Abstract
Objective:
The aim of this study was to analyze the survival of patients with peritoneal dissemination of appendiceal malignancy having incomplete cytoreductive surgery.
Summary Background Data:
Cytoreductive surgery plus perioperative intraperitoneal chemotherapy has emerged as a new and potentially curative treatment option for patients with peritoneal dissemination of appendiceal mucinous tumors. The goal of surgery is to remove all visible disease. Nevertheless, in some patients, complete cytoreduction is not possible.
Methods:
Over a 30-year period, 645 patients with epithelial peritoneal surface malignancy of appendiceal origin were treated with cytoreductive surgery and intraperitoneal chemotherapy by a single surgeon. One hundred seventy-four (27.1%) of these patients had an incomplete cytoreduction. A critical statistical analysis of the impact of selected clinical features on survival was performed from a prospective database.
Results:
Mortality and morbidity rates were 0% and 33.3%, respectively. Median survival of these 174 patients was 20.5 months and their 1-year, 3-year, and 5-year survival rates were 71%, 34%, and 15%, respectively. By multivariate analysis, the presence of signet ring cells and lymph node involvement were independent prognostic indicators of poor survival (P = 0.047 and P < 0.001, respectively). Patients who underwent more than 1 cytoreduction or repeat intraperitoneal chemohyperthermia showed significant improvement in survival (P = 0.018 and P < 0.001, respectively)
Conclusion:
Incomplete cytoreduction plus perioperative intraperitoneal chemotherapy of peritoneal dissemination from appendiceal malignancy results in limited long-term survival. Patients with signet ring histology or lymph node involvement have an especially poor outcome. Repeat cytoreduction and intraperitoneal chemohyperthermia may improve outcome.
Incomplete cytoreductive surgery plus intraperitoneal chemotherapy of peritoneal dissemination from appendiceal malignancy results in limited long-term survival. In 174 patients with a median survival of 20.5 months, the presence of signet ring cells and lymph node involvement were independent poor prognostic indicators. Repeat cytoreduction and intraoperative chemohyperthermia may improve outcome.
Malignant tumors of the appendix are rare tumors, accounting for 0.2% to 0.5% of all cancers of the gastrointestinal tract and diagnosed in 0.9% to 1.4% of appendectomy specimens.1,2 Peritoneal surface dissemination of appendiceal malignancy arises from a perforated appendiceal tumor.3 The primary tumor in the appendix invades through the appendiceal wall or produces a mucocele that eventually ruptures. In both situations, mucus-producing adenomatous epithelial cells are disseminated throughout the abdomen and pelvis. This results in extensive accumulation of mucinous tumor at characteristic sites within the peritoneal cavity. Despite relatively bland histology and better understanding of its etiology and prognostic features, the long-term survival of patients with peritoneal carcinomatosis from appendiceal malignancy remains poor, with reported 5-year and 10-year survival rates of 50% to 75% and 10% to 30%, respectively.4
The best therapeutic approach remains controversial. Over the past decade, repeated surgical debulking and evacuation of all free mucus with vigorous irrigation has been the sole therapeutic modality.4,5 All macroscopic tumor was removed if possible, but a complete cure could never be achieved. Following repeated interventions, tumor entrapment on bowel surfaces resulted in failure of subsequent therapeutic options. All patients eventually succumbed to progressive bowel obstruction and terminal starvation.6,7
Beginning in the 1980s, an interest in therapeutic options for peritoneal carcinomatosis emerged. A comprehensive multimodality approach has been proposed that combines extensive cytoreductive surgery using peritonectomy procedures8 with adjuvant intraperitoneal and systemic chemotherapy,9,10 phototherapy,11 radiotherapy,9 or intraperitoneal chemohyperthermia (IPCH).12–14 For peritoneal surface malignancies arising from appendix cancer, the most promising survival results have been observed in patients treated with complete cytoreductive surgery combined with IPCH. These results need to be confirmed by a longer follow-up.12,15 It has become clear that the appendix as a primary cancer site for cancer seeding on peritoneal surfaces is unique and allows for a curative approach to peritoneal dissemination.15,16 For this reason, peritoneal surface spread from appendiceal malignancy is considered a paradigm for treatment of a subset of patients with abdominal and pelvic dissemination of gastrointestinal malignancy limited to peritoneal surfaces.
Every study of combined treatment of disseminated appendiceal mucinous malignancy clearly shows improved survival in the group of patients in whom a complete cytoreduction is achieved.3,7,10,12,13,15 Unfortunately, even with the most meticulous radiologic study preoperatively, some patients cannot be completely cytoreduced. Also, some patients with extensive abdominal distension by mucus and mucinous tumor require palliation by a debulking procedure. Elective surgery in this group of patients has been discouraged by some surgeons because of a high morbidity and mortality rate associated with extensive procedures.4,14,17
No prior study directed specifically at the outcome of patients with incomplete cytoreduction has been published. The aim of this study was to analyze the survival of patients with peritoneal surface spread of appendiceal malignancy who had an incomplete cytoreductive surgery for better management of this group of patients.
MATERIALS AND METHODS
Patients
From May 1983 to February 2003, 645 patients with the diagnosis of an epithelial peritoneal surface malignancy of appendiceal origin were treated with a curative intent using a standardized management plan with cytoreductive surgery and perioperative intraperitoneal chemotherapy.18 This group of patients represents a single surgeon's complete operative experience with this disease during this period of time with no patients eliminated from the data analysis. No patients were lost to follow-up. A total of 174 of these patients (27%) underwent an incomplete cytoreductive surgery and had residual tumor nodules more than 0.25 mm after surgery. A statistical analysis of these 174 patients constitutes the basis of our study, focused on survival and the impact of prognostic factors on survival.
Overall Treatment Strategy
The treatments used in a curative approach to appendiceal malignancy involved 2 major components. The intent of treatment of all patients was maximal surgery combined with maximal regional chemotherapy, and these 2 therapies were blended together into a single treatment plan. Using extensive electrosurgery, 1 to 6 peritonectomy procedures and appropriate gastrointestinal resections were performed in an attempt to remove all visible tumor.8,19 Intraperitoneal chemotherapy was withheld perioperatively if small bowel loops could not be separated to allow intraperitoneal access to a majority of peritoneal surfaces.18 This perioperative intraperitoneal chemotherapy was supplemented postoperatively by additional cycles of combined intraperitoneal and systemic chemotherapy.
Chemotherapy
Of the 174 patients, 37 patients did not receive perioperative intraperitoneal chemotherapy because of the impossibility to achieve near complete separation of bowel loops. The remaining 137 patients received perioperative intraperitoneal chemotherapy with mitomycin C and 5-fluorouracil. Before March 1993, patients were given normothermic mitomycin C as early postoperative intraperitoneal chemotherapy (EPIC) on postoperative day 1 at a dose of 12.5 mg/m2 for males and 10 mg/m2 for females. The 5-fluorouracil has always been given at 650 mg/m2 on postoperative days 2 to 6 or 1 to 5.18 Seventy-six received EPIC. As hyperthermic intraoperative chemotherapy became available after March 1993, the same dose of mitomycin C was given in the operative room with 41 to 42°C heat and manual distribution of the chemotherapy solution.18 Sixty-one patients were given IPCH. This was given in the absence of 5-fluorouracil in 11 patients if there was a high risk for fistula formation. Intraperitoneal chemohyperthermia was combined with EPIC 5-fluorouracil in 50 patients.
Twenty-four patients had been previously treated with intraperitoneal chemotherapy and 56 patients with systemic chemotherapy. Thirty-three patients also received 3 cycles of combined intravenous mitomycin C and intraperitoneal 5-fluorouracil at monthly intervals postoperatively. Fifty-six patients received postoperative systemic chemotherapy as clinically indicated with various regimens involving 5-fluorouracil, leucovorin, oxaliplatin, mitomycin C, paclitaxel, irinotecan, and cisplatin. The information regarding preoperative and adjuvant chemotherapy was incomplete; a great majority of the systemic chemotherapy treatments were not given at our institution.
Clinical Data
The clinical features selected for analysis with survival as an end point were age, gender, prior surgical score (PSS), completeness of cytoreduction (CC) score, morphologic type of tumor (adenomucinosis, hybrid type, mucinous carcinoma), presence versus absence of lymph node involvement, presence versus absence of signet ring cells, and number of operative interventions. Also, treatment variables were treatment versus no treatment by preoperative systemic chemotherapy, preoperative intraperitoneal chemotherapy, IPCH, EPIC, long-term intraperitoneal chemotherapy, and adjuvant systemic chemotherapy. The significance of each of these clinical features was analyzed for each group.
Quantitative Prognostic Indicators for Peritoneal Surface Malignancy
The PSS allowed an assessment of the anatomic locations in which prior surgery had been performed. When the previous operative notes on the patients were reviewed, a judgment was made regarding the anatomic sites of prior surgical dissections. The summation of these dissections was recorded on a diagram of the abdominopelvic regions.20 In patients with a PSS of 0, diagnosis of peritoneal carcinomatosis was obtained through biopsy only, or by laparoscopy plus biopsy. A PSS of 1 indicated only a prior exploratory laparotomy. Prior surgical score 2 indicated exploratory laparotomy with some resections. Usually this was a greater omentectomy or greater omentectomy plus a right colectomy. A PSS of 3 indicated patients had undergone a prior attempt at complete cytoreduction. This was usually greater omentectomy, right colectomy, hysterectomy, and bilateral salpingo-oophorectomy, with the possibility of other resections from both abdominal organs or parietal peritoneal regions.
The CC score was used to estimate residual disease after the definitive cytoreduction. Patients with a CC score of 0 (no visible tumor remained) or 1 (tumor implants less than 0.25 cm) were designated as complete cytoreduction and were excluded from this analysis. The score was determined by the size of the largest tumor mass left behind at the completion of the resection.20 A score of 2 (CC-2) indicated tumor implants of 0.25 to 2.5 cm in greatest dimension. A score of 3 (CC-3) indicated tumor nodules of greater than 2.5 cm in greatest dimension or a layering of disease from a coalescence of cancer nodules.
The morphologic type of mucinous tumor was read as disseminated peritoneal adenomucinosis (grade 1), hybrid type (grade 2), or mucinous adenocarcinoma (grade 3).21 Disseminated peritoneal adenomucinosis indicated scant epithelial cells existed as single cells or as a single layer surrounding a glandular structure. Fibrous matrix was minimal. Epithelial cells appeared bland without atypia or mitoses. Hybrid type tumor contained both the morphology of adenomucinosis and mucinous adenocarcinoma. The great majority (95% or more) of the tumor mass in these patients was typical of adenomucinosis, but areas of cellular atypia or a confluence of glandular structures were apparent. Mucinous adenocarcinoma indicated that signet ring morphology, cellular atypia, or invasion was evident in the large part of the specimen.
Lymph node status was determined within ileocolic and right colic nodes at the time of initial appendiceal malignancy treatment and at the time of cytoreduction. The presence versus absence of appendiceal malignancy in lymph nodes as well as the presence versus absence of signet ring cells were analyzed for their impact on survival.
Statistical Analysis
Data were recorded and analyzed on a commercially available program (Staview 4.5, Abacus Inc., Berkeley, CA). Survival was analyzed from the time of the definitive cytoreduction at this institution. The survival curve distribution was calculated by the Kaplan-Meier method. Univariate analysis with the log-rank test was performed for each clinical and therapeutic variables. The response variable was survival in months for the clinical features that were compared. The P values were calculated for each analysis. The follow-up for survival was 100%, and no patients were censored from the data analysis. A multivariate analysis using a Cox regression was performed to determine which variables were most strongly correlated with survival. All variables that were close to significance (P < 0.01) by univariate analysis were included in the model. Two-tailed P < 0.05 was considered statistically significant.
RESULTS
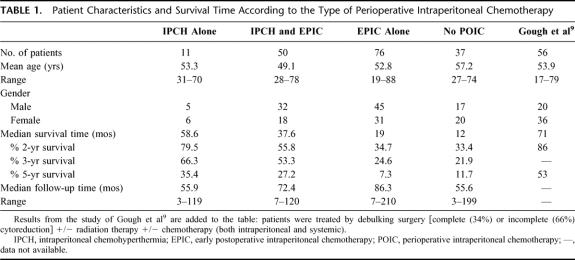
Of the 645 patients treated for peritoneal surface spread of an appendiceal malignancy, 174 (27%) underwent an incomplete cytoreductive surgery: 37 patients underwent a CC-2 resection and 137 a CC-3 resection. There were 99 males and 75 females with a mean age of 52.7 years (range 19–88). Forty-eight patients had a PSS of 0 to 2, and the other 126 patients had a PSS of 3. The median survival of these 174 patients was 20.5 months and their overall 2-year, 3-year, and 5-year survivals were 42.8%, 34%, and 15.3%. The Kaplan-Meier survival distribution is shown in Figure 1.
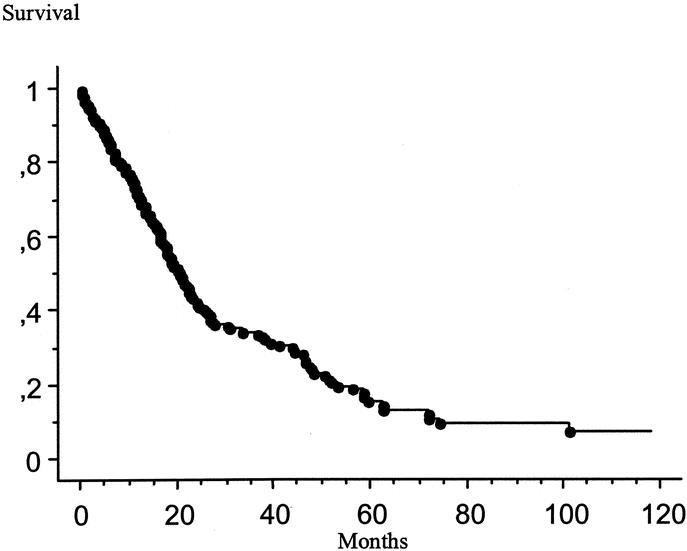
FIGURE 1. Kaplan-Meier survival distribution for 174 patients treated by incomplete cytoreductive surgery. Survival was computed from the initial cytoreduction performed at our institution.
Survival by Perioperative Intraperitoneal Chemotherapy
The patient characteristics and their survival according to the type of perioperative intraperitoneal chemotherapy used are summarized in Table 1. The median survivals were 58.6 months for patients treated by IPCH alone, 37.6 months for patients treated by IPCH combined with EPIC, 34.7 months for patients treated by EPIC alone, and 33.4 months for patients who did not receive perioperative intraperitoneal chemotherapy (P < 0.001). The Kaplan-Meier survival distribution for these 4 groups of patients is shown in Figure 2. The 61 patients treated by IPCH had a median survival of 39.4 months compared with 18.1 month for the 113 patients treated without IPCH (P < 0.001). The 126 patients treated with EPIC had a median survival of 20.9 months compared with 18 months for patients treated without EPIC. The survival difference was not significant (P = 0.589).
TABLE 1. Patient Characteristics and Survival Time According to the Type of Perioperative Intraperitoneal Chemotherapy
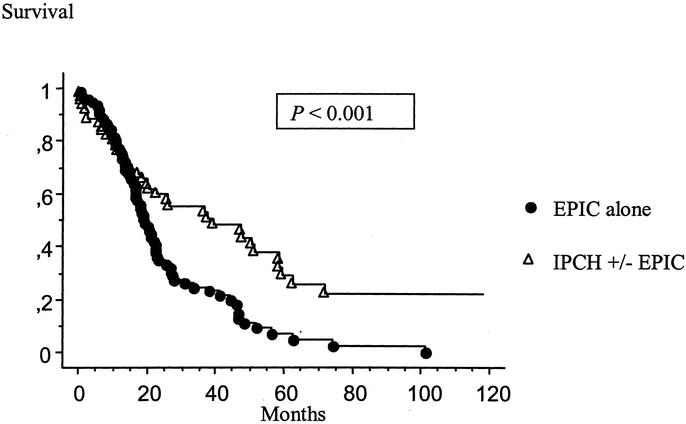
FIGURE 2. Kaplan-Meier survival distribution for 137 patients treated by incomplete cytoreductive surgery according to the type of perioperative intraperitoneal chemotherapy. Survival was computed from the initial cytoreduction performed at our institution. IPCH, intraperitoneal chemohyperthermia; EPIC, early postoperative intraperitoneal chemotherapy.
Survival by Number of Operative Procedures
Of these 174 patients, 141 underwent 1 operative intervention with a median survival of 18.1 month. Thirty-three patients (19%) treated with more than 1 operative intervention had a median survival of 36.8 months (P = 0.003). Patients were selected for additional attempts of combined treatment by clinical criteria. If there was systemic disease, reoperation was not performed. If the preoperative computed tomography (CT) of the abdomen and pelvis showed recurrent disease diffusely layering on small bowel, effective debulking was thought to be impossible and reoperation was not performed. The performance status of the patient was also considered. The Kaplan-Meier survival distribution for patients based on number of operative interventions is shown Figure 3. Twenty-eight of these 33 patients had 2 operative interventions, 3 patients had 3, 1 patient had 4, and 1 patient had 6.
FIGURE 3. Kaplan-Meier survival distribution based on number of operative interventions. Survival was computed from the initial cytoreduction performed at our institution.
Survival by Assessment of Tumor Histology
Forty-one patients had a disseminated peritoneal adenomucinosis with a median survival of 30.8 months, 64 patients had an hybrid type with a median survival of 20.1 months, and 69 patients had mucinous adenocarcinoma with a median survival of 16.4 months (P = 0.001). The Kaplan-Meier distribution of these 3 groups of patients is shown in Figure 4. Thirty-six patients presented with signet ring cells on histopathologic examination. The presence of signet ring cells had a significantly negative influence on survival (P = 0.004) (Fig. 5). Lymph node involvement was observed in 22 patients and also had a significantly negative influence on survival (P < 0.001) (Fig. 6). No patient with lymph node involvement was alive at 2 years.
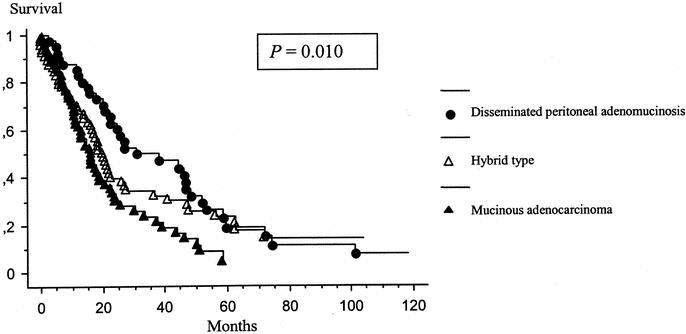
FIGURE 4. Kaplan-Meier survival distribution based on the morphologic type of the tumor. Survival was computed from the initial cytoreduction performed at our institution.
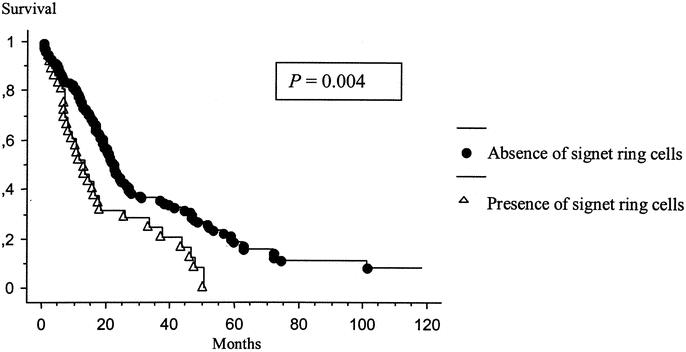
FIGURE 5. Kaplan-Meier survival distribution based on the presence of signet ring cells. Survival was computed from the initial cytoreduction performed at our institution.
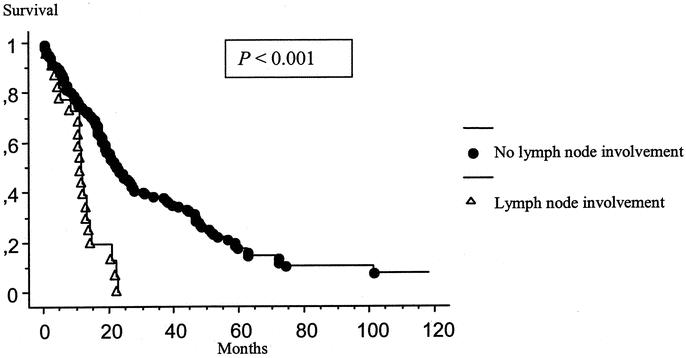
FIGURE 6. Kaplan-Meier survival distribution based on the presence of lymph node involvement. Survival was computed from the initial cytoreduction performed at our institution.
Other statistical evaluations including age, gender, PSS, CC score, treatment versus no treatment with preoperative systemic chemotherapy, preoperative intraperitoneal chemotherapy, long-term postoperative intraperitoneal chemotherapy, and adjuvant systemic chemotherapy did not have a significant impact on survival.
Multivariate Analysis
By multivariate analysis, the presence of signet ring cells, lymph node involvement, the number of procedures performed, and the use of hyperthermia were the variables that had a significant influence on survival (P = 0.047, P < 0.001, P = 0.018, and P < 0.001, respectively).
Morbidity and Mortality
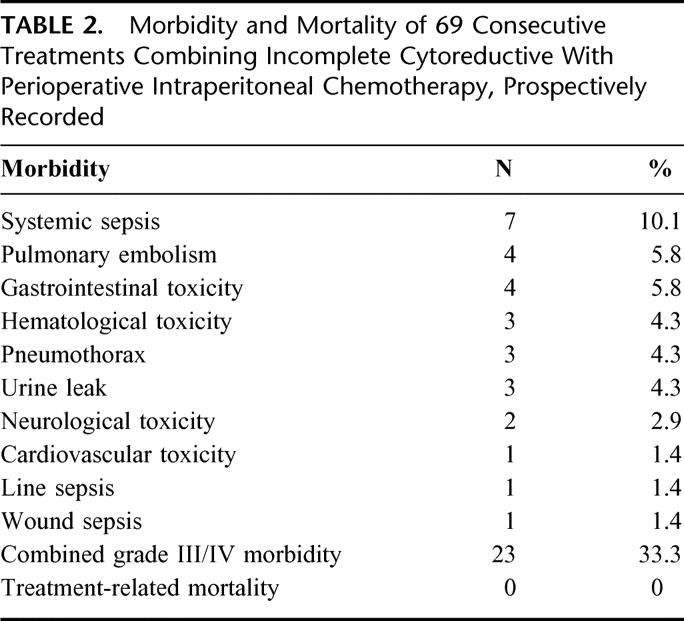
The information concerning morbidity and mortality was prospectively recorded since 1998 and was available on 69 patients. Grade III/IV postoperative complications (National Cancer Institute Common Toxicity Criteria) occurred in 23 (33.3%) of these patients. Two patients suffered combined grade III/IV complications. The most frequent complication was systemic sepsis and was due to urine infection in 6 of 7 cases. There were no postoperative intestinal fistulas. Three patients required a return to the operative room: 1 for postoperative intraabdominal bleeding and 2 for urine leaks. There was no postoperative death. The postoperative complications are summarized in Table 2.
TABLE 2. Morbidity and Mortality of 69 Consecutive Treatments Combining Incomplete Cytoreductive With Perioperative Intraperitoneal Chemotherapy, Prospectively Recorded
DISCUSSION
It is difficult to define the optimal management of peritoneal surface malignancy from appendiceal mucinous tumors because of a lack of controlled studies, difficulty with uniform histologic classification, and the rare nature of this entity.4,5,22 In addition, the long natural history makes prolonged follow-up necessary to determine the impact of therapeutic maneuvers. Compared to colon cancer, appendiceal mucinous tumors with peritoneal spread possess a unique tumor biology characterized by a paucity of dissemination to lymph nodes, liver, or systemic sites; this makes them amenable to aggressive local-regional treatment.10 These malignancies rarely cause treatment failure outside the abdomen or pelvis. As specialized centers report larger numbers of patients, the use of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy appears to be the most effective therapeutic strategy, with 5-year and 10-year survival rates that approach 80% and 60%, respectively, in patients who have a complete cytoreduction.9,12,15 The 5-year and 10-year survival rates from the literature for serial debulking or after incomplete cytoreduction are only 20% and 0%, respectively. These results are confirmed by our analysis with 5-year and 10-year survival rates of 15.3% and 8.3%. The many publications concerning mucinous appendiceal tumors with peritoneal dissemination and with pseudomyxoma peritonei establish that this disease is a lethal one with few survivors at 10 years. Our data are nearly identical to those published by Gough et al in 1994.9 In 56 patients treated by serial debulking at the Mayo Clinic, there was a 5.9-year median survival and only 32% alive at 10 years. Even though the histopathology suggests a tumor of borderline malignancy, the surgeon must not expect a favorable long-term outcome in the absence of special treatments. The patient should not be advised that this is benign disease, nor should the patient be told that serial debulking in the absence of combined treatment (complete cytoreduction plus perioperative intraperitoneal chemotherapy) can result in a cure. In the absence of the surgical skills required for cytoreductive surgery and an institutional capability for performing perioperative chemotherapy, all of these patients require referral to a specialized center. Treatment nihilism for mucinous appendiceal tumors with peritoneal dissemination is no longer defensible.
The morbidity and mortality rates of 69 consecutive procedures combining incomplete cytoreductive surgery and perioperative intraperitoneal chemotherapy were 33.3% and 0%, respectively. These results are comparable to those previously reported by other teams using the same therapeutic strategy for the treatment of peritoneal surface malignancies, with morbidity rates ranging between 24% and 39% and mortality rates ranging between 0% and 14%.12,23–27 In the 2 most important trials dedicated to the analysis of the complications following cytoreductive surgery and perioperative intraperitoneal chemotherapy, the duration of surgery and the number of peritonectomy procedures and resections were the most common predictors of morbidity.23,24 In the present study, the morbidity rate appears to be high, but we observed no digestive fistula or postoperative deaths, and most complications were not surgical. Seven of 23 patients presented with systemic sepsis of urinary tract or pulmonary origin successfully treated with antibiotics. The low morbidity and mortality noted in this group of patients reflects the unwillingness of the surgical team to risk a major complication in patients who are having a palliative intervention. There was an avoidance of extensive dissections after limited survival benefits were confirmed by surgical exploration. The low incidence of serious surgical complications could be explained by an aggressive but comprehensive cytoreductive surgery, which was not too extensive in these cases of palliative approach. Surgical expertise and judgment are required to find a balance between the postoperative risk of extensive surgery and benefit in survival and quality of life. When surgical exploration shows evidence of an invasive and unresectable disease process, cytoreductive surgery needs to be limited and comprehensive.
Ronnett et al22 have already reported the strong influence of histologic features on the prognosis of this disease. The 5-year survival rates after cytoreductive surgery and perioperative intraperitoneal chemotherapy for patients with disseminated mucinous adenomucinosis, hybrid type, and mucinous adenocarcinoma were 86%, 33.6%, and 6.7%, respectively. In the group of patients treated by incomplete cytoreductive surgery, the influence of the histologic type was also found, but was less significant and not an independent prognostic factor. The 3-year survival rate of patients with disseminated mucinous adenomucinosis was 49.7% compared to 34% for those with hybrid type and 23.5% for those with mucinous adenocarcinoma. Two factors seemed to be the strongest indicators of survival: the presence of signet ring cells and the lymph node involvement. No patients with lymph node involvement were alive at 2 years, and the 2-year survival of patients with signet ring cells was less than 30%. The current therapeutic approach does not seam to be adequate for these groups of patients. The use of palliative systemic chemotherapy or neoadjuvant chemotherapy with new promising chemotherapeutic agents such as oxaliplatin or irinotecan need to be considered and evaluated in controlled studies. Lymph node involvement reveals the systemic dissemination of the disease and needs to be treated with systemic chemotherapeutic agents. An incomplete cytoreductive surgery and the time needed to recover delay the administration of this systemic chemotherapy. The presence of signet ring cells denotes a very aggressive type of tumor, reducing the hope of prolonged survival after incomplete cytoreduction. It is possible, as a result of these data, to eliminate some patients from elective surgical interventions for mucinous appendiceal cancer. Only limited surgical interventions designed to manage specific adverse symptoms, such as debilitating ascites, should be contemplated if patients have lymph node metastases or the signet ring morphology. These clinical types of mucinous appendiceal tumors have a rapidly progressing natural history; interventions would be suggested as with colorectal cancer with carcinomatosis. A peritoneal cancer index of 20 or less and the possibility of a complete cytoreduction would be requirements for an elective intervention.
Our data show that patients who underwent more than 1 procedure had a better prognosis. Esquivel and Sugarbaker reported a 5-year survival of 74% for patients with peritoneal surface spread of appendiceal malignancy treated with second look surgery plus intraperitoneal chemotherapy. They concluded that in patients with peritoneal dissemination from appendiceal tumors, second-look surgery resulted in a significant survival benefit.28 More recently, Mohamed et al29 reported a 5-year survival rate of 70% in 45 patients with the same disease treated with 3 or more reoperations. In this group of patients, prolonged survival was significantly associated with a complete cytoreduction. The completeness of cytoreduction was an important determinant of survival except for the initial cytoreduction. This aberration may be due to the surgeon's tendency to stage the initial cytoreduction (gross disease intentionally left behind) in patients with massive mucinous tumor volumes. In these selected patients, the cytoreduction score at the second operation would be the important determinant.
When treatment-related variables were analyzed in this study, patients who received hyperthermic intraperitoneal chemotherapy had a superior survival to those who received normothermic intraperitoneal chemotherapy. Of course, these treatment options were not randomized, and therefore, selection factors may account for the differences in survival between hyperthermic chemotherapy and normothermic chemotherapy. Certainly, because the patients who did not receive 5-FU had the longest survival, one cannot postulate that the more local-regional chemotherapy that was given, the better the outcome. Rather, one might suspect that intraperitoneal chemotherapy in this group of patients with a large volume of residual disease would have little or no effect; the very limited penetration of drugs into large tumor nodules has been repeatedly demonstrated. However, in the laboratory, large tumor nodules are treated very effectively by hyperthermia.30 Also, the effects of hyperthermia at higher temperatures are increased in larger tumor nodules.31 It is quite possible that these data suggest that the local-regional hyperthermia induced in these largely avascular tumors resulted in an effective debulking of the major tumor mass and was thereby an active component of a treatment response.
These studies suggest that in the future, these patients might be treated with aggressive hyperthermia that would be expected to cause a heat-induced cytotoxicity (43°C for 90 minutes). These treatments would be similar to those originally proposed by Shiu and Fortner in 1980.32
Intraperitoneal chemohyperthermia appears to be the most effective adjuvant treatment of patients with peritoneal carcinomatosis from other origins and is used by most of the teams specialized in the management of peritoneal surface malignancy.13,26,33–35 To determine the precise effect of IPCH after radical surgery, randomized studies are needed. However, in a rare disease such as peritoneal carcinomatosis from appendiceal malignancy, this seems impossible to realize. The question of quality of life is also important, especially in patients following incomplete cytoreduction with a palliative intent. McQuellon et al36 reported a better or similar quality of life in 64 patients with peritoneal carcinomatosis from digestive origin, 3 months after IPCH. The same authors recently reported a good quality of life in 17 long-term survivors after the aggressive combination of cytoreductive surgery with IPCH.37 Again, prospective studies are needed to more clearly evaluate the toxicity and the benefit of this therapeutic approach.
Unfortunately, the sites of treatment failure following cytoreductive surgery plus perioperative intraperitoneal chemotherapy were not recorded in this group of patients. This is a rare disease with patients referred from all part of the United States and some from other countries. Because of the distances involved, follow-up studies were only sporadically recorded, sites of treatment failure were not always known, and cause of death was difficult (usually impossible) to determine. Future prospective data on this important aspect in the treatment of peritoneal surface malignancy are expected.
Long-term survival and cure are not expected for patients with peritoneal carcinomatosis from appendiceal malignancies following incomplete cytoreduction. The combination of comprehensive surgical debulking with IPCH may allow 1-year, 2-year, and 3-year survival rates of 71%, 43%, and 34%, respectively. For patients with signet ring histology or lymph node involvement, new therapeutic strategies are required.
Footnotes
Reprints: P. H. Sugarbaker, MD, Washington Cancer Institute, 110 Irving Street, NW, Suite CG-185, Washington, DC 20010-2975. E-mail: paul.sugarbaker@medstar.net.
Olivier Glehen is supported by a grant from Fondation de France.
REFERENCES
- 1.Lyss AP. Appendiceal malignancies. Semin Oncol. 1988;15:129–137. [PubMed] [Google Scholar]
- 2.McCusker ME, Cote TR, Clegg LX, et al. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer. 2002;94:3307–3312. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Ronnett BM, Archer A, et al. Pseudomyxoma peritonei syndrome. Adv Surg. 1996;30:233–280. [PubMed] [Google Scholar]
- 4.Hinson FL, Ambrose NS. Pseudomyxoma peritonei. Br J Surg. 1998;85:1332–1339. [DOI] [PubMed] [Google Scholar]
- 5.Nitecki SS, Wolff BG, Schlinkert R, et al. The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg. 1994;219:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wertheim I, Fleischhacker D, McLachlin CM, et al. Pseudomyxoma peritonei: a review of 23 cases. Obstet Gynecol. 1994;84:17–21. [PubMed] [Google Scholar]
- 7.Smith JW, Kemeny N, Caldwell C, et al. Pseudomyxoma peritonei of appendiceal origin. The Memorial Sloan-Kettering Cancer Center experience. Cancer. 1992;70:396–401. [DOI] [PubMed] [Google Scholar]
- 8.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sindelar WF, DeLaney TF, Tochner Z, et al. Technique of photodynamic therapy for disseminated intraperitoneal malignant neoplasms. Phase I study. Arch Surg. 1991;126:318–324. [DOI] [PubMed] [Google Scholar]
- 12.Witkamp AJ, de Bree E, Kaag MM, et al. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei. Br J Surg. 2001;88:458–463. [DOI] [PubMed] [Google Scholar]
- 13.Glehen O, Mithieux F, Osinsky D, et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21:799–806. [DOI] [PubMed] [Google Scholar]
- 14.Deraco M, Gronchi A, Mazzaferro V, et al. Feasibility of peritonectomy associated with intraperitoneal hyperthermic perfusion in patients with Pseudomyxoma peritonei. Tumori. 2002;88:370–375. [DOI] [PubMed] [Google Scholar]
- 15.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–731. [DOI] [PubMed] [Google Scholar]
- 16.Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirtzfeld DA, Rodriguez-Bigas M, Weber T, et al. Disseminated peritoneal adenomucinosis: a critical review. Ann Surg Oncol. 1999;6:797–801. [DOI] [PubMed] [Google Scholar]
- 18.Sugarbaker PH. Intraperitoneal Chemotherapy and Cytoreductive Surgery: Manual for Physicians and Nurses. 3rd ed. Grand Rapids, MI: Ludann; 1998. [Google Scholar]
- 19.Sugarbaker PH. Laser-mode electrosurgery. In: Sugarbaker PH, ed. Peritoneal Carcinomatosis: Diagnosis and Treatment. Boston, MA: Kluwer Academic; 1996:375–385. [DOI] [PubMed] [Google Scholar]
- 20.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In: Sugarbaker PH, ed. Peritoneal Carcinomatosis: Principles of Management. Boston, MA: Kluwer Academic Publishers; 1996:359–374. [DOI] [PubMed] [Google Scholar]
- 21.Yan H, Pestieau SR, Shmookler BM, et al. Histopathologic analysis in 46 patients with pseudomyxoma peritonei syndrome: failure versus success with a second-look operation. Mod Pathol. 2001;14:164–171. [DOI] [PubMed] [Google Scholar]
- 22.Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19:1390–1408. [DOI] [PubMed] [Google Scholar]
- 23.Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790–796. [DOI] [PubMed] [Google Scholar]
- 24.Glehen O, Osinski D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863–869. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto S, Takahashi M, Mutou T, et al. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529–534. [PubMed] [Google Scholar]
- 26.Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–76. [DOI] [PubMed] [Google Scholar]
- 27.Glehen O, Beaujard AC, Arvieux C, et al. Les carcinoses péritonéales. Traitement chirurgical, péritonectomies et chimiohyperthermie intrapéritonéale. Gastroenterol Clin Biol. 2002;26:210–215. [PubMed] [Google Scholar]
- 28.Esquivel J, Sugarbaker PH. Second-look surgery in patients with peritoneal dissemination from appendiceal malignancy: analysis of prognostic factors in 98 patients. Ann Surg. 2001;234:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed F, Chang D, Sugarbaker PH. Third look surgery and beyond for appendiceal malignancy with peritoneal dissemination. J Surg Oncol. 2003;83:5–12. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed F, Marchettini P, Stuart OA, et al. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol. 2003;10:463–468. [DOI] [PubMed] [Google Scholar]
- 31.Sugarbaker PH, Sugarbaker C, Stephens AD, et al. Radiofrequency hyperthermia in the palliative treatment of mucinous carcinomatosis of appendiceal origin: optimizing and monitoring heat delivery in western patients. Int J Hyperthermia. 2000;16:429–441. [DOI] [PubMed] [Google Scholar]
- 32.Shiu MH, Fortner JG. Intraperitoneal hyperthermic treatment of implanted peritoneal cancer in rats. Cancer Res. 1980;40:4081–4084. [PubMed] [Google Scholar]
- 33.Shen P, Levine EA, Hall J, et al. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg. 2003;138:26–33. [DOI] [PubMed] [Google Scholar]
- 34.Yonemura Y, Fujimura T, Nishimura G, et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery. 1996;119:437–444. [DOI] [PubMed] [Google Scholar]
- 35.Zoetmulder FAN, Verwaal V, Ruth S. Hyperthermic intra peritoneal chemotherapy (HIPEC) with mitomycin C significantly improves survival in patients with peritoneal carcinomatosis of colorectal origin. ASCO 2002; abstract no. 586.
- 36.McQuellon RP, Loggie BW, Fleming RA, et al. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27:65–73. [DOI] [PubMed] [Google Scholar]
- 37.McQuellon RP, Loggie BW, Lehman AB, et al. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2003;10:155–162. [DOI] [PubMed] [Google Scholar]