Abstract
Objective:
We reported here a series of 49 patients with unresectable hepatocellular carcinoma (HCC) who underwent nonsurgical treatment to downstage the disease followed by salvage surgery, their long-term outcome, and pattern of recurrence.
Summary Background Data:
Most HCC patients present with unresectable disease and are treated with chemotherapy or intra-arterial therapy with a palliative intent. Occasionally, there are good responses to treatment so that salvage surgery becomes feasible afterward. However, long-term outcomes of these patients are seldom reported.
Methods:
Patients with unresectable hepatocellular carcinoma, from September 1993 to June 2002, who received salvage surgery after downstaging by systemic chemotherapy, intra-arterial yttrium-90 microspheres, or sequential treatment were included in this study. Systemic chemotherapy consisted of combination doxorubicin, cisplatin, interferon-alpha and 5-fluorouracil (5-FU), or single-agent doxorubicin. The choice of treatment was according to stage of disease and contemporary clinical trial protocol. Survival, recurrence pattern, and surgical outcome were studied.
Results:
There were 49 patients in this study with 40 males and 9 females, age ranged from 12 to 69 years. Forty patients (81.6%) were hepatitis B positive. Thirty-two patients had combination chemotherapy alone (65.3%), 8 patients had single agent chemotherapy alone (16.3%), 4 patients received intra-arterial yttrium-90 microspheres alone (8.2%), and 5 patients received sequential therapy (10.2%). Twenty-eight (57.1%) patients received major hepatic resection. Thirteen patients (26.5%) had complete necrosis of the tumor after treatment. Twenty-one patients (42.9%) had recurrence after surgery, and 14 of them were intrahepatic recurrence. The median survival was 85.9 months. The 1-year, 3-year, and 5-year survival rates were 98%, 64%, and 57%, respectively.
Conclusions:
Salvage surgery after successful downstaging can provide long-term control of disease in a small proportion of patients with unresectable hepatocellular carcinoma.
Nonsurgical treatment with systemic chemotherapy, intra-arterial yttrium-90 microspheres either alone or sequentially, can effectively downstage some patients with unresectable hepatocellular carcinoma and allow salvage surgery afterward. Combined modality treatment of unresectable hepatocellular carcinoma can be curative in some patients.
It is a generally accepted principle that a cure for cancer is possible only if complete extirpation of tumor is achieved with either a single or combined modalities. Cure is rarely possible for hepatocellular carcinoma (HCC) when it is unresectable, making the prognosis dismal. Unfortunately, unresectable HCC, either because of the local extent of disease or metastases, is also unsuitable for orthotopic liver transplantation or local ablative therapy.1 The treatment of these unresectable HCCs is mainly palliative, aiming to relieve symptoms, and if possible, prolong survival. There have been occasional reports in the medical literature showing that some palliative procedures can downstage HCC, thus allowing unresectable HCC to become resectable.2–6 Two of these reports came from our center using either systemic chemotherapy5 or intra-arterial yttrium 90 microspheres.6 With more experience using these modalities of treatment, we started to use one modality followed by another if the HCC did not respond well enough for resectional surgery, if the patient's condition allowed us to do so. Surgical resection of the residual lesion was offered to patients at any stage if surgery could potentially resect all gross lesions shown radiologically with a margin. Our experience using salvage surgery following downstaging of unresectable HCC with systemic chemotherapy and/or intra-arterial yttrium 90 microspheres formed the basis of this study.
MATERIALS AND METHODS
Patients with a diagnosis of HCC referred to the Joint HCC Clinic at the Prince of Wales Hospital (Hong Kong) were seen by a team of clinicians consisting of hepatologists, oncologists, interventional radiologists, and liver surgeons. Patients with unresectable or metastatic HCC were considered for treatment with either systemic chemotherapy or intra-arterial yttrium 90 microspheres, according to contemporary phase II or III clinical trials. These trials were approved by the clinical ethics committee of the Chinese University of Hong Kong. Patients had to give written consent before entry into the respective clinical trial for unresectable HCC. Unresectable disease was defined as extensive bilobar involvement of the liver by a large solitary tumor or by multiple tumors, or invasion of major blood vessels including the main portal vein, hepatic veins, inferior vena cava, and main hepatic artery. The diagnosis of HCC was made either by histologic examination of tumor tissue obtained by percutaneous needle biopsy or imaging evidence of a space occupying lesion in the liver compatible with HCC together with a serum alpha fetoprotein (AFP) concentration of over 500 ng/mL (normal value < 10 ng/mL) in a patient who was known to have a high risk for HCC (known carrier of hepatitis B virus surface antigen or positive for hepatitis C antibody). Routine investigation for any patient undergoing active anticancer treatment included full blood counts, liver and renal function tests, clotting profile, serological markers for hepatitis B, serum AFP, chest radiography, ultrasound scan, and computed tomography (CT) of the abdomen.
Patients with extrahepatic disease were treated with systemic chemotherapy. Patients with localized disease to the liver who also satisfied criteria for intra-arterial yttrium-90 microsphere treatment would receive the said treatment; otherwise, systemic chemotherapy was given.
Criteria for Hepatic Intra-arterial Yttrium 90 Microspheres Treatment
Additional inclusion criteria included no extrahepatic spread of disease, Karnosfsky performance score >70%, adequate liver function with a total bilirubin <50 ìmol/L, and no tumor invasion into the portal vein, hepatic artery, hepatic veins, or inferior vena cava. There was no age limit for entry. Suitable patients were then subjected to selective hepatic angiography (HAG). Gamma scintigraphy for assessment of percentage lung shunting and tumor-to-normal (T/N ratio) was performed after infusion of technetium-99m macroaggregated albumin (99m Tc-MAA, Amersham Pulmonate II, UK). This technique has been reported previously7,8 and is summarized below. Selective HAG was performed with the Seldinger technique. The tip of the angiographic catheter was placed in the hepatic artery distal to the origin of the gastroduodenal artery. A total of 111 MBq (3mCi) of 99mTc-MAA in 1 mL was injected over a period of 10 seconds. After hemostasis of the catheter puncture site, the patient was transported to the gamma camera suite, and images over the liver and lungs were obtained. The percentage of lung shunting and T/N ratio were determined from the counts over the various regions of interest. Patients were offered selective internal radiation (SIR) therapy using yttrium 90 microspheres if their lung shunting was <15% and the average T/N ratio equal or greater than 2, with no obvious necrotic area within the tumor. Patients were also excluded if there was a significant amount (>5%) of radioactivity shunted into the gastroduodenal region or when complicated arterial blood supply to the liver that precluded superselection of the hepatic artery was shown on HAG. Suitable patients were then treated with yttrium 90 microspheres supplied by Australian Radioisotope (Lucas Height, Sydney, Australia). After quality assurance tests that included checking the specific gravity and percentage of activity leaching followed by sterilization, the microspheres, in aliquots of 1.0 and 1.5 GBq (27.0–40.5 mCi), were suspended in a mixture of equal volumes of water for injection and Omnipaque® 300 (Nycomed, Oslo, Norway) to a final volume of 5 mL. The Omnipaque was added to make the vehicle carrying the microspheres radiopaque so that its flow could be monitored under fluoroscopy. The infusion of the microspheres was given through a 4-way valve of an injection bracket made of 1-cm thick Perspex.9 An activity of yttrium 90 microspheres, determined by the tumor size and the Tc-MAA scintigraphy findings, was given in fractions of 1.0 and 1.5 GBq. The radiation doses to the lungs, the tumor, and the nontumorous liver were estimated using the partition model first described by us.9 Computed tomography was done routinely before and every 2 months after the infusion to monitor the response.
Criteria for Systemic Therapy
Inclusion criteria included patients below the age of 70 years, Karnofsky performance score >70%, platelet count >100 × 109/L, white blood cell (WBC) count >3 × 109/L, total bilirubin <50 ìmol/L, and creatinine clearance >50 mL/minute. Previous treatment, including surgery, radiotherapy, systemic chemotherapy, or chemoembolization, was not a cause for excluding patients, but a washout period of at least 4 weeks was required. Cases with isolated bone metastasis or pleural effusion were considered inevaluable and excluded.
We used the combination chemotherapy PIAF (cisplatin, doxorubicin, 5-FU, and alpha-IFN), which was previously described.10,11 The regimen consisted of cisplatin (20 mg/m2) (days 1–4), 5-FU (400 mg/m2) (days 1–4), recombinant alpha-interferon-2b (5 MU/m2, Intron A, Schering-Plough Inc., Kenilworth, NJ) given subcutaneously (days 1–4), and intravenous doxorubicin (40 mg/m2) given on day 1 only. Treatment was repeated every 3 weeks or delayed by 1 week until the platelet count was over 100 × 109/L and WBC count was over 3 × 109/L. The maximum number of cycles was 6. A dose reduction of 25% was allowed for grade 3 bone marrow suppression from the previous cycle, but treatment was stopped if either grade 4 toxicity or clinical deterioration considered not suitable for further treatment was present. Some of the patients received single agent doxorubicin, 60 mg/m2, once every 3 weeks for 6 cycles, because they were participating in a phase III prospective randomized study comparing single agent and combination chemotherapy. Computed tomography was done before treatment, after 3 cycles, and at the end of treatment of assessment of response for all patients who received systemic therapy.
Sequential Therapy
Patients who received systemic chemotherapy but the lesion was still unresectable and localized were considered for intra-arterial yttrium-90 microspheres treatment.
Salvage Surgery
Resectability of the tumor was assessed by the same liver surgeons before, during, and after the completion of the different modalities of treatment either alone or in combination. All CT scans were assessed by the surgeons with the radiologist. Surgical resection of residual lesions was offered to patients at any stage of the treatment if surgery could potentially resect all gross lesions with a margin as shown radiologically. This is a policy of treatment and not part of the clinical trial. The reasons why the HCC was initially unresectable but became resectable were disappearance of some of the lesions, shrinkage of large HCC, and enlargement of nontumorous part of the liver. For liver resection, an anatomic resection based on the segments described by Couinaud was the preferred operation, aiming at a gross resection margin of 1 cm from the edge of the lesions. Nonanatomic resection of the liver was only performed when the tumor was at the edge of the liver. If at operation, the residual lesion was considered not resectable because of additional lesions that were not shown on preoperative imaging, a debulking surgery using liver resection, microwave coagulation, cryosurgery, or alcohol injection was performed.12 The aim of the debulking surgery was to remove all macroscopic tumors, probably leaving behind microsatellite lesions to be dealt with later by locoregional or systemic therapy.
RESULTS
From September 1993 to June 2002, we undertook 3 clinical trials using yttrium-90 microspheres or systemic chemotherapy for unresectable HCC. The first study was the use of intra-arterial yttrium-90 microspheres in 71 patients with localized unresectable HCC. Four patients in this study received salvage surgery after treatment. This study was reported in 1998.6 The second study was the use of systemic PIAF for 50 patients with unresectable or metastatic HCC in a phase II study and the results published in 1999.10 The third study was a prospective randomized study comparing single-agent doxorubicin and PIAF, which is still ongoing. The latter 2 studies treated a total of 128 patients with PIAF and 76 patients with single-agent doxorubicin. In the present series, all the patients were trial participants from these 3 studies. These 49 patients with initially unresectable HCC received salvage surgery after the HCC was downstaged to become resectable. There were 40 males and 9 females (ratio 4.4:1). The age ranged from 12 to 69 years (median 45). Forty patients were hepatitis B surface antigen positive (81.6%), 1 was hepatitis C antibody positive (2%), and another was positive for both (2%). The number of patients with Pugh-Child A, B, and C were 42, 6, and 1, respectively. The AFP on first presentation was over 500 ng/mL in 28 patients (57.1%) and below 10m ng/mL in 14 patients (28.6%) (range 3–610, 431, median 1707). The AFP just before the salvage surgery was over 500 ng/mL in 14 patients (28.6%) and below 10 ng/mL in 7 patients (14.3%) (range 4–12612, median 25). The AFP showed a drop in 35 patients (71.4%).
Out of the 49 patients, the reasons for unresectable disease at time of presentation were bilobar disease (34 patients, 69.4%), main portal vein thrombosis (7 patients, 14.3%), and extrahepatic metastases (8 patients, 16.3%).
The types of surgery the 49 patients underwent are listed in Table 1. Most patients underwent major resectional surgery involving 3 Couinaud liver segments or more (n = 28, 57.1%). In 2 patients, the primary tumor showed complete radiologic response to the treatment. Solitary residual metastatic deposits were demonstrated in these patients, 1 patient underwent a pulmonary segmentectomy, whereas another patient underwent excision of an omental deposit. The former patient survived for 38.4 months and the latter 30.1 months. Both died of disseminated metastatic disease. One patient developed progressive liver failure, although the tumor responded to systemic PIAF. He underwent orthotopic liver transplantation. There was a solitary tumor of 3 cm in diameter in the explanted liver. Histology showed complete necrosis of the tumor. He was still alive and tumor free at the time of censor, 58.7 months after the surgery. Five patients underwent debulking surgery. One patient was still alive and tumor free at censor, 57.7 months after surgery. The other 4 patients had died, surviving 10.2 months, 12.2 months, 17.4 months, and 21.6 months after surgery, respectively.
TABLE 1. Types of Surgery
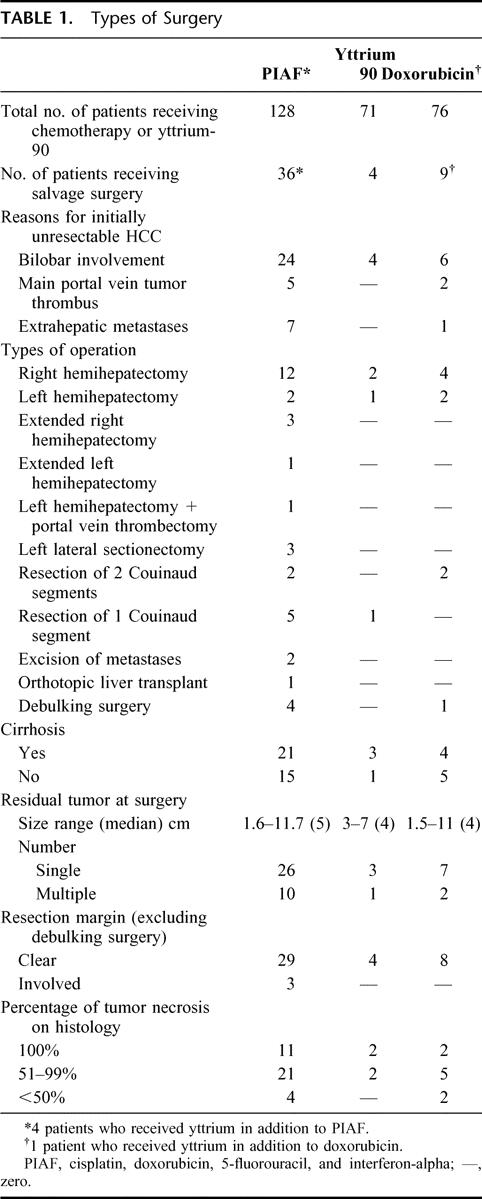
There were 2 in-hospital mortalities. They died 4 days and 66 days from time of surgery, respectively, and both deaths were due to postoperative complications (liver failure and sepsis). Other postoperative morbidity occurred in 12.2% of patients (3 bile leaks, 3 intra-abdominal collections and pleural effusions). The median stay in hospital after surgery was 9 days (range 5–66 days).
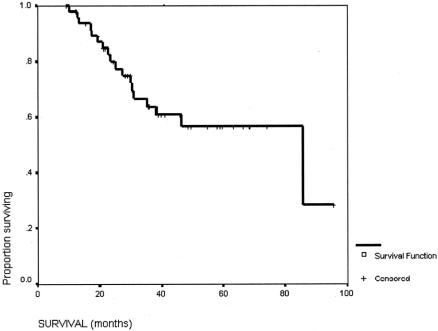
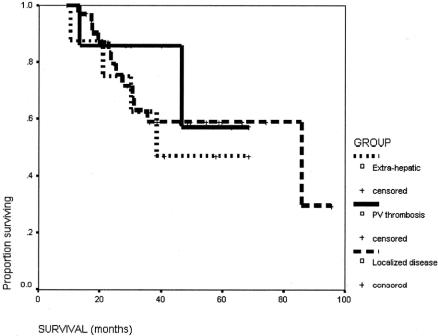
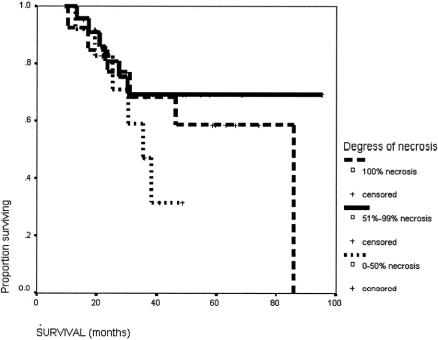
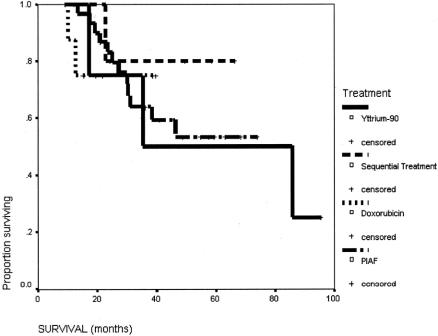
The survival of this group of patients is shown in Figure 1. The 5-year survival rate from the start of the treatment of the overall group was 57%. The median survival from the time of surgery was 71.5 months (95% confidence interval [CI] 25.9–117.1 months), and the 5-year survival rate was 55.2%. The initial reason why the HCC was not resectable had no influence on the long-term survival after the salvage surgery (Fig. 2). The degree of necrosis of the tumor in response to the treatment again had no impact on the long-term survival (Fig. 3). The number of patients was too small to analyze for the impact of the initial therapy to the long-term survival (Fig. 4).
FIGURE 1. Overall survival for the whole group of patients (n = 49).
FIGURE 2. Survival of patients with initially unresectable HCC because of extensive bilobar liver involvement (n = 34), main portal vein tumor thrombus (n = 7), or extrahepatic metastases (n = 8).
FIGURE 3. Survival of patients with different degrees of tumor necrosis to the therapy before surgery: 100% necrosis (n = 15); 51% to 99% necrosis (n = 28), and 0% to 50% necrosis (n = 6).
FIGURE 4. Survival of patients with initial therapy being PIAF alone (n = 32), yttrium-90 (n = 4), doxorubicin (n = 8), and sequential therapy (n = 5).
DISCUSSION
Partial hepatectomy, orthotopic liver transplantation, and local ablative therapy in the form of radiofrequency ablation, microwave coagulation, and alcohol injection are the only treatment options that can offer the possibility of a cure for HCC.1,13 Unfortunately, the majority of symptomatic patients with HCC cannot be treated with these options, and the prognosis is dismal.
A number of procedures have been shown to be able to shrink HCC, thus allowing unresectable tumors to become resectable.1 Procedures with the potential to downstage HCC for a secondary resection include transarterial chemoembolization2; combined chemotherapy, external beam irradiation combined in some patients with internal radiation using iodine-131-antiferritin4; a combination of hepatic artery ligation with hepatic artery infusion of chemotherapeutic agent, radioimmunotherapy, and fractionated regional radiotherapy3; transarterial yttrium 90 microspheres6; and systemic chemotherapy.5 The main problem with tumor shrinkage is that only a small proportion of patients respond well enough to the treatment to allow secondary liver resection following therapy, and the responders cannot be predicted. However, the fact that some patients can still be cured in a previously dismal situation gives great hope to patients with unresectable HCC. The 5-year survival of 57% in our patients who received salvage surgery after tumor downstaging compares favorably to that in patients who underwent liver resection when they first presented with resectable tumors. The 5-year survival reported in the medical literature for the latter group of patients ranges from 11%14 to 76%,15 with a median of 30%.1 The favorable results in our series may well reflect that selection of biologically less aggressive tumor, which also responded better to systemic and/or intra-arterial treatment.
The selection of the procedure with a potential to downstage unresectable HCC to become resectable in a particular patient would depend on many factors, including the general condition of the patient, stage of the disease, the liver function of the patient, the choice of the patient, as well as the treatment protocol and availability of expertise of the individual medical center. For disease which has spread beyond the liver, intra-arterial treatments2–5 would not be useful, and systemic chemotherapy6 is the only remaining option. For patients with tumor thrombus in the main portal vein, transarterial chemoembolization2 or yttrium-90 microspheres6 are contraindicated. For disease still confined to the liver, some procedures that involve internal radiation of the tumor using radioactive isotopes3,4,6 are possible in selected patients, but the delivery requires specialized facilities. Some treatment has significant toxicity,5 whereas other treatments are complicated and difficult to administer.3,4 In our hands, systemic chemotherapy, either using doxorubicin alone or a combination of cisplatin, interferon alpha, doxorubicin, and 5-FU,5 is now used as the first line of treatment in patients who present with unresectable tumor because systemic chemotherapy has a wider indication and is simpler to use. We now reserve yttrium-90 microspheres as a second-line treatment in patients who do not respond well enough to systemic chemotherapy.
One common criticism about studies on salvage surgery following downstaging of the tumors using either intra-arterial or systemic therapy is that the original tumors before the downstaging were resectable. We agree that the criteria to determine whether a tumor is resectable or not are subjective. In our series, the resectability of the tumor was determined by a single surgeon (W.Y.L.) who made the decision as to whether the tumor was resectable before, during, and after the tumor downstaging. Many of the tumors in the liver shrunk remarkably with disappearance of the satellite nodules and portal vein tumor thrombus and enlargement of the nontumorous liver to make the HCC resectable. Furthermore, in this series of patients, the reasons for unresectability were extrahepatic metastases in 8 patients (16.3%) and main portal vein tumor thrombus in 7 patients (14.2%), these being the commonly accepted criteria used for unresectability.
We are surprised that the degree of necrosis in the tumor caused by the downstaging therapy has no impact on the long-term survival of our patients. The only logical explanation is some resistant clones of tumor cell escaped the cytotoxic effects of these therapies, and they were not detected radiologically before and at the salvage surgery. An alternative explanation, which is less likely, is that a second tumor developed in the liver remnant after the salvage surgery. It is also worthwhile to take note that of the 5 patients who underwent debulking surgery, 1 survived for almost 5 years, and he was still alive and tumor free at the time of censor. This supports our policy of doing debulking surgery at the time of the operation, when unexpected lesions that are not shown radiologically are found intraoperatively.
In conclusion, salvage surgery following tumor-downstaging using systemic chemotherapy and/or regional therapy has low operative mortality and morbidity. It gives good long-term outcome.
Footnotes
Reprints: Professor W.Y. Lau, MD, FRCS, FRACS, FACS, Department of Surgery, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, New Territories, Hong Kong SAR, China. E-mail: josephlau@surgery.cuhk.edu.hk.
REFERENCES
- 1.Lau WY. Management of hepatocellular carcinoma. J R Coll Surg Edinb. 2002;47:389–399. [PubMed] [Google Scholar]
- 2.Fan J, Tang ZY, Yu YQ, et al. Improved survival with resection after transcatheter arterial chemoembolisation (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15:674–678. [DOI] [PubMed] [Google Scholar]
- 3.Tang ZY, Yu YQ, Zhou XD, et al. Cytoreduction and sequential resection for surgically verified unresectable hepatocellular carcinoma: evaluation with analysis of 72 patients. World J Surg. 1995;19:784–789. [DOI] [PubMed] [Google Scholar]
- 4.Sitzmann JV, Abrams R. Improved survival for hepatocellular cancer with combination surgery and multimodality treatment. Ann Surg. 1993;217:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau WY, Leung TW, Lai BS, et al. Preoperative systemic chemoimmunotherapy and sequential resection for unresectable hepatocellular carcinoma. Ann Surg. 2001;233:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau WY, Ho S, Leung TW, et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90 yttrium microspheres. Int J Radiat Oncol Biol Phys. 1998;40:583–592. [DOI] [PubMed] [Google Scholar]
- 7.Lau WY, Leung TWT, Ho S, et al. Diagnostic pharmacoscintigraphy with technetium-99m macroaggregated albumin in the prediction of tumor to normal uptake ratio during therapy of inoperable hepatocellular carcinoma with yttrium-90 microspheres. Br J Radiol. 1994;67:136–139. [DOI] [PubMed] [Google Scholar]
- 8.Leung WT, Lau WY, Ho SKW, et al. Measurement of lung shunting in hepatocellular carcinoma with intrahepatic-arterial technetium-99m macroaggregated albumin. J Nucl Med. 1994;35:70–73. [PubMed] [Google Scholar]
- 9.Ho S, Lau WY, Leung WT, et al. Partition model for estimating radiating doses from yttrium 90 microspheres in treating hepatic tumors. Eur J Nucl Med. 1996;23:947–952. [DOI] [PubMed] [Google Scholar]
- 10.Leung TW, Patt YZ, Lau WY, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676–1681. [PubMed] [Google Scholar]
- 11.Leung TW, Tang AMY, Zee B, et al. Factors predicting response and survival in 149 patients with resectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer. 2002;94:421–427. [DOI] [PubMed] [Google Scholar]
- 12.Lau WY, Leung WT, Leung KL, et al. Cytoreductive surgery for hepatocellular carcinoma. Surg Oncol. 1994;3:161–166. [DOI] [PubMed] [Google Scholar]
- 13.Lau WY, Leung TW, Yu SC, et al. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai EC, Ng IO, You KT, et al. Hepatic resection for small hepatocellular carcinoma: the Queen Mary Hospital experience. World J Surg. 1991;15:654–659. [DOI] [PubMed] [Google Scholar]
- 15.Takenaka Shimade M, Higashi H, et al. Liver resection for hepatocellular carcinoma in the elderly. Arch Surg. 1994;129:846–850. [DOI] [PubMed] [Google Scholar]