic value of disease history, clinical presentation, and inflammatory parameters of appendicitis. World J Surg. 1999;23:133–140. [DOI] [PubMed] [Google Scholar]
- 23.McGuire W, Hill AV, Allsopp CE, et al. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–510. [DOI] [PubMed] [Google Scholar]
- 24.O'Keefe GE, Hybki DL, Munford RS. The G–>A single nucleotide polymorphism at the −308 position in the tumor necrosis factor-alpha promoter increases the risk for severe sepsis after trauma. J Trauma. 2002;52:817–826. [DOI] [PubMed] [Google Scholar]
- 25.LeVan TD, Bloom JW, Bailey TJ, et al. A common single nucleotide polymorphism in the CD14 promoter decreases the affinity of Sp protein binding and enhances transcriptional activity. J Immunol. 2001;167:5838–5844. [DOI] [PubMed] [Google Scholar]
- 26.Hubacek JA, Rothe G, Pit'ha J, et al. C(-260)–>T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction. Circulation. 1999;99:3218–3220. [DOI] [PubMed] [Google Scholar]
- 27.Hubacek JA, Stuber F, Frohlich D, et al. The common functional C(-159)T polymorphism within the promoter region of the lipopolysaccharide receptor CD14 is not associated with sepsis development or mortality. Genes Immun. 2000;1:405–407. [DOI] [PubMed] [Google Scholar]
- 28.Koch W, Kastrati A, Mehilli J, et al. CD14 gene −159C/T polymorphism is not associated with coronary artery disease and myocardial infarction. Am Heart J. 2002;143:971–976. [DOI] [PubMed] [Google Scholar]
- 29.Gibot S, Cariou A, Drouet L, et al. Association between a genomic polymorphism within the CD14 locus and septic shock susceptibility and mortality rate. Crit Care Med. 2002;30:969–973. [DOI] [PubMed] [Google Scholar]
- 30.Smirnova I, Poltorak A, Chan EK, et al. Phylogenetic variation and polymorphism at the toll-like receptor 4 locus (TLR4). Genome Biol. 2000;1:RESEARCH002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorenz E, Mira JP, Frees KL, et al. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–1032. [DOI] [PubMed] [Google Scholar]
- 32.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauramaa R, Väisänen SB, Luong LA, et al. Stromelysin-1 and interleukin-6 gene promoter polymorphisms are determinants of asymptomatic carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2657–2662. [DOI] [PubMed] [Google Scholar]
- 34.Hulkkonen J, Pertovaara M, Antonen J, et al. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjogren's syndrome and correlate with the clinical manifestations of the disease. Rheumatology (Oxford). 2001;40:656–661. [DOI] [PubMed] [Google Scholar]
- 35.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 37.Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Hooge AS, Van de Loo FA, Arntz OJ, et al. Involvement of IL-6, apart from its role in immunity, in mediating a chronic response during experimental arthritis. Am J Pathol. 2000;157:2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neumann FJ, Ott I, Marx N, et al. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol. 1997;17:3399–3405. [DOI] [PubMed] [Google Scholar]
- 40.Biffl WL, Moore EE, Moore FA, et al. Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J Trauma. 1996;40:575–578. [DOI] [PubMed] [Google Scholar]
- 41.Bank U, Reinhold D, Kunz D, et al. Effects of interleukin-6 (IL-6) and transforming growth factor-beta (TGF-beta) on neutrophil elastase release. Inflammation. 1995;19:83–99. [DOI] [PubMed] [Google Scholar]
- 42.Nadel S, Newport MJ, Booy R, et al. Variation in the tumor necrosis factor-alpha gene promoter region may be associated with death from meningococcal disease. J Infect Dis. 1996;174:878–880. [DOI] [PubMed] [Google Scholar]
- 43.Wilson AG, Symons JA, McDowell TL, et al. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Speiser W, Kapiotis S, Kopp CW, et al. Effect of intradermal tumor necrosis factor-alpha-induced inflammation on coagulation factors in dermal vessel endothelium: an in vivo study of human skin biopsies. Thromb Haemost. 2001;85:362–367. [PubMed] [Google Scholar]
- 45.Horie Y, Chervenak RP, Wolf R, et al. Lymphocytes mediate TNF-alpha-induced endothelial cell adhesion molecule expression: studies on SCID and RAG-1 mutant mice. J Immunol. 1997;159:5053–5062. [PubMed] [Google Scholar]
- 46.Mangan DF, Wahl SM. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines PG-. J Immunol. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 47.Shornick LP, De Togni P, Mariathasan S, et al. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J Exp Med. 1996;183:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lohmueller KE, Pearce CL, Pike M, et al. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. [DOI] [PubMed] [Google Scholar]
- 49.Ioannidis JP, Ntzani EE, Trikalinos TA, et al. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. [DOI] [PubMed] [Google Scholar]
- 50.Andersson R, Hugander A, Thulin A, et al. Indications for operation in suspected appendicitis and incidence of perforation. Br Med J. 1994;308:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]