Abstract
Objective:
Since albumin has the ability to detoxify, we assessed whether low-dose albumin could protect against trauma/hemorrhagic shock (T/HS)-induced endothelial cell, lung, gut, and red blood cell (RBC) injury in vivo and endothelial cell injury in vitro.
Summary Background Data:
T/HS cause ischemic insult to the gut, resulting in the release of biologically active factors into the mesenteric lymph, which then cause injury to multiple distant organs.
Methods:
In vitro experiments tested the ability of albumin to reduce the cytotoxicity of mesenteric lymph from male rats subjected to T/HS (laparotomy + MAP 30 mm Hg for 90 minutes) for human umbilical vein endothelial cell (HUVEC). In subsequent in vivo experiments, the ability of albumin given as part of the resuscitation regimen to protect against T/HS-induced injury was tested by comparing the magnitude of injury in T/HS rats receiving human albumin (shed blood + 0.12, 0.24, or 0.36 g/kg) or lactated Ringer's solution (shed blood + 2 × volume of shed blood as LR) with that observed in rats subjected to trauma/sham shock. Rats were killed after a 3-hour recovery period and had lung permeability evaluated by bronchoalveolar lavage and myeloperoxidase assays, intestinal microvillous injury by histology, and RBC deformability using ektacytometry.
Results:
Both bovine and human albumin prevented T/HS lymph-induced HUVEC cytotoxicity in vitro, even when added 30 minutes after the lymph (viability 15 ± 4% to 88 ± 3%, P < 0.01). In vivo RBC deformability was better preserved by blood plus albumin than blood plus lactated Ringer's solution (P < 0.01). Likewise, albumin administration reduced T/HS-induced lung permeability and neutrophil sequestration in a dose-dependent fashion, with 0.36 g/kg of albumin effecting total lung protection (P < 0.01). In contrast, albumin treatment did not prevent T/HS-induced gut injury.
Conclusions:
Low-dose albumin protects against gut lymph-induced lung, HUVEC, and RBC injury by neutralizing T/HS lymph toxicity.
Trauma and hemorrhagic shock cause ischemic insult to the gut, resulting in the release of biologically active factors into the mesenteric lymph, which then cause injury to multiple distant organs. In vitro and in vivo, low-dose albumin protected against trauma/hemorrhagic shock (T/HS)-induced endothelial cell, lung and red blood cell injury, but not gut injury, by neutralizing T/HS lymph toxicity.
Trauma and hemorrhagic shock can cause the adult respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS). Although ARDS and MODS are common in ICUs, therapy remains largely supportive.1 The failure to identify effective therapies for ARDS and MODS is largely due to our incomplete understanding of the biology of these syndromes. Recently, we and others have shown experimentally that gut-derived factors carried in the mesenteric lymph, but not the portal blood, are responsible for trauma-hemorrhagic shock (T/HS)-induced lung injury,2–4 and that these factors are cytotoxic for endothelial cells,5,6 activate neutrophils,5,7 and cause defects in red blood cell (RBC) deformability.8 Although the exact nature of the biologically active factors in shock lymph remain to be determined, there is information that both lipid and protein factors are involved.9,10 Albumin has been shown to protect endothelial cells from a range of toxic substances11,12 as well as bind fatty acids, scavenge free radicals, and improve microvascular permeability.13 This concept that albumin may have protective properties in shock states is supported by recent studies documenting that albumin infusions may improve the hemodynamic response and reduce inflammation after hemorrhagic shock.14 We therefore tested the ability of albumin to protect endothelial cells from injury by T/HS lymph in vitro, as well as to reduce T/HS-induced gut, lung, and RBC injury in vivo.
MATERIALS AND METHODS
Animals
Adult male specific pathogen-free Sprague-Dawley rats weighing 310 to 440 g were used in all experiments. Animals underwent a 7-day acclimation period with 12-hour light/dark cycles under barrier-sustained conditions, during which time they had free access to water and Harlan Teklad Laboratory Chow (Teklad 22/5 Rodent Diet [W] 8640, Harlan Teklad, Madison, WI). The rats were maintained in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the New Jersey Medical School Animal Care Committee.
Experimental Design
Our goals were to test the hypotheses that albumin 1) neutralizes the toxicity of T/HS mesenteric lymph to human umbilical vein endothelial cells (HUVECs) in vitro and 2) protects against T/HS-induced lung injury and RBC injury in vivo. Thus, in the first set of experiments, we collected mesenteric lymph from rats subjected to T/HS (details below). We then incubated HUVECs with the T/HS 5% mesenteric lymph in the presence or absence of albumin for 18 hours. Both bovine serum albumin (BSA) and human albumin (HSA) were used. The albumin concentrations used (BSA, 4 mg/mL; HAS, 5 mg/mL) are approximately 10% of normal plasma albumin concentrations. At the end of the incubation period, HUVEC viability was assessed. Control experiments were performed using T/HS lymph alone, albumin alone, ovalbumin (a biologically inactive form of albumin), and medium.
In a second set of experiments, we tested the ability of albumin given at the end of the T/HS period to protect against lung, gut, and RBC injury in vivo. To do this, we created three experimental groups. A control group underwent laparotomy and sham shock (T/SS). Animals undergoing T/HS were then resuscitated with their shed blood plus either twice the volume of shed blood as lactated Ringer's (LR, Baxter Healthcare Corp., Deerfield, IL), or plus albumin. To insure that any protection due to albumin was unrelated to greater resuscitation, animals in the albumin group received a maximum of 3 mL (150 mg) of 5% human albumin (Baxter Healthcare Corp., Glendale, CA) in addition to their shed blood. This amount (0.36 g/kg body weight) is the clinical equivalent of a 25-g dose of albumin (500 mL of 5% or 100 mL of 25%) given to a 70-kg human. Doses of 1 and 2 mL (50 and 100 mg) of 5% albumin were also tested to assess dose responsiveness. The T/HS model is based on prior work, which shows significantly greater immune suppression following laparotomy in addition to hemorrhagic shock when compared with either insult alone.15
The crystalloid group received shed blood plus twice the volume of their shed blood as LR. The mean volume of shed blood was 9 mL, and thus the average volume of LR administered was 18 mL per rat. This was 6 times the volume of 5% albumin administered in the 3 mL/rat group. Based on our prior radionuclide studies of plasma volume expansion after LR and albumin,16 the LR group therefore received 150%, 225%, and 450% of the plasma expansion that the three albumin groups did in addition to shed blood.
Three hours after resuscitation from T/HS, rats were killed. Lung injury was assessed as lung permeability to Evans Blue and as pulmonary leukosequestration measured by lung myeloperoxidase (MPO) levels. Intestinal injury was assessed morphologically by light microscopy. RBC injury was determined by comparing pre-shock with post-shock red cell deformability as measured by ektacytometry.
Trauma/Hemorrhagic Shock Model
As previously described,3 animals were anesthetized intraperitoneally with 50 mg/kg sodium pentobarbital. Body temperature was maintained at 37°C with a heat lamp or a heating pad as required. Rats were prepared in a sterile fashion and underwent vascular cannulation with minimal dissection. A minimally heparinized polyethylene catheter (PE-50) was introduced into the left femoral artery for blood pressure monitoring. A right jugular vein catheter (50-gauge silicone) was similarly inserted for blood withdrawal and resuscitation. The animals then underwent 5-cm midline laparotomy (trauma) with a 2-layer closure using 3–0 silk. The T/HS animals were subjected to hemorrhagic shock (90 minutes at a mean arterial pressure of 30 mm Hg) by withdrawing blood through the jugular vein catheter into a heparinized syringe until their mean arterial blood pressure reached 30 mm Hg. The mean arterial pressure was maintained at 30 mm Hg for 90 minutes by withdrawing or reinfusing the shed blood (kept at 37°C) as required. At the end of the 90-minute shock period, the T/HS animals were resuscitated by reinfusion of their shed blood plus 5% albumin or LR as described above. T/SS animals underwent vascular cannulation and laparotomy but had no blood withdrawn and received no resuscitation.
Mesenteric Lymph Collection
Rats were prepared for laparotomy as above. A left medial visceral rotation was performed to expose the mesenteric lymph duct. A silastic catheter was inserted into the main efferent mesenteric lymphatic duct, secured in place with cyanomethacrylate glue (Super Glue; Duro, CO), and brought out through a right flank stab wound. A gastrostomy tube was inserted into the greater curvature of the stomach and advanced into the duodenum. The gastrostomy was secured with glue and brought out through the left flank. The laparotomy incision was closed in two layers. The artery and vein were cannulated and the animals subjected to hemorrhagic shock as above. During the experiment, the animals received a constant infusion of 5% dextrose in saline through the gastrostomy at 5 mL/hr. At the end of the 90-minute shock period, the T/HS animals were resuscitated by reinfusion of their shed blood only. Previous work done in our laboratory has shown no difference between resuscitation of rats with their own shed blood alone, as compared with shed blood plus twice the volume as LR.6
Mesenteric lymph was continuously collected into heparin wetted iced tuberculin syringes in 1-hour increments. The collected lymph was centrifuged at 400g for 15 minutes to remove formed elements, diluted 1:1 with phosphate-buffered saline, aliquoted, and flash frozen at −80°C. Lymph samples collected during the first, second, and third post-shock hours were used in the endothelial cell viability experiments.
Endothelial Cells
HUVECs (Clonetics/BioWhittaker, Walkersville, MD) were harvested between the second and sixth passages, seeded at 2 × 104 cells per well in 96-well plates and grown to confluence over a 24-hour period. The HUVECs were grown at 37°C in 95% air, 5% carbon dioxide using complete medium (EGM Bullet Kit, Clonetics/BioWhittaker), containing Dulbecco modified eagle medium, 2% fetal bovine serum, 90 μg/mL heparin, 60 μg/mL endothelial cell growth factor, 2 mm l-glutamine, and 1% antibiotic-antimycotic solution. The antibiotic-antimycotic solution contains 10,000 units penicillin, 10 mg streptomycin, and 25 μg amphotericin B per 1 mL.
HUVEC Injury and Viability Assay
HUVECs were incubated for 18 hours in 1) medium, 2) medium plus 5% T/HS lymph with or without BSA or HSA albumin, and 3) medium plus BSA or HSA albumin only. Albumin was added either at the same time as the T/HS lymph, or 30 minutes after the addition of lymph to the HUVECs. Additional experiments were done with HUVECs incubated in medium plus 5% T/HS lymph plus 5 mg/mL ovalbumin, a biologically inactive form of albumin (Sigma, St. Louis, MO). After incubation, cell viability was measured by the mitochondrial tetrazolium test (MTT). This assay recognizes viable cells by detecting a color change in cells with active mitochondrial enzyme activity. MTT was measured spectrophotometrically using a kit (Sigma, MO).
Lung Permeability Assay
Three hours after the end of the shock period, the animals were injected with 10 mg of Evans blue dye via the internal jugular catheter. After 5 minutes, a 1-mL blood sample was withdrawn through the femoral artery catheter and centrifuged at 1500 rpm at 4°C for 20 minutes. This resultant plasma was serially diluted to create a standard curve. Twenty minutes after injection of the Evans blue dye, the rats were killed and the tracheobronchial tree and lungs were harvested as a unit. Bronchial alveolar lavage (BAL) was performed with 5 mL normal saline instilled and collected 3 times. The recovered BAL fluid (BALF) was then centrifuged at 1500 rpm at 4°C for 20 minutes to remove any cells. The supernatant fluid was then assayed spectrophotometrically at 620 nm for dye concentration. The dye concentration in the BALF was then plotted on the standard curve and the percentage relative to the plasma concentration was determined.
BALF and Plasma Protein Assay
The total protein content (g/dL) of both the BALF and plasma was determined for all groups using a hand refractometer (Milton Roy Co, Rochester, NY), as another measure of lung protein permeability.
Myeloperoxidase Assay
Frozen lung tissue was homogenized and processed for MPO level measurement as previously described.3 One unit of MPO activity represents the amount of enzyme that will reduce 1 μmol/min of peroxide.
Red Blood Cell Ektacytometry
Blood samples (0.1 mL) were withdrawn prior to induction of shock, at the end of the 90-minute shock period prior to resuscitation, and at 3 hours post-shock before death. RBC deformability was quantified at a shear stress of 0.30 Pa by laser diffraction analysis using an ektacytometer (LORCA; RR Mechatronics, Hoorn, The Netherlands) as previously described.8 RBC deformability under shear stress is expressed as the elongation index, which is calculated by dividing the difference between the long and short axes by the sum of the 2 axes: A − B/A + B.
Intestinal Histology
A segment of the terminal ileum was harvested at death and processed for histologic analysis by fixation in 10% buffered formalin. The intestinal samples were then embedded in paraffin, processed into 4- to 5-μm slices, and stained with hematoxylin and eosin. As described previously,17 ileal villi were graded based on degree of injury, as evidenced by submucosal edema or villus tip necrosis. Five random fields were examined for each animal at 100× magnification. Villous damage was assessed as the number of injured villi divided by the total number of villi counted. A minimum of 150 villi were counted for each animal. The histologic evaluations were done in a blinded manner.
Statistics
Analysis of variance (ANOVA) with the post hoc Tukey-Kramer Multiple Comparison test was used for comparisons between groups. Results are expressed as mean ± sd. Linear regression was used to analyze the in vivo dose-response data. P values less than 0.05 were considered statistically significant.
RESULTS
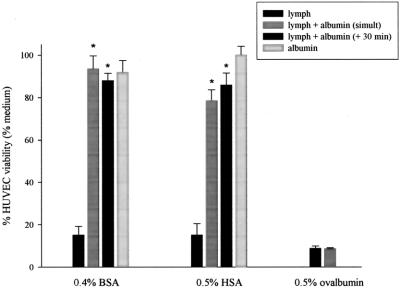
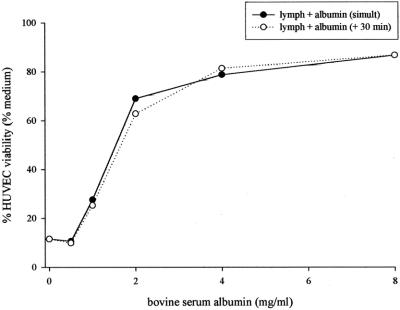
The addition of either bovine (5 mg/mL) or human albumin (4 mg/mL) to HUVEC cultures significantly decreased the toxicity of the T/HS lymph (Fig. 1). Both types of albumin were protective either when added simultaneously or when added 30 minutes after the HUVECs were exposed to T/HS lymph (P = 0.0001). No differences were noted between the effects of albumin added simultaneously to or after the T/HS lymph. To better define the protective effects of albumin on T/HS lymph-induced HUVEC cytotoxicity, BSA was also tested over a range of doses (Fig. 2). BSA's protective effect on HUVECs was dose-dependent, with protection observed at doses as low as 2 mg/mL. To discern whether this protection was unique to albumin, ovalbumin was used as an additional control. The inability of ovalbumin to protect the HUVECs from T/HS lymph's toxicity argues that the observed protective effect of albumin is due to its biologic properties rather than a nonspecific protein effect.
FIGURE 1. The incubation of T/HS lymph with HUVECs results in markedly decreased viability, whereas the addition of either BSA or HSA significantly prevents this cytotoxicity, added either simultaneously or after 30 minutes (n = 4). Ovalbumin, however, clearly does not show this same protection. Neither type of albumin alone is cytotoxic to HUVECs. Viability is expressed as % medium control. *P = 0.0001.
FIGURE 2. In vitro, HUVECs treated with T/HS lymph demonstrate a dose-response effect to BSA. Linear regression of albumin added simultaneously yielded r2 value of 0.5918 and P < 0.01. Albumin added after 30 minutes showed r2 = 0.5906 and P < 0.01.
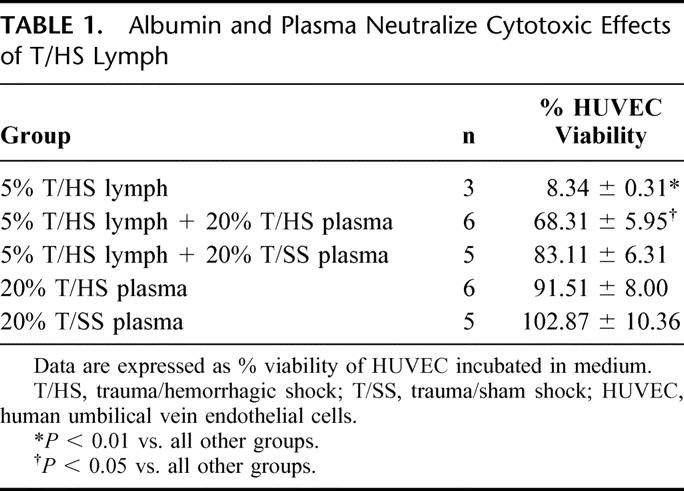
Our previous studies indicated that portal vein plasma from rats subjected to T/HS does not injure endothelial cells when tested at the same concentrations as T/HS lymph.6 Moreover, plasma contains albumin. We therefore tested the effects of plasma on T/HS lymph-induced HUVEC cytotoxicity. We noted that neither 20% T/HS nor 20% T/SS plasma was directly toxic to HUVECs. Also, both T/HS and T/SS plasma at 20% concentration reduced the toxicity of 5% T/HS lymph to HUVECs (Table 1). The protective effect of T/HS plasma, however, was less than that of T/SS plasma, suggesting that plasma from T/HS rats had less capacity to neutralize the toxic properties of T/HS lymph than did plasma from sham-shock rats. These differences in protection were clearly unrelated to T/HS-induced loss of plasma proteins, since plasma protein levels were the same in T/SS (6.5 ± 1.2 g/dL) and T/HS rats resuscitated with LR (6.3 ± 0.5 g/dL).
TABLE 1. Albumin and Plasma Neutralize Cytotoxic Effects of T/HS Lymph
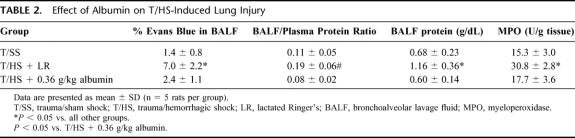
Having demonstrated that albumin reverses the toxicity of T/HS lymph to endothelial cells, we next tested whether albumin administration would protect against T/HS-induced lung, gut, and/or RBC injury in vivo. As previously reported,3,6 rats subjected to T/HS and resuscitated with shed blood plus LR showed increased lung permeability, as reflected by increased Evans blue dye in the BALF as well as an increased BALF-to-plasma protein ratio (Table 2). Similarly, pulmonary leukosequestration increased in T/HS rats resuscitated with LR (Table 2).
TABLE 2. Effect of Albumin on T/HS-Induced Lung Injury
Albumin administered at 0.36 g/kg totally prevented the increases in lung permeability and pulmonary neutrophil sequestration observed in T/HS rats resuscitated with LR (Table 2). As the dose of albumin was decreased progressively to 0.12 g/kg or 0.24 g/kg, the lung permeability and lung MPO levels increased (Fig. 3). Thus, as was observed in the in vitro HUVEC studies, the protective effects of albumin were dose-dependent. Furthermore, although the plasma protein levels of the T/HS rats receiving 0.36 g /kg of albumin (7.3 ± 0.6 g/dL) were higher than that observed in the T/HS rats receiving 0.24 g/kg (7.2 ± 0.6 g/dL) or 0.12 g/kg (7.1 ± 0.4 g/dL) of albumin, these differences were not significantly different.
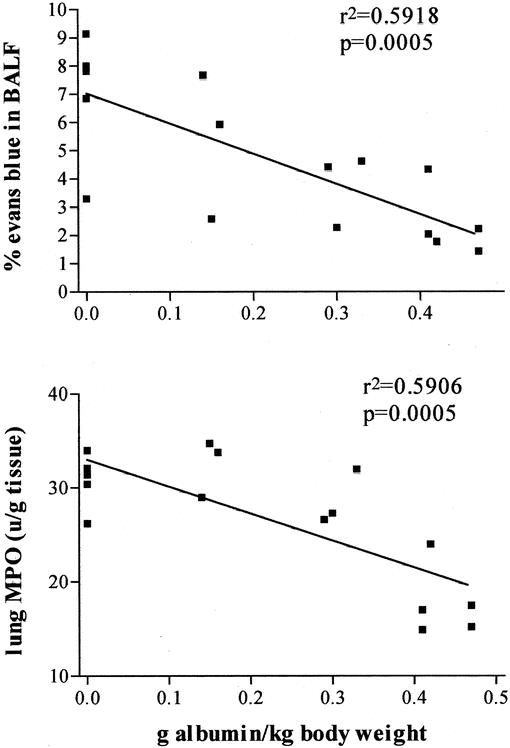
FIGURE 3. In vivo, lung permeability following T/HS and resuscitation with albumin is dose-dependent, as shown in these representative linear regressions of % Evans blue in BALF and lung MPO. Absolute BALF protein and BALF/plasma protein ratio data yielded similar curves (data not shown).
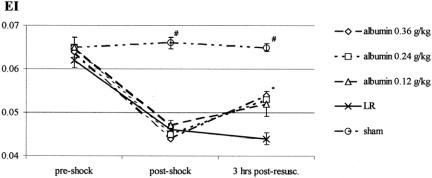
RBC deformability did not decrease after T/SS (Fig. 4), whereas RBC deformability was significantly decreased in all T/HS groups at the end of shock, prior to reperfusion. Three hours after resuscitation, all T/HS albumin-treated rats showed improvement in RBC deformability, although the values did not return to preshock levels. No improvements in RBC deformability were observed in T/HS rats resuscitated with LR.
FIGURE 4. In vivo, all groups began with similar deformability, and induction of T/HS resulted in significantly lowered elongation index (EI). The presence of albumin improved deformability, whereas the LR group showed no improvement (P < 0.01). There was no difference observed between the different albumin doses.
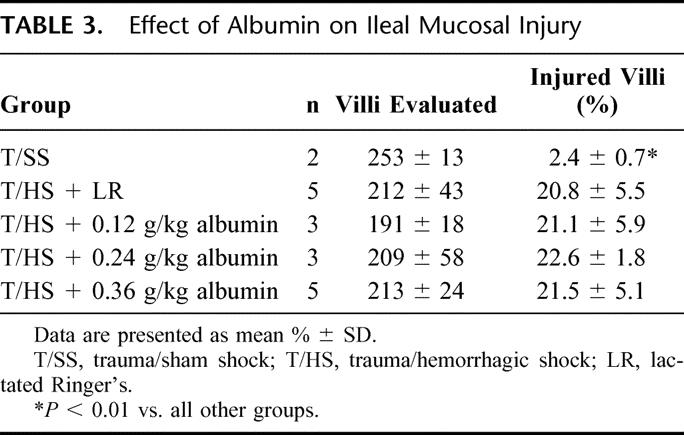
One mechanism by which albumin could protect against lung and RBC injury could be by preventing or reducing gut injury. We therefore assessed the effects of albumin on T/HS-induced gut injury. Histologic evaluation of the gut indicated that albumin did not prevent T/HS-induced gut injury at any dose (Table 3). These results suggest that albumin's protective effects on the lung and on the red cells are related to its ability to neutralize the biologic activity of T/HS lymph rather than by preventing gut injury and the subsequent production of toxic T/HS lymph.
TABLE 3. Effect of Albumin on Ileal Mucosal Injury
DISCUSSION
Over the last 20 years, understanding the role of the gut in the pathogenesis of ARDS and MODS has evolved considerably. For example, the gut hypothesis of MODS, as originally proposed, stated that gut barrier failure led to systemic inflammation and MODS through the translocation of bacteria and their products from the gut into the systemic circulation.1 Although the concept of bacterial translocation as the mechanism of gut-origin sepsis was conceptually attractive and supported by extensive animal and some human studies, the failure to find bacteria or endotoxin in the portal veins of high-risk trauma patients cast doubt on the clinical relevance of bacterial translocation.18 Indeed, as more data accumulated, it became clear that the idea of translocating bacteria and endotoxin being the major and only cause of gut-induced ARDS and MODS became less and less tenable.2 On the other hand, there were compelling data from clinical trials of early enteral feeding19,20 as well as from clinical studies of high-risk patients,21–24 including clinical trials testing splanchnic-directed therapies,25,26 strongly indicating that gut dysfunction is playing a major role in the pathogenesis of systemic sepsis and organ failure in high-risk patients. Motivated by a desire to clarify these conflicting data, work carried out over the last several years has expanded the concept of gut-origin sepsis and MODS and provided information that has begun to resolve these previously conflicting results. For example, studies in burn and trauma-hemorrhagic models documenting that gut-induced systemic inflammation and distant organ dysfunction are due to nonbacterial gut-derived factors carried in the mesenteric lymph rather than the portal blood3,6,9,10,27,28 has helped to explain why neither endotoxin nor bacteria was found in the portal blood of trauma and other patient populations. Furthermore, the observation that biologically active mesenteric lymph in these rodent models is sterile and does not contain endotoxin is similar to results of a recent study examining thoracic duct lymph in ICU patients.29
Having documented that hemorrhagic shock-induced pulmonary injury and systemic inflammation are due to gut-derived nonbacterial factors carried in the mesenteric lymph, it is now important to determine the identity of these factors, their mechanisms of action, as well as potential treatment options. There is evidence that the biologic activity of T/HS mesenteric lymph is due to both lipid and protein factors.9,10 Albumin has been shown to protect endothelial cells from a range of toxic substances as well as bind fatty acids, scavenge free radicals, and improve microvascular permeability.13 We therefore tested the ability of low-dose albumin to protect endothelial cells from the injurious effects of shock lymph in vitro as well as to reduce T/HS-induced gut, lung, and RBC injury in vivo.
The current studies document that albumin neutralizes the cytotoxic effects of T/HS lymph on HUVECs in vitro and that albumin administration during resuscitation protects against T/HS-induced lung and RBC injury in vivo. Both the in vitro and in vivo protective effects of albumin were dose-dependent. Nonetheless, viewed as a resuscitation regimen, even the highest doses of albumin administered in vivo effected much less volume expansion than the crystalloids given to LR controls. Moreover, the increases in plasma protein levels found after albumin administration were insignificant. Thus, it is clear that albumin did not exert its in vivo protective effects either through its hemodynamic or its colloid osmotic properties. In further support of this assessment, albumin administration failed entirely to prevent histologic gut injury, a consistent marker for gut ischemia-reperfusion. These data strongly support the conclusion that albumin's protective effects were not due to better maintenance of gut perfusion, prevention of ischemia-reperfusion injury, or the subsequent avoidance of the production of biologically active mesenteric lymph.
Rather, we believe that albumin exerts both its in vitro and in vivo protective effects through immunopharmacologic and not hemodynamic mechanisms. An important feature of albumin, which distinguishes it from other colloids and crystalloids, is its unique ability to bind biologically active substances. These include fatty acids, hormones, enzymes, dyes, trace metals, and drugs.13 Furthermore, substances that are toxic in the unbound or free state are generally not toxic when bound to albumin. For example, while unbound plasma free fatty acids are toxic, free fatty acids bound to albumin are not.30 These unique binding properties allow albumin to regulate the extracellular concentrations of endogenous as well as exogenously administered substances.13 Consequently, we think it likely that albumin protects against T/HS-induced lung and RBC injury as well as T/HS lymph-induced endothelial cell cytotoxicity by binding to and neutralizing a variety of active factors in shock lymph. Since albumin has the ability to scavenge free radicals and limit lipid peroxidation,13,31 another mechanism by which albumin may have exerted its protective effects is through its oxygen radical scavenging properties. In addition to its direct free radical scavenging properties, albumin has been documented to reduce neutrophil-endothelial interactions both in vivo14 and in vitro.32,33 Thus, since trauma-hemorrhagic shock can be viewed as a global ischemia-reperfusion injury in which neutrophil-endothelial interactions contribute to lung and other organ injuries,1 albumin's antioxidant and anti-inflammatory properties may have contributed to its ability to prevent T/HS-induced increases in lung permeability and pulmonary leukosequestration as well as RBC damage. Additionally, albumin has been shown to inhibit both cytokine-induced34 as well as stress-induced12 endothelial apoptosis in vitro, and this protective effect is likely to be mediated by specific endothelial cell surface receptors that bind albumin.35 These in vitro observations are consistent with albumin being a survival factor for endothelial cells12,13 as are studies documenting that removal of albumin from vascular perfusates increases endothelial permeability in both capillary36 and whole-organ preparations.37 Thus, an additional mechanism by which albumin could have limited shock lymph-induced endothelial cell cytotoxicity and/or T/HS-induced increases in lung permeability is by binding to specific albumin receptors on endothelial cells.35,38
Determining which of these potential mechanisms may contribute to the in vitro and in vivo protective effects of albumin observed in this study will require further investigation. Nonetheless, the relative inability of plasma from T/HS rats to neutralize the cytotoxic effects of T/HS lymph compared with T/SS plasma raises the strong possibility that albumin in T/HS plasma already has gut-derived factors bound to it; thus, it has fewer binding sites available to bind the biologically active factors in T/HS lymph. Albumin in shock plasma may also be modified and less able to bind either to the putative toxic factors in lymph or to the albumin receptors on the endothelial cells. Supplementation with exogenous nonshock plasma or with albumin restores the ability of the blood to buffer toxic lymph.
Currently, the clinical utility of albumin administration to patients is an area of controversy. On the one hand, one must consider the results of the Cochrane meta-analysis on albumin administration, which suggested that the administration of albumin to patients may be harmful.39 However, this conclusion has been contested for a number of reasons, including the heterogeneous nature of the patient populations studied. Indeed, since the Cochrane study was published, two additional meta-analyses have been published,40,41 both of which dispute the conclusions of the Cochrane study. The first meta-analysis found no increased risk to the administration of albumin to patients,40 while the second meta-analysis found an association between hypoalbuminemia and a poor clinical outcome and concluded that there is no reason to withhold albumin therapy.41 Although it would be premature to directly apply our results to the clinical arena, our results do suggest that the timing of albumin administration may be an important variable as well as the patient population being treated. Specifically, to prevent the adverse effects of toxic T/HS lymph on organ and cellular function, albumin must be administered in the very early post-shock period, preferably as part of the initial resuscitation regimen. This is critical since the biologically active factors present in mesenteric lymph appear to peak during the first few hours post-shock and subsequently disappear over the next several hours.6
In summary, we have observed that relatively low doses of albumin protect against the toxic effects of shock lymph in vitro and limit T/HS-induced lung and RBC dysfunction in vivo. The data suggest that this protection is not due to colloidal or hemodynamic properties. Rather, it is probably due to albumin's scavenging, antioxidant, or endothelial protective properties. Although the potential merits of albumin's oncotic and hemodynamic effects during resuscitation have been thoroughly studied and debated, its immunomodulatory effects in shock states remain relatively unexplored. Our findings support the concept that early resuscitation from T/HS with low-dose albumin, even after the shock period, may be beneficial due to its protection from the deleterious effects of gut-derived products. Lastly, the ability of albumin to neutralize T/HS mesenteric lymph toxicity in vitro and in vivo adds further support to the gut hypothesis of MODS.
CONCLUSION
Even though low-dose albumin resuscitation does not protect the gut from ischemia, it can protect the organism from the inflammatory effects of shock. It appears to do this by scavenging factors in toxic mesenteric lymph generated after T/HS, thereby decreasing secondary lung and RBC injury.
Footnotes
Supported in part by NIH grant no. GM 59841 (E.A.D.).
Reprints: Edwin A. Deitch, MD, Department of Surgery, New Jersey Medical School, PO Box 1709, Newark, NJ 07101-1709. E-mail: edeitch@umdnj.edu.
REFERENCES
- 1.Deitch EA. Multiple organ failure: pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131:241–244. [DOI] [PubMed] [Google Scholar]
- 3.Magnotti LJ, Upperman JS, Xu DZ, et al. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zallen G, Moore EE, Johnson JL, et al. Post-hemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res. 1999;83:83–89. [DOI] [PubMed] [Google Scholar]
- 5.Upperman JS, Deitch EA, Guo W, et al. Post-hemorrhagic shock mesenteric lymph is cytotoxic to endothelial cells and activates neutrophils. Shock. 1998;10:407–414. [DOI] [PubMed] [Google Scholar]
- 6.Deitch EA, Adams CA, Lu Q, et al. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129:39–47. [DOI] [PubMed] [Google Scholar]
- 7.Adams JM, Hauser CJ, Adams CA, et al. Entry of gut lymph into the circulation primes rat neutrophil respiratory burst in hemorrhagic shock. Crit Care Med. 2001;29:2194–2198. [DOI] [PubMed] [Google Scholar]
- 8.Zaets S, Berezina T, Morgan C, et al. Effect of trauma-hemorrhagic shock on red blood cell deformability and shape. Shock. 2003;19:268–273. [DOI] [PubMed] [Google Scholar]
- 9.Dayal SD, Hauser CJ, Feketeova E, et al. Shock mesenteric lymph-induced rat polymorphonuclear neutrophil activation and endothelial cell injury is mediated by aqueous factors. J Trauma. 2002;52:1048–1055. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez RJ, Moore EE, Biffl WL, et al. The lipid fraction of post-hemorrhagic shock mesenteric lymph (PHSML) inhibits neutrophil apoptosis and enhance cytotoxic potential. Shock. 2000;14:404–408. [DOI] [PubMed] [Google Scholar]
- 11.Ueda T, Takeyama Y, Takase K, et al. Hematin is one of the cytotoxic factors in pancreatitis-associated ascitic fluid that causes hepatocellular injury. Surgery. 2002;131:66–74. [DOI] [PubMed] [Google Scholar]
- 12.Zoellner H, Hofler M, Beckmann R, et al. Albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci. 1996;109:2571–2580. [DOI] [PubMed] [Google Scholar]
- 13.Emerson TE. Unique features of albumin: a brief review. Crit Care Med. 1989;17:690–694. [DOI] [PubMed] [Google Scholar]
- 14.Horstick G, Lauterbach M, Kempf T, et al. Early albumin infusion improves global and local hemodynamics and reduces inflammatory response in hemorrhagic shock. Crit Care Med. 2002;30:851–855. [DOI] [PubMed] [Google Scholar]
- 15.Zellweger R, Ayala A, DeMaso CM, et al. Trauma-hemorrhage causes prolonged depression in cellular immunity. Shock. 1995;4:149–153. [DOI] [PubMed] [Google Scholar]
- 16.Hauser CJ, Shoemaker WC, Turpin I, et al. Oxygen transport responses to colloids and crystalloids in critically ill surgical patients. Surg Gynecol Obstet. 1980;6:811–816. [PubMed] [Google Scholar]
- 17.Adams CA Jr, Magnotti LJ, Xu DZ, et al. Acute lung injury after hemorrhagic shock is dependent on gut injury and sex. Am Surg. 2000;66:905–913. [PubMed] [Google Scholar]
- 18.Moore FA, Moore EE, Poggetti R, et al. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991;31:629–638. [DOI] [PubMed] [Google Scholar]
- 19.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore FA, Feliciano DV, Andrassey RJ, et al. Early enteral feeding compared with parenteral, reduces postoperative septic complications: the results of a meta-analysis. Ann Surg. 1992;216:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedman PC, MacFie J, Sagar P, et al. The prevalence of gut translocation in humans. Gastroenterology. 1994;107:643–649. [DOI] [PubMed] [Google Scholar]
- 22.Doig CJ, Sutherland LR, Sandham JD, et al. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–451. [DOI] [PubMed] [Google Scholar]
- 23.MacFie J, O'Boyle C, Mitchell CJ, et al. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora and septic morbidity. Gut. 1999;45:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivatory RR, Simon RJ, Islam S, et al. A prospective randomized study of end points of resuscitation after major trauma: global oxygen transport indices versus organ-specific gastric mucosal pH. J Am Coll Surg. 1996;183:145–154. [PubMed] [Google Scholar]
- 25.Barquist E, Kirton O, Windsor J, et al. The impact of antioxidant and splanchnic-directed therapy on persistent uncorrected gastric mucosal pH in the critically injured trauma patient. J Trauma. 1998;44:355–360. [DOI] [PubMed] [Google Scholar]
- 26.Porter JM, Ivatory RR, Azimuddin K, et al. Antioxidant therapy in the prevention of organ dysfunction syndrome and infectious complications after trauma: early results of a prospective randomized study. Am Surg. 1999;65:478–483. [PubMed] [Google Scholar]
- 27.Magnotti LJ, Xu DZ, Lu Q, et al. Gut-derived mesenteric lymph: a link between burn and lung injury. Arch Surg. 1999;143:1333–1341. [PubMed] [Google Scholar]
- 28.Adams CA, Xu DZ, Lu Q, et al. Factors larger than 100 kd in post-hemorrhagic shock mesenteric lymph are toxic for endothelial cells. Surgery. 2001;129:351–362. [DOI] [PubMed] [Google Scholar]
- 29.Lemaire JC, van Lanschot JB, Stoutenbeck CP, et al. Thoracic duct in patients with multiple organ failure: no major route of bacterial translocation. Ann Surg. 1999;229:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broe PJ, Toung TJ, Margolis S, et al. Pulmonary injury caused by free fatty acid: evaluation of steroid and albumin therapy. Surgery. 1981;89:582–587. [PubMed] [Google Scholar]
- 31.Caraceni P, Gasbarrini A, van Thiel DH, et al. Oxygen free radical formation by rat hepatocytes during post-anoxic reoxygenation: scavenging effect of albumin. Am J Physiol. 1994;266:G451–G458. [DOI] [PubMed] [Google Scholar]
- 32.Nathan C, Xie QW, Halbwachs-Mecarelli L, et al. Albumin inhibits neutrophil spreading and hydrogen peroxide release by blocking the shedding of CD43. J Cell Biol. 1993;122:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bignold LP, Rogers SD, Harkin DG. Effects of plasma proteins on the adhesion, spreading, polarization in suspension, random motility and chemotaxis of neutrophil leukocytes on polycarbonate filtration membrane. Eur J Cell Biol. 1990;53:27–34. [PubMed] [Google Scholar]
- 34.Emanuel C, Foo E, Medbury HJ, et al. Synergistic induction of apoptosis in human endothelial cells by tumor necrosis factor and transforming growth factor. Cytokine. 2001;18:237–241. [DOI] [PubMed] [Google Scholar]
- 35.Schnitzer JE, Carley WW, Palade GE. Albumin interacts specifically with a 60-kDa microvascular endothelial glycoprotein. Proc Natl Acad Sci USA. 1998;85:6773–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huxley VH, Curry FE. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol. 1985;248:H264–H273. [DOI] [PubMed] [Google Scholar]
- 37.Schneeberger EE, Hamelin M. Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol. 1994;247:H206–H217. [DOI] [PubMed] [Google Scholar]
- 38.He P, Curry FE. Albumin modulation of capillary permeability: role of endothelial cell [Ca++I]. Am J Physiol. 1993;265:H74–H82. [DOI] [PubMed] [Google Scholar]
- 39.Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. Br Med J. 1998;317:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkes MM, Navickis. Patient survival after albumin administration: a meta-analysis of randomized controlled trials. Ann Intern Med. 2001;135:149–165. [DOI] [PubMed] [Google Scholar]
- 41.Vincent JL, Dubois MJ, Navickis RJ, et al. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Crit Care Med. 2003;237:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]