Abstract
Objective:
To investigate the effects of IL-18 therapy on severe and mild bacterial infection after burn injury.
Summary Background Data:
IL-18 therapy restores IFN-γ production in immunosuppressive mice following burn injury and up-regulate host response to LPS and experimental bacterial peritonitis. On the other hand, the overproduction of IFN-γ could induce an exaggerated inflammation. Therefore, in this study, we focus on the beneficial and deleterious effects of IL-18-induced IFN-γ and investigate the behavior of IL-18 in infections.
Methods:
Burn injury was induced in C57BL/6 mice and then they were i.p. injected with IL-18 (0.2 μg) on alternate days. After 1 week, severe and mild infections were made in mice by an Escherichia coli challenge (5 × 108 CFU and 1 × 108 CFU i.v., respectively).
Results:
IL-18 therapy decreased the mortality of burn-injured mice followed by a severe infection, whereas it unexpectedly increased the mortality of burned mice with a mild infection. The IL-18 therapy increased the number of liver mononuclear cells (MNCs), especially NK cells, and greatly up-regulated the impaired IFN-γ production from the liver and spleen MNCs in mice with severe infection. Both the serum IFN-γ concentrations recovered while the bacterial count in the liver decreased. In contrast, the serum IFN-γ concentrations of the burned mice with mild infection did not decrease in comparison to the unburned mice, whereas IL-18 therapy greatly up-regulated the serum IFN-γ levels in burned mice. However, IL-18 therapy significantly elevated the serum ALT and creatinine levels, thus suggesting that the mortality was induced by an exaggerated form of shock/multiorgan failure. These beneficial and deleterious effects of IL-18 therapy in mice with severe and mild infections, respectively, were all inhibited by anti-IFN-γ Ab pretreatment.
Conclusion:
IL-18 therapy can be a potent therapeutic tool against severe bacterial infection in immunocompromised hosts, but careful attention should also be paid to its adverse effects.
IL-18 therapy up-regulates impaired IFN-γ production in mice with Escherichia coli infections after burn injury. IL-18 therapy decreased mouse mortality in severe infection, whereas it unexpectedly increased the mortality of mild infection because excessively produced IFN-γ by IL-18 is beneficial in severe infection but deleterious in mild infection.
Many trauma surgeons as well as us have known that most cases of death that occur among severe burn patients who survive the initial resuscitation are the direct result of multiple organ dysfunction following severe sepsis.1–3 Therefore, the up-regulation of the immune dysfunction against bacterial infections after burn injury might play a crucial role in improving the mortality of severe burn patients. The suppression of interferon (IFN)-γ production, which strongly up-regulates the TH1 immune response, has been observed after burn injury.4–6 Interleukin (IL)-18 has been reported to induce a potent IFN-γ production from NK cells and T cells in the presence of IL-12.7,8 We have recently shown that IL-18 therapy up-regulates IFN-γ production and decreases the mouse mortality after experimental bacterial peritonitis following burn injury, which was made by a cecal ligation and puncture (CLP).9 Although burn wound infections are mainly caused by gram-positive bacteria, they are not important sources of severe sepsis because gram-positive bacteria infections account for less than 10% of all burn-related deaths.10 In contrast, secondary infections with gram-negative bacteria often cause sepsis and occasional mortality in the burn patients. Pseudomonas is a common gram-negative bacterium that causes infections following burn injury, whereas Escherichia coli should not be ignored because of its virulency.11–13 Although IFN-γ decreases the mouse mortality in infection under immunosuppressive conditions by up-regulating the TH1 immune response, other reports have also indicated that it may increase the mortality after infection because IFN-γ induces exaggerated inflammatory reactions.14 We have also reported that IFN-γ is an essential cytokine for mouse mortality in the shock induced by IL-12/lipopolysaccharide (LPS)15 or a synthetic ligand of NK1.1 Ag+ T cells (NKT cells), α-galactosylceramide.16 Therefore, an excessive up-regulation of IFN-γ production by IL-18 therapy may possibly be harmful to the hosts. In the present study, we found that the up-regulation of the IFN-γ production induced by IL-18 injections decreases the mortality of burn-injured mice with a severe E. coli infection, whereas it surprisingly increases the mortality of burned mice with a mild E. coli infection. IL-18 therapy can therefore be a potent therapeutic tool against severe bacterial infection in immunocompromised hosts; however, careful attention should also be paid to its adverse effects.
MATERIALS AND METHODS
This study was conducted according to the guidelines of the Institutional Review Board for the Care of Animal Subjects at the National Defense Medical College, Japan.
Mice and Burn or Sham Injury
Male C57BL/6 mice were studied (8 weeks old, 20 g, Charles River Inc, Yokohama, Japan). The mice were anesthetized using an intraperitoneal injection with pentobarbital (1 mg/body, Abbott Laboratories, North Chicago, IL). Once the mice were fully anesthetized, the dorsa were shaved and the mice were placed in a plastic mold that exposes 20% of their total body surface area. The mice were then subjected to full-thickness burn injury by pressing a heated brass blade to the skin. Immediately after burn injury, phosphate-buffered saline (PBS) (1 mL/body) was intraperitoneally administered for fluid resuscitation. The control unburned mice only had their backs shaved without any burn injury followed by PBS administration.
Reagents
E. coli strain B (ATCC 11303, Sigma Co., St. Louis, MO) and mouse recombinant IL-18 (MBL, Nagoya, Japan) were used for the experiments. The intravenous injection dose of E. coli that produced a 50% lethality (LD50) was 7.5 × 108 colony-forming units (CFU).
IL-18 Treatment (Alternate-Day Injections of IL-18)
IL-18 treatment was performed by intraperitoneal injections of IL-18 (0.2 μg/0.5 mL/body) on alternate days for 7 days (1, 3, 5, and 7 days after injury). Sham treatment was injected with PBS (0.5 mL) in the same way as the IL-18 treatment.
Models of Lethal Infection, Severe (Sublethal) Infection, and Mild (Nonlethal) Infection by a Systemic E. coli Challenge
The lethal infection model was made by an intravenous injection of 1 × 109 CFU of E. coli into the mice 7 days after either the burn injury or sham handling. The severe (sublethal) infection model was also made by an injection of 5 × 108 CFU of E. coli, and a mild (nonlethal) infection model was made by an injection of 1 × 108 CFU of E. coli.
Isolation of the Liver and Spleen Mononuclear Cells (MNCs)
Under deep ether anesthesia, the mice were killed to remove the livers and spleens. The liver and spleen MNCs were obtained as previously described.15,17,18 Briefly, the liver was minced and incubated in a RPMI 1640 medium containing 0.05% collagenese (Wako, Osaka, Japan) for 20 minutes at 37°C. The liver specimen was passed through a stainless steel mesh. The cells were suspended in 33% Percoll solution and centrifuged at 500g for 20 minutes to obtain the liver MNCs. Splenocytes were also passed through the mesh to obtain the spleen MNCs.
Phenotypical Analysis of Liver and Spleen MNCs
Extracted MNCs from the liver and spleen were stained with Turk solution (Wako, Osaka, Japan) and then were counted using a microscope. A two-color immunofluorescence test was performed using FITC conjugated anti-mouse αβ TCR monoclonal Ab (Caltag Labo. Burlingame, CA) and PE conjugated anti-NK1.1 monoclonal Ab (Pharmingen, San Diego, CA). The percentage of fluorescence-positive cells was analyzed using EPICS XL (Coulter, Miami, FL). The NK cells were positive for NK1.1 staining but negative for αβ TCR staining, while the NKT cells were positive for both NK1.1 staining and αβ TCR staining.
In Vivo Depletion of NK Cells, NKT Cells, and IFN-γ
Anti-NK1.1 Ab (PK136) (200 μg/mouse) or anti-asialo GM1 (AGM1) Ab (Wako, Tokyo) (50 μg/mouse) was i.v. injected into the mice 3 days before the E. coli challenge. Anti-NK1.1 Ab depletes both NK cells and NKT cells, and anti-AGM1 Ab depletes NK cells for approximately 1 week, as we previously reported.19,20 To deplete IFN-γ, anti-IFN-γ Ab (500 μg/mouse) (R4-6A2, rat Ig G1; IBL, Gunma, Japan) was i.v. injected into mice at 1 hour before the E. coli challenge. Rat Ig G1 (500 μg/mice, Sigma Chemical Co.) was also injected into the mice as an isotype control.
Measurements of Cytokine, Alanine Aminotransferase (ALT), and Creatinine Levels Using Culture Supernatants and Sera
After counting the cells, 5 × 105 of the liver or the spleen MNCs in 200 μL of 10% FBS RPMI 1640 medium were cultured in 96 well flat-bottomed plates in 5% CO2 at 37°C for 24 hours and then the culture supernatants were stocked at −80°C. Blood samples were obtained from the retro-orbital plexus of mice. The sera were also stocked at −80°C until assays. IFN-γ levels of the culture supernatants or IFN-γ, IL-10, and total IL-12 levels of the sera were measured by cytokine-specific ELISA kits (Endogen, Woburn, MA). The serum ALT and creatinine levels were measured by a FUJI dry-chem system (FUJIFILM, Tokyo).
Viable Bacterial Counts
The livers were aseptically removed to produce a homogenized PBS suspension. The bacterial suspensions were 1 × 104-fold diluted by PBS, placed by a spiral platter on brain heart infusion agar plates, and incubated at 37°C for 24 hours. The number of viable bacteria in the liver was then counted according to the observed colonies on the agar plates.
Histologic Examinations
The mice were killed to remove the lungs, livers, and kidneys. The livers and kidneys were then immersed in 20% formalin for 2 days. The lungs were also immersed in 20% formalin for 2 days, after gentle intratracheal instillation of 20% formalin with a pressure of about 10 cm H2O. From the specimens, slides were prepared and stained with hematoxylin and eosin.
Statistical Analysis
The data are presented as the mean values ± se. Statistical analyses were performed using an iMac computer (Apple, Cupertino, CA) and the Stat View 4.02J software package (Abacus Concepts, Berkeley, CA). The survival rates were compared using the Wilcoxon rank test, and other statistical evaluations were compared using the standard one-way analysis of variance followed by the Bonferroni post hoc test. P < 0.05 was considered to indicate a significant difference.
RESULTS
IL-18 Therapy Increases the Liver NK Cells in Both the Unburned and Burn-Injured Mice
IL-18 treatment greatly increased proportions of NK cells in the liver MNCs of both the unburned and burn-injured mice (Fig. 1). In addition, IL-18 treatment greatly increased the number of liver MNCs in both the unburned and burn-injured mice (2.9 × 106 vs. 9.2 × 106 and 2.7 × 106 vs. 10.4 ×106, respectively). In the spleen, IL-18 treatment slightly increased MNC count only in the unburned mice but did not increase the NK and NKT cells ratio (data not shown).
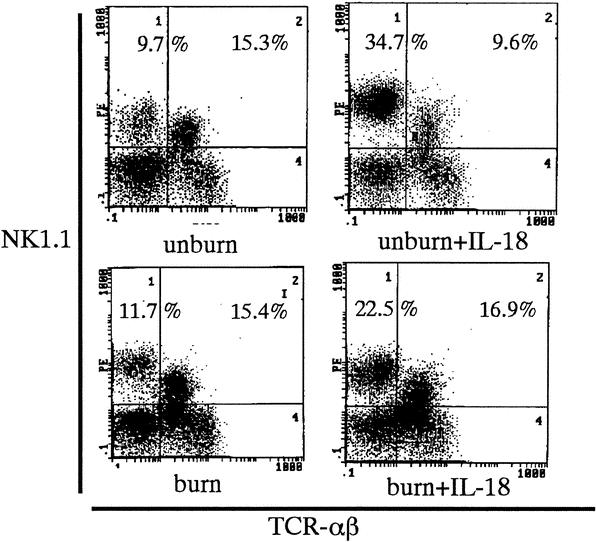
FIGURE 1. Proportions of NK and NKT cells in the liver MNCs. The number of upper quadrants are the mean % of NK cells and NKT cells of the liver MNCs from 6 to 10 mice of each group.
IL-18 Therapy Increases Survival in Severe E. coli Infection but Decreases Survival in Mild E. coli Infection of Burn-Injured Mice
After the 1 × 109 CFU of E. coli challenge (i.v.), any mice including IL-18 treated burned mice did not survive beyond 1 day (Fig. 2A). After the 5 × 108 CFU of E. coli challenge (severe infection), the burned mice significantly decreased the survival rate than the unburned mice, whereas IL-18 treatment following burn injury significantly improved the survival rate in the burned mice (Fig. 2B). After the 1 × 108 CFU of E. coli challenge (mild infection), no difference in the survival rate was found between the burned mice and the unburned mice, while unexpectedly IL-18 treatment significantly decreased the survival in the burned mice (Fig. 2C).
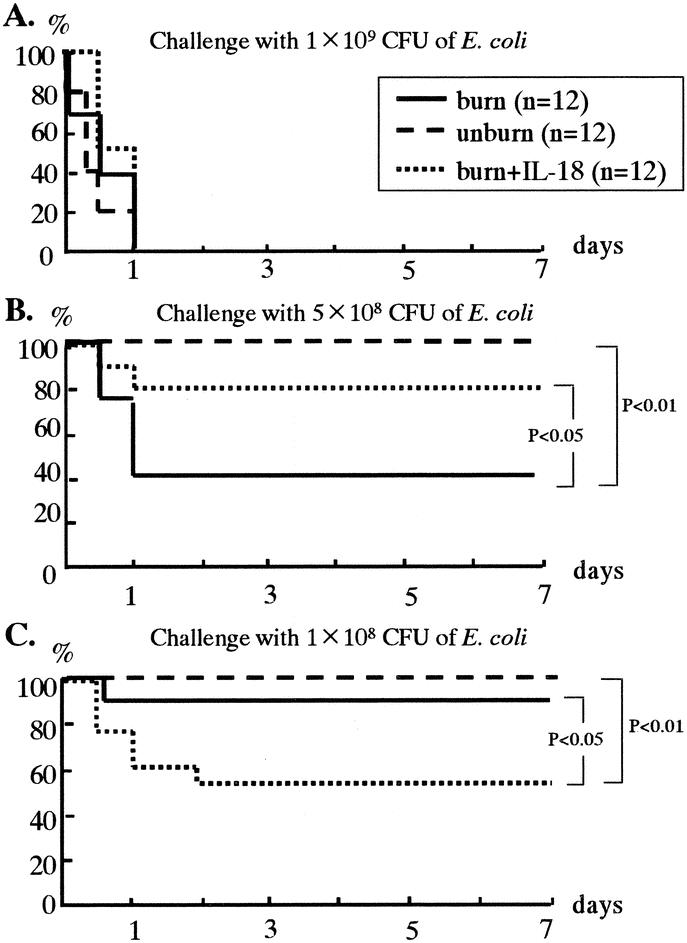
FIGURE 2. The effects of IL-18 therapy on mouse survival after various doses of systemic E. coli challenge (A, 1 × 109 CFU; B, 5 × 108 CFU; and C, 1 × 108 CFU) following burn injury.
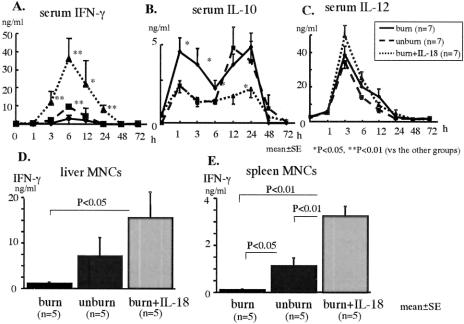
IL-18 Therapy Increases Serum IFN-γ Levels but Decreases Serum IL-10 Levels After Severe E. coli Infection Following Burn Injury
The mice were i.v. inoculated with E. coli (5 × 108 CFU) 7 days after the burn or sham injury. Although the serum IFN-γ peak was significantly suppressed in the burned mice than in the unburned mice, the IL-18 treatment significantly increased the serum IFN-γ peak in burned mice (Fig. 3A). The burned mice showed a significantly higher initial peak of IL-10 at 1 hour than that of the unburned mice, but no difference was observed in a later peak of IL-10 at 12 to 24 hours between the burned mice and the unburned mice. However, the IL-18 treatment significantly decreased both the initial and the later peaks in burned mice (Fig. 3B). No differences in the change of the serum IL-12 levels were observed among all of the mice (Fig. 3C). Serum IL-18 level peaked at 12 to 24 hours in the burned and the unburned mice, and no significant difference of serum IL-18 levels was observed between the burned mice and the unburned mice. As expected, IL-18 treated burned mice showed the high serum IL-18 level, while it decreased to a basal level at 48 hours (data not shown).
FIGURE 3. The effects of IL-18 therapy on the changes of serum IFN-γ (A), IL-10 (B), and IL-12 (C) and on IFN-γ production from the liver MNCs (D), and the spleen MNCs (E) at 2 hours after the severe E. coli challenge.
IL-18 Therapy Increases IFN-γ Production in the Liver and Spleen MNCs Obtained at 2 Hours After the Severe E. coli Infection Following Burn Injury
The mice were i.v. inoculated with E. coli (5 × 108 CFU) 7 days after the burn. At 2 hours after E. coli challenge, the liver and spleen MNCs were isolated and were cultured for 24 hours to examine IFN-γ production. The IL-18 treatment significantly increased the IFN-γ production from both the liver and spleen MNCs in the burned mice, although those IFN-γ productions of the burned mice were severely impaired compared with the control unburned mice (Fig. 3D, E).
Beneficial Effects of IL-18 Therapy in the Burned Mice With the Severe E. coli Infection Were Inhibited by Anti-IFN-γ Ab Pretreatment
Anti-IFN-γ Ab or rat Ig G1 (isotype Ab as a control) was i.v. injected into the IL-18 treated burned mice at 1 hour before E. coli challenge. Mice were then i.v. inoculated with E. coli (5 × 108 CFU) 7 days after the burn or sham injury. Anti-IFN-γ Ab pretreatment significantly decreased the survival of the IL-18 treated burned mice (Fig. 4A). Similarly, although IL-18 treatment suppressed the viable bacterial counts in the liver at 24 hours as well as the elevation of serum ALT levels at 12 hours after the severe E. coli challenge in the burned mice, the inhibition of IFN-γ abrogated these effects (Fig. 4B, C).
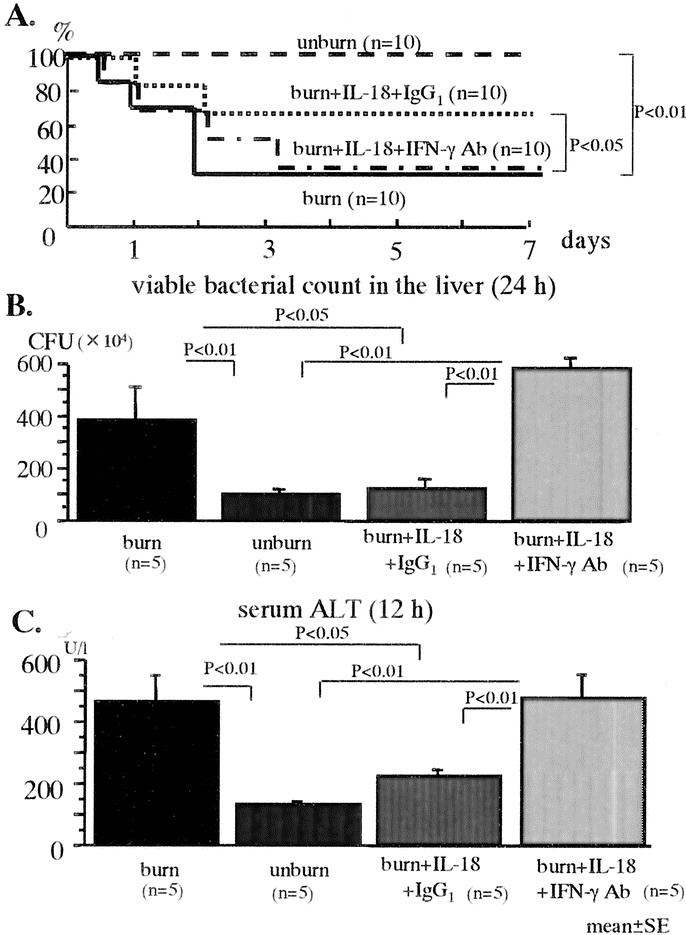
FIGURE 4. The effects of anti-IFN-γ Ab on the mortality: A, viable bacterial counts in the liver at 24 hours; B, serum ALT levels at 12 hours; and C, after the severe E. coli challenge in the IL-18-treated burned mice.
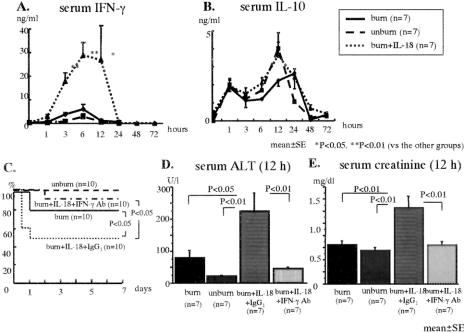
Anti-IFN-γ Ab Pretreatment Decreases the Mortality, Serum ALT Levels, and Creatinine Levels of the IL-18-Treated Burned Mice With Mild E. coli Infection and Improves the Organ Injuries
The mice were i.v. inoculated with E. coli (1 × 108 CFU) 7 days after the burn. Although no significant difference of serum IFN-γ peak was observed between the burned mice and the unburned mice, the IL-18 treatment significantly increased serum IFN-γ after E. coli challenge in the burned mice (Fig. 5A). The serum IL-10 levels showed no significant difference among all mouse groups (Fig. 5B). Although IL-18 treatment decreased the survival in burned mice, in contrast to E. coli severely infected mice, anti-IFN-γ Ab pretreatment increased the mouse survival after the mild E. coli challenge (Fig. 5C). As expected, in all groups, the viable bacterial counts in the liver at 12 hours after the mild E. coli challenge were remarkably lower than those receiving a severe E. coli challenge (data not shown). However, IL-18 injections increased both the serum ALT and creatinine levels in the burned mice at 12 hours after the mild E. coli challenge (Fig. 5D, E). The IL-18 injections also tended to increase the lung wet/dry ratio (an indicator of the pulmonary congestion) at 24 hours after the mild E. coli challenge (data not shown). Interestingly, anti-IFN-γ Ab pretreatment inhibited all these deleterious effects of IL-18 therapy. According to a pathologic examination, IL-18-treated burned mice showed coagulation necrosis in the liver (Fig. 6 A-c, indicated by arrows), acute tubular necrosis in the kidney (Fig. 6B-c, indicated by arrows), and septum thickness and intra-alveolar edema in the lung (Fig. 6 C-c) at 24 hours after the mild E. coli challenge, whereas the depletion of IFN-γ remarkably suppressed those pathologic findings in the IL-18-treated burned mice (Fig. 6A-d, B-d, C-d).
FIGURE 5. The effects of IL-18 treatment on the changes of serum IFN-γ (A) and IL-10 (B) in the burned mice after the mild E. coli challenge. The effects of anti-IFN-γ Ab on the mortality: C, serum levels of ALT at 12 hours; D, and creatinine at 12 hours; E, after the mild E. coli challenge in the IL-18-treated burned mice.
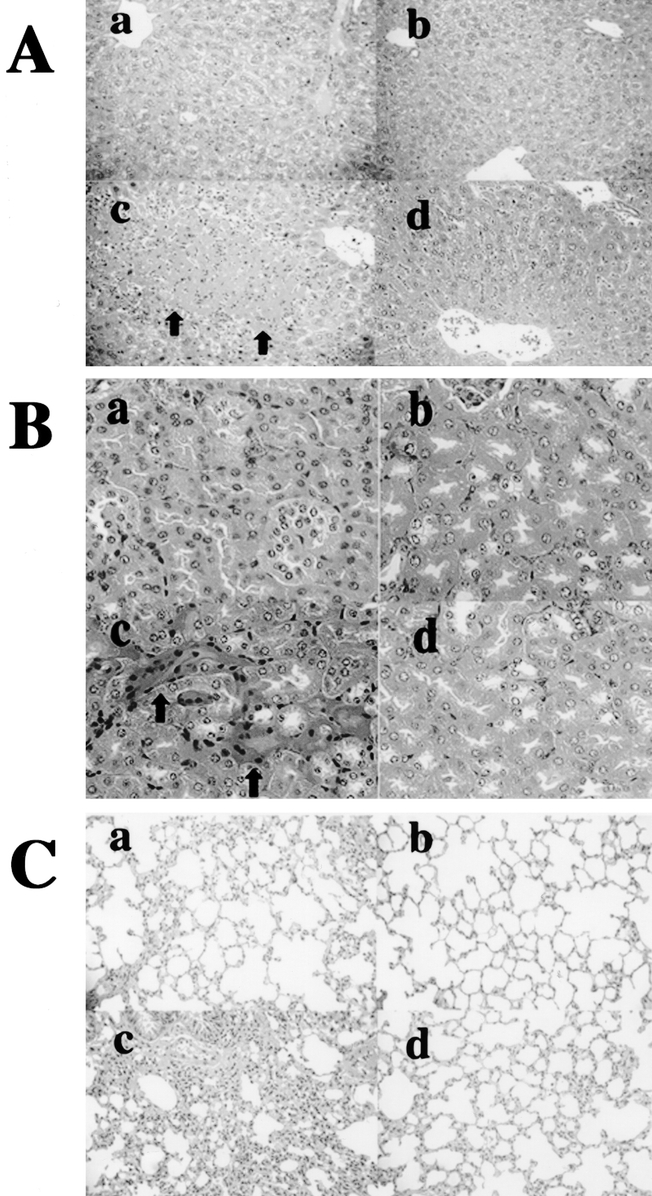
FIGURE 6. The pathologic findings of the IL-18-treated burned mice at 24 hours after the mild E. coli challenge. The liver is shown as A (hematoxylin and eosin, original magnification ×200), the kidney is B (hematoxylin and eosin, original magnification ×300) and the lung is C (hematoxylin and eosin, original magnification ×200). The nontreated burned mice (n = 4) are labeled as a, nontreated (control) unburned mice (n = 4) as b, IL-18-treated burned mice (n = 4) as c, and IFN-γ Ab + IL-18-treated burned mice (n = 4) as d, in each panel.
Depletion of NK Cells Increases the Survival, Decreases Serum IFN-γ Levels, and Improves the Liver and Kidney Injury After the Severe E. coli Infection in the IL-18-Treated Unburned Mice
The IL-18 injections increased the mortality after the severe E. coli challenge (5 × 108 CFU) in the unburned mice (approximately 40%), while none of mice depleted of NK/NKT cells using anti-NK1.1 Ab died (not shown). The IL-18 injections greatly increased serum IFN-γ levels in the unburned mice, while the depletion of NK/NKT cells suppressed the elevation of serum IFN-γ levels (Fig. 7A). The depletion of only NK cells using anti-AGM1 Ab also suppressed the mortality (not shown) and the elevation of serum IFN-γ levels (Fig. 7A). Furthermore, although the IL-18 injections increased serum ALT and creatinine levels at 12 hours after the severe E. coli challenge in the unburned mice, the depletion of NK/NKT cells suppressed these values (Fig. 7B, C). IL-18 injections did not induce mortality after the mild E. coli challenge (1 × 108 CFU), and the peak of serum IFN-γ levels at 6 hours after the mild E. coli challenge was lower (30 ng/mL) than that in the mice with the severe E. coli challenge.
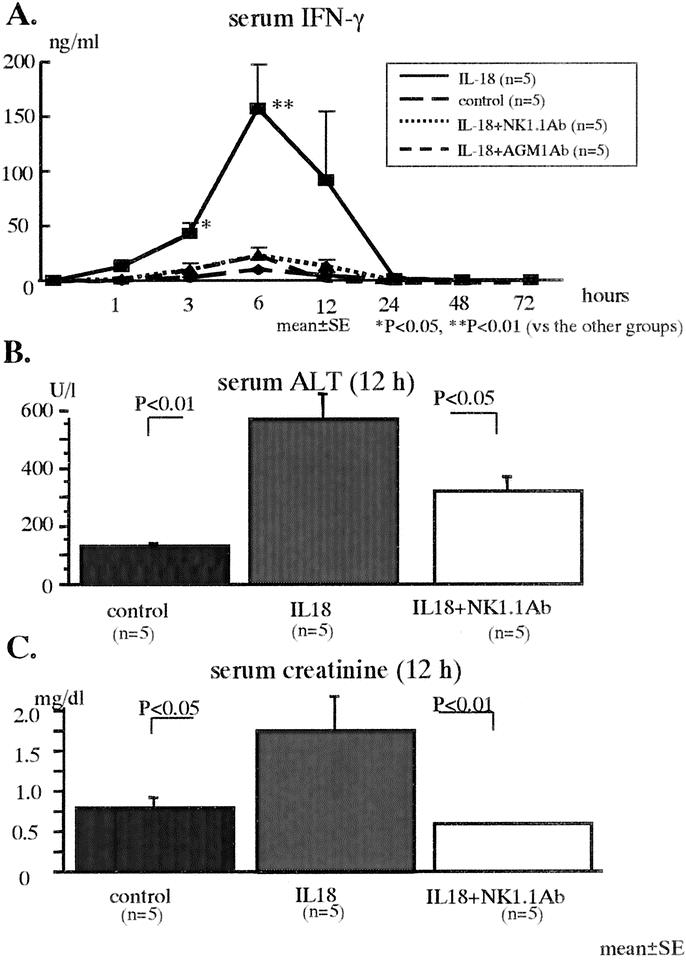
FIGURE 7. The effect of IL-18 treatment on the serum levels of IFN-γ (A), ALT at 12 hours (B), and creatinine at 12 hours (C) after the severe E. coli challenge in the unburned control mice.
DISCUSSION
In the case of a severe E. coli infection in mice after burn injury, which is a condition of IFN-γ hyporesponsiveness, alternate-day injections of IL-18 remarkably augmented the E. coli clearance in the liver by up-regulating IFN-γ production from NK cells and thereby significantly decreased the mortality. On the other hand, in the case of mild E. coli infection in mice after burn injury, which is a condition without IFN-γ hyporesponsiveness, IL-18 injections induced multiple organ injury and increased the mortality due to the large amount of IFN-γ induced by IL-18 therapy.
Alternate-day injections of IL-18 increased in the number and proportion of liver NK cells despite the fact that the IL-18 treatment did not increase the spleen NK cells. Liver NK cells were recently shown to be main IFN-γ producers in septic bacterial peritonitis19 and LPS injection.19,21 The increased serum IFN-γ concentrations induced by IL-18 injections reflect a markedly enhanced IFN-γ production from the liver and spleen NK cells, and IFN-γ activates the phagocytosis of E. coli by Kupffer cells in a positive feedback loop. IL-18 injections also improved the liver injury and the mortality induced by the severe E. coli infection. Anti-IFN-γ Ab pretreatment abrogated all these beneficial effects of IL-18 therapy, thus indicating that IFN-γ plays an important role in the IL-18-induced host defense. In mild E. coli infection, the burned mice exhibited normal response of IFN-γ production, and then most burned mice survived the infection without IL-18 therapy. IL-18 pretreatment to the burned mice with mild infection also greatly increased serum IFN-γ concentration, whereas IL-18 therapy unexpectedly induced multiple organ injury and decreased the survival of the burned mice. Interestingly, anti-IFN-γ Ab pretreatment also abrogated these deleterious effects of IL-18 injections. These findings suggest that the effectiveness of IL-18 therapy in bacterial infections associated with burn injury depends on the severity of the infection and the associated degree of IFN-γ hyporesponsiveness. Indeed, although the bacterial counts in the liver of mice with severe infection were approximately 100-fold larger than that of mice with mild infection, the maximum serum IFN-γ levels (approximately 30 ng/mL) induced by IL-18 therapy were similar in both mouse groups. The high IFN-γ level induced by IL-18 therapy may thus be needed in burned mice to overcome a severe infection while a normal IFN-γ production may be sufficient to resolve a mild infection. On the other hand, the high IFN-γ level induced by IL-18 therapy may be excessive and harmful in burned mice with a mild infection. In line with the deleterious effects of IL-18 in the burned mice after the mild E. coli infection, alternate-day injections of IL-18 into the unburned mice increased the mortality after the severe E. coli infection. IL-18 injections into the unburned mice greatly increased the serum IFN-γ concentrations and induced both liver and kidney injury as assessed by an elevation of the serum ALT and creatinine levels, respectively. Notably, the depletion of NK cells suppressed an elevation of the serum IFN-γ concentrations and improved the organ injury after infection and decreased mortality. Therefore, the large amount of IFN-γ produced by NK cells during IL-18 therapy might induce organ injury presumably as a result of endotoxin (LPS)-induced shock, and it thereby induces mouse mortality in healthy control mice. We also tried to examine the effect of NK/NKT cell depletion on IL-18 treatment after burn injury. However, the treatment of burned mice with either anti-NK1.1 Ab or AGM1 Ab could not effectively deplete NK/NKT cells presumably because burn injury has been reported to induce a depletion/inactivation of complements22,23 and therefore NK/NKT cells could not be depleted by the combination of Ab and complements. Nevertheless, NK cells are suggested to be responsible for the augmented IFN-γ production by IL-18 therapy both in burned mice and unburned mice. It is noteworthy, however, that the unburned mice with IL-18 therapy were all able to survive the mild E. coli infection while the serum IFN-γ level was similar to that seen in the IL-18-treated burned mice with a mild infection but it was far below those observed in the IL-18-treated unburned mice with a severe E. coli infection. These findings suggest that hosts with severe burn injury are both immunologically and physiologically damaged and thus are more susceptible to either bacterial infections themselves or endotoxin/IL-18/IFN-γ induced death than unburned mice.
IL-12 production has been reported to be suppressed in severe infections following burn injury, and IL-12 treatment improves the suppression of IFN-γ production and decreases the mortality these mice.24–27 We also observed a significant suppression of the serum IL-12 levels in the lethal E. coli challenge after burn injury (unpublished observation). However, in the severe E. coli challenged mice, we did not find any significant difference in the serum IL-12 levels between the burned mice and the unburned mice despite a significant suppression of IFN-γ. In an experimental bacterial peritonitis model in unburned mice, we recently reported that IL-12 as well as IFN-γ induction was suppressed in lethal peritonitis as compared with mild peritonitis.28 As a result, the IL-12 production was speculated to be impaired in such severe infections that induce 100% mortality. Nevertheless, the present findings suggest that the IFN-γ production from NK cells could be suppressed with an unimpaired production of IL-12 and IL-18 in the bacterial infections following burn injury. NK cells thus appear to be anergic to these cytokines and cannot produce IFN-γ, whereas exogenous IL-18 can augment their IFN-γ production. However, the present results also suggest that the macrophages in the burned mice may produce more IL-10 than those in unburned mice. In addition, if macrophages can produce a larger amount of endogenous IL-18 to induce IFN-γ, the mice with burn injury could inhibit bacterial infections, thus implying that both macrophages and NK cells would be affected by burn injury.
Finally, it should be noted that the sensitivity to the LPS-induced shock and the susceptibility to gram-negative bacteria infections are reciprocally related to each other. LPS-unresponsive mice are highly resistant to the LPS-induced lethality but are extremely susceptible to gram-negative bacteria infections themselves.29 Similarly, IL-18-deficient mice are quite resistant to LPS-induced lethality but are susceptible to various gram-positive and -negative bacteria infections.7,30 Endogenous IL-18 may therefore normally regulate the positive and negative responses precisely of the hosts in the bacterial infections while burn injury disturbs the cross-talk between IL-18 and IFN-γ and the immune balance.
Footnotes
Reprints: Shuhji Seki, MD, Department of Microbiology, National Defense Medical College, Namiki 3-2, Tokorozawa 359-8513, Japan. E-mail: btraums@res.ndmc.ac.jp.
REFERENCES
- 1.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. [DOI] [PubMed] [Google Scholar]
- 2.Shackford SR, Mackersie RC, Davis JW, et al. Epidemiology and pathology of traumatic deaths occurring at a Level I Trauma Center in a regionalized system: the importance of secondary brain injury. J Trauma. 1989;29:1392–1397. [DOI] [PubMed] [Google Scholar]
- 3.Baker CC, Oppenheimer L, Stephens B, et al. Epidemiology of trauma deaths. Am J Surg. 1980;140:144–150. [DOI] [PubMed] [Google Scholar]
- 4.De AK, Kodys KM, Pellegrini J, et al. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol. 2000;96:52–66. [DOI] [PubMed] [Google Scholar]
- 5.Peter FW, Schuschke DA, Barker JH, et al. The effect of severe burn injury on proinflammatory cytokines and leukocyte behavior: its modulation with granulocyte colony-stimulating factor. Burns. 1999;25:477–486. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki F, Pollard RB. Mechanism for the suppression of gamma-interferon responsiveness in mice after thermal injury. J Immunol. 1982;129:1811–1815. [PubMed] [Google Scholar]
- 7.Nakanishi K, Yoshimoto T, Tsutsui H, et al. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. [DOI] [PubMed] [Google Scholar]
- 8.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. [DOI] [PubMed] [Google Scholar]
- 9.Ami K, Kinoshita M, Yamauchi A, et al. IFN-gamma production from liver mononuclear cells of mice in burn injury as well as in postburn bacterial infection models and the therapeutic effect of IL-18. J Immunol. 2002;169:4437–4442. [DOI] [PubMed] [Google Scholar]
- 10.Alexander JW, Meakins JL. A physiological basis for the development of opportunistic infections in man. Ann Surg. 1972;176:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen SR, Umphred E, Warden GD. The incidence of bacteremia following burn wound excision. J Trauma. 1982;22:274–279. [DOI] [PubMed] [Google Scholar]
- 12.Gang RK, Bang RL, Sanyal SC, et al. Pseudomonas aeruginosa septicaemia in burns. Burns. 1999;25:611–616. [DOI] [PubMed] [Google Scholar]
- 13.Nagoba BS, Deshmukh SR, Wadher BJ, et al. Bacteriological analysis of burn sepsis. Indian J Med Sci. 1999;53:216–219. [PubMed] [Google Scholar]
- 14.Miles RH, Paxton TP, Dries DJ, et al. Interferon-gamma increases mortality following cecal ligation and puncture. J Trauma. 1994;36:607–611. [DOI] [PubMed] [Google Scholar]
- 15.Ogasawara K, Takeda K, Hashimoto W, et al. Involvement of NK1+ T cells and their IFN-gamma production in the generalized Shwartzman reaction. J Immunol. 1998;160:3522–3527. [PubMed] [Google Scholar]
- 16.Inui T, Nakagawa R, Ohkura S, et al. Age-associated augmentation of the synthetic ligand- mediated function of mouse NK1.1 ag(+) T cells: their cytokine production and hepatotoxicity in vivo and in vitro. J Immunol. 2002;169:6127–6132. [DOI] [PubMed] [Google Scholar]
- 17.Dobashi H, Seki S, Habu Y, et al. Activation of mouse liver natural killer cells and NK1.1(+) T cells by bacterial superantigen-primed Kupffer cells. Hepatology. 1999;30:430–436. [DOI] [PubMed] [Google Scholar]
- 18.Habu Y, Seki S, Takayama E, et al. The mechanism of a defective IFN-gamma response to bacterial toxins in an atopic dermatitis model, NC/Nga mice, and the therapeutic effect of IFN-gamma, IL-12, or IL-18 on dermatitis. J Immunol. 2001;166:5439–5447. [DOI] [PubMed] [Google Scholar]
- 19.Seki S, Osada S, Ono S, et al. Role of liver NK cells and peritoneal macrophages in gamma interferon and interleukin-10 production in experimental bacterial peritonitis in mice. Infect Immun. 1998;66:5286–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa R, Nagafune I, Tazunoki Y, et al. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J Immunol. 2001;166:6578–6584. [DOI] [PubMed] [Google Scholar]
- 21.Seki S, Habu Y, Kawamura T, et al. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1. 1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. [DOI] [PubMed] [Google Scholar]
- 22.Farrell MF, Day NK, Tsakraklides V, et al. Study of lymphocyte depletion and serum complement perturbations following acute burn trauma. Surgery. 1973;73:697–705. [PubMed] [Google Scholar]
- 23.Gelfand JA, Donelan M, Burke JF. Preferential activation and depletion of the alternative complement pathway by burn injury. Ann Surg. 1983;198:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goebel A, Kavanagh E, Lyons A, et al. Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann Surg. 2000;231:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Sullivan ST, Lederer JA, Horgan AF, et al. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–490; discussion 490–492. [DOI] [PMC free article] [PubMed]
- 26.O'Suilleabhain C, O'Sullivan ST, Kelly JL, et al. Interleukin-12 treatment restores normal resistance to bacterial challenge after burn injury. Surgery. 1996;120:290–296. [DOI] [PubMed] [Google Scholar]
- 27.O'Sullivan ST, O'Connor TP. Immunosuppression following thermal injury: the pathogenesis of immunodysfunction. Br J Plast Surg. 1997;50:615–623. [DOI] [PubMed] [Google Scholar]
- 28.Ono S, Ueno C, Aosasa S, et al. Severe sepsis induces deficient interferon-gamma and interleukin-12 production, but interleukin-12 therapy improves survival in peritonitis. Am J Surg. 2001;182:491–497. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien AD, Rosenstreich DL, Scher I, et al. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980;124:20–24. [PubMed] [Google Scholar]
- 30.Hochholzer P, Lipford GB, Wagner H, et al. Role of interleukin-18 (IL-18) during lethal shock: decreased lipopolysaccharide sensitivity but normal superantigen reaction in IL-18-deficient mice. Infect Immun. 2000;68:3502–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]