Abstract
Objective:
Developing strategies for transfusion-free live donor liver transplantation in Jehovah's Witness patients.
Summary Background Data:
Liver transplantation is the standard of care for patients with end-stage liver disease. A disproportionate increase in transplant candidates and an allocation policy restructuring, favoring patients with advanced disease, have led to longer waiting time and increased medical acuity for transplant recipients. Consequently, Jehovah's Witness patients, who refuse blood product transfusion, are usually excluded from liver transplantation. We combined blood augmentation and conservation practices with live donor liver transplantation (LDLT) to accomplish successful LDLT in Jehovah's Witness patients without blood products. Our algorithm provides broad possibilities for blood conservation for all surgical patients.
Methods:
From September 1998 until June 2001, 38 LDLTs were performed at Keck USC School of Medicine: 8 in Jehovah's Witness patients (transfusion-free group) and 30 in non-Jehovah's Witness patients (transfusion-eligible group). All transfusion-free patients underwent preoperative blood augmentation with erythropoietin, intraoperative cell salvage, and acute normovolemic hemodilution. These techniques were used in only 7%, 80%, and 10%, respectively, in transfusion-eligible patients. Perioperative clinical data and outcomes were retrospectively reviewed. Data from both groups were statistically analyzed.
Results:
Preoperative liver disease severity was similar in both groups; however, transfusion-free patients had significantly higher hematocrit levels following erythropoietin augmentation. Operative time, blood loss, and postoperative hematocrits were similar in both groups. No blood products were used in transfusion-free patients while 80% of transfusion-eligible patients received a median of 4.5+/− 3.5 units of packed red cell. ICU and total hospital stay were similar in both groups. The survival rate was 100% in transfusion-free patients and 90% in transfusion-eligible patients.
Conclusions:
Timely LDLT can be done successfully without blood product transfusion in selected patients. Preoperative preparation, intraoperative cell salvage, and acute normovolemic hemodilution are essential. These techniques may be widely applied to all patients for several surgical procedures. Chronic blood product shortages, as well as the known and unknown risk of blood products, should serve as the driving force for development of transfusion-free technology.
This study analyzes the blood conservation techniques used for live donor liver transplantation in Jehovah's Witness patients. There was no statistical difference in complication and definitive outcome between these patients and the general population. These results suggest that live donor liver transplantation may be safely performed without the usage of blood products. Extrapolation of these techniques may have a profound impact in general surgical practice.
Since first performed by Thomas Starzl in 1963, liver transplantation has become mainstream surgical therapy for patients with end-stage liver disease, with an operative mortality of less than 5% and 1- and 5-year survivals of 85% and 65%.1–5 Despite the successes of liver transplantation, however, end-stage liver disease from hepatitis C has led to a disproportionate increase in patients awaiting transplantation in relation to available organs.6,7 The resultant medical consequences for transplant candidates include longer waiting times, frequent hospitalizations with increased costs of care, transplantation during a state of higher medical acuity, and increased mortality risk. Responses by the transplant community to this need have included expanding organ utilization to include older donors, splitting of cadaveric livers for two recipients, living donor liver transplantation (LDLT) for children and adults, and remodeling of the organ allocation system for maximal utilization.
Because the current allocation system gives preference to sicker patients, cadaveric transplantation is usually performed within the physiologic context of various system failures. Renal insufficiency, hypersplenism, portal hypertension, lowered production of pro-coagulant factors, and heightened fibrinolysis are proportionate to the severity of liver disease, contribute to bleeding during transplantation, and make transfusion of blood products likely. These factors have made liver transplantation prohibitive in Jehovah's Witness (JW) patients who refuse the transfusion of blood and blood products for religious reasons. We report successful LDLT in 8 adult JW patients. This group was compared with non-JW patients who underwent LDLT during the same period of time. Although live donation from JW patients may raise some ethical issues, several lessons may be learned from the management of these patients and applied to non-JW patients.
We have successfully combined the technique of LDLT with transfusion-free strategies to accomplish LDLT in JW recipients. Based on our experience with blood augmentation and conservation practice, we elaborate an algorithm for elective LDLT in JW patients and report a comparative hematologic record in transfusion-eligible LDLT recipients. Our approach provides broad possibilities for LDLT timing and blood resource conservation with applications for all surgical patients.
METHODS
Demographics
From April 1999 to January 2002, 38 adult patients underwent LDLT at the University of Southern California. Eight JW recipients formed the transfusion-free (TF) group, while the remaining 30 patients were classified as transfusion-eligible (TE). Both groups underwent the same core pretransplant evaluation process. There were 6 males and 2 females in the TF group with a mean age of 42 years (range, 17–62 years) and 24 males and 6 females in the TE group with a mean age of 54 years (range, 38–67 years). TF group etiologies included hepatitis C (n = 3), primary sclerosing cholangitis (n = 3), and cryptogenic (n = 2). For the TE group, hepatitis C (n = 14), primary sclerosing cholangitis (n = 3), cryptogenic (n = 4), hepatitis B (n = 4), alcohol (n = 1), primary biliary cirrhosis (n = 1), á-1-antitrypsin deficiency (n = 1), polycystic liver (n = 1), and fulminant hepatic failure (n = 1) were causative.
Our management strategy for TF-LDLT recipients is divided into a pretransplant period, which is heavily administrative, and medical, an operative, and a posttransplant phase.
Pretransplant Management Phase
Administrative Preparation
The University of Southern California University Hospital has an active program in Transfusion Free Medicine and Surgery. At the matriculation of TF patients into the liver transplant program, the administrative liaison for the TF program, the physicians, and the patient discuss the strategies of 1) blood augmentation and conservation, 2) utilization of acute normovolemic hemodilution, 3) intraoperative cell salvage, and 4) individual component therapies. The first three are required therapies for TF-LDLT candidates, although patients are allowed choice on the individual component therapies that they will accept. Important component therapies include albumin, cryoprecipitate, factor VIIa, aprotinin, and aminocaproic acid.
When a candidate enrolls in the TF-LDLT program, he/she documents refusal of allogeneic blood and its primary components, eg, red cells, platelets, and plasma on a “Refusal to Permit Blood Transfusion” form, which serves as a legal release of liability. In addition, the patient is given the “Personal Decision and Release” form that specifies his/her consent for the use of various secondary blood fractions (mentioned above) and autologous procedures.
Acceptance of a candidate into the TF-LDLT program is dependent on factors that reflect bleeding risk, coagulation performance, and tolerance of anemia. These include prior operations, severity of liver disease, body habitus, age, degree of portal hypertension, cardiovascular risk factors, portal vein patency, hypersplenism, and overall physiologic status. The collective interpretation of these multiple factors is subjective in that no formal scoring/weighting of these parameters has yet been established.
During the administrative phase of the program, several contingencies are explained to the potential recipients that require their understanding and agreement. These include: 1) proceeding with TF-LDLT is dependent upon successful blood augmentation or a satisfactory hemoglobin prior to operation; 2) once the operation has begun in the recipient, excessive bleeding or prohibitive anatomy may require cancellation prior to beginning the donor operation; and 3) the blood product restrictions and inclusions agreed upon during the administrative phase are legally binding during the operative phase, even to the point of death.
Medical Preparation
Medical preparation is aimed at increasing red cell mass to a normal or hypernormal level and decreasing portal hypertension. Erythropoietin in conjunction with iron sulfate and folic acid was used to support blood augmentation.8,9 Transjugular intrahepatic portal-systemic shunt was performed either as therapy for upper gastrointestinal bleeding or as prophylaxis to decrease portal hypertension in patients with pronounced varices.10
Operative Management Phase
Hematologic Manipulation and Monitoring
Acute normovolemic hemodilution (ANH) is a single term for a broad therapeutic initiative that involves simultaneously removing the patient's blood and replacing it with a non-blood product.11,12 ANH is performed on all TF-LDLT patients according to the following protocol: Blood is removed from the central line and drained via gravity to a citrate-phosphate-dextrose (CPD) bag before the surgical incision, and the patient's intravascular volume is maintained by infusion of colloid and/or crystalloid solutions. The patient's red cell mass is diluted to the allowable minimum based on medical considerations and maintained at that level by reinfusion of the collected blood and non-red cell containing solutions until surgical loss is complete. This ensures that all surgically lost blood contains the minimum red cell level deemed acceptable to the patient.
The ANH blood is kept at room temperature for up to 6 hours to preserve platelet function. After that, it is placed in a blood cooler at 4°C to 6°C. ANH blood can be reinfused at the same rate as any other transfused blood. If needed urgently, it can be reinfused via rapid transfuser or it can be given like a normal blood transfusion over a 2-hour period. The volume of blood withdrawn for ANH usually ranges between 400 and 1500 mL, depending upon anticipated surgical blood loss, the original hemoglobin, and patient tolerance. In adults, the hemoglobin will decrease 1 g/dL for each unit of blood removed. ANH blood remains in the operating room and as such is not required to be registered according to blood banking regulations. The CPD tubing remains connected to the patient at all times conforming to the patients’ religious beliefs. This CPD circuit is checked and approved by the hospital TF coordinator. In our patient study, the lowest hematocrit following ANH was 21%.
Hemodynamic monitoring of heart rate, blood pressure, arterial blood gases, and pulmonary artery and central venous pressure is routine (Fig. 1) during the ANH collection as a measure of tolerance to the procedure. Intraoperative transesophageal echo continuously monitors cardiac contractility and ventricular filling.13 The displaced ANH blood volume is replaced with 5% albumin and plasmalyte. All ANH blood collected during this part of the procedure is reinfused during operation as needed or routinely following liver implantation.

FIGURE 1. Illustration of acute normovolemic hemodilution
Intraoperative cell salvage (ICS) is also used throughout the procedure to collect the operative blood losses that are reinfused after washing.14,15
Coagulation profiles are tracked using the thromboelastogram16 and approved component therapies such as factor VIIA,17 antifibrinolytics, or protamine are added as indicated.18
Surgical Timing and Procedure
As a routine, in both groups the recipient is taken to the operating room prior to the donor to rule out any contraindication to transplantation such as the presence of tumor. In addition, the operating surgeon performs an analysis on the feasibility of successful hepatectomy and transplantation without transfusion in the TF group. This was done in the context of the liver mobilization, facility of exposure, and blood loss prior to the final commitment to hilar vascular ligation. This process on an average takes less than half an hour. Once this recipient threshold has been crossed, the donor operation is allowed to begin. The donor operation was a standard right lobectomy. The anatomy and volume determination of these donors were evaluated preoperatively by an MRI. In addition, all donors underwent an intraoperative cholangiogram to determine the biliary tree anatomy prior to parenchymal transection.
Recipient hepatectomy and right liver lobe implantation are performed using the piggy-back technique as previously described.19 The recipient dissection is performed with electrocautery while topical hemostatics and the argon beam laser are used for local hemostasis.
Postoperative Phase
Postoperative management in TF-LDLT patients consisted of blood conservation by minimization of blood testing and the use of pediatric tube phlebotomy. Blood augmentation with erythropoietin was used in selected TF-LDLT patients depending on the postoperative hemoglobin. Induction and maintenance immunosuppression were similar in both groups with calcineurin inhibitors and steroids. An attempt was made to avoid bone marrow suppressant medications in the TF group.
Data from both groups were analyzed by using Mann-Whitney-Rank-Sum test for mean difference and Fisher's 2-tailed exact tests for percent differences.
RESULTS
Preoperative Preparation
Hepatic function and hematic profiles for both groups are presented in Table 1. There were no significant differences between the groups with respect to the Childs-Pugh-Turcotte scores at transplantation; however, TF-LDLT patients had significantly higher hematocrit levels as a result of their pretransplant preparation with erythropoietin. Despite the frequent anemia in liver disease patients, the targeted hematocrit in the majority of our patients was achieved within 2 months. Recombinant erythropoietin therapy in conjunction with iron sulfate and folic acid was used in 6 TF patients (75%) but in only 2 TE patients (7%). The median total dose of erythropoietin used per patient was 180,000 units (0–1,808,000 units) over a median time of 21 days (0–226 days). For 2 patients, the starting hematocrit prior to treatment was 15% and 16% respectively, both experienced an episode of upper GI bleeding and were treated with transjugular intrahepatic portal-systemic shunt. Clotting parameters as measured by prothrombin time and platelet values were not statistically different. Transjugular intrahepatic portal-systemic shunt was performed in 5 TF patients (63%): 2 as therapy for upper gastrointestinal bleeding and 3 as prophylaxis to decrease portal hypertension in patients with pronounced varices. None was performed prophylactically for TE patients.
TABLE 1. Comparison of Clinical Characteristics Between Transfusion-Free (TF) and Transfusion-Eligible (TE) Groups

United Network of Organ Sharing (UNOS) status at the time of transplantation reflects the UNOS allocation system in effect when these patients were transplanted. Most were UNOS status 2B (TF 25%, TE 13%) or status 3 (TF 75%, TE 67%) at the time of elective LDLT. There were 2 patients (6%) in the TE group of higher acuity (status 1, 1 patient; status 2A, 1 patient). There were no status 1or 2A patients in the TF group.
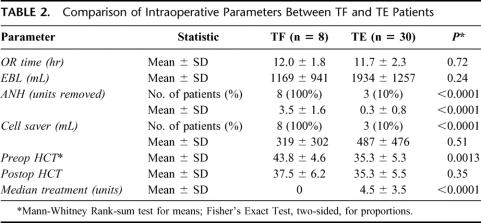
Data pertaining to operative time and hematics are presented in Table 2. LDLT was successfully completed in all 38 patients, without intraoperative complications. Mean operative times were 12.0 ± 1.8 hours (range, 9.8–15.1 hours) in the TF group and 11.7 ± 2.3 hours (range, 7.8–18.1 hours) in the TE patients. ANH and ICS were used on all of the TF patients but in only 10% and 80%, respectively, of the TE group. The mean estimated blood loss in the TF group was 1169 mL (range, 300–2900 mL), and 1934 mL (range, 500–5000 mL) in the TE patients (P = 0.24). The mean volume of reinfused ICS blood in the TF and TE groups was 319 mL and 487 mL, respectively (P = 0.51). None of the TF patients received blood, fresh frozen plasma, or platelet transfusions. However, in accordance with their religious beliefs, they were open to receiving human derived products such as albumin, cryoprecipitate, factor VIIA, and aminocaproic acid. Of the 8 patients in TF group, one patient received a single unit of cryoprecipitate, one received 4.8 mg (80 μg/kg) of factor VII A, and aminocaproic acid was used in 6 patients. In the TE group, 80% of patients received an average of 4.3 PRBC units. Postoperative hematocrit levels were similar in both groups.
TABLE 2. Comparison of Intraoperative Parameters Between TF and TE Patients
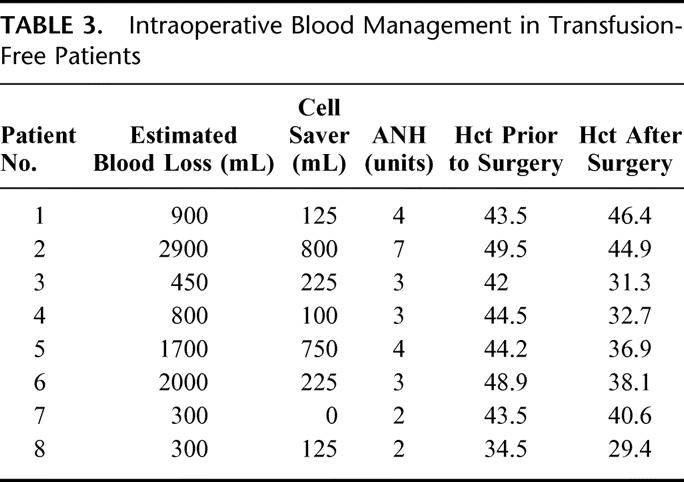
Table 3 further details the hematics in individual TF patients. In 4 of the TF patients (50%), the blood loss was 900 mL or greater. Patient 2 represents the most extreme case in which, despite an estimated blood loss of 2900 mL, the use of ICS and the removal and subsequent reinfusion of 7 units of ANH resulted in a postoperative hematocrit of 44.9%.
TABLE 3. Intraoperative Blood Management in Transfusion-Free Patients
Despite substantial blood losses in the TF group, there were no episodes of sustained hypotension or indirect signs of organ hypoperfusion (acidosis, oliguria, sustained tachycardia) in any of the TF patients.
The surgical and anesthesia management of the donors were similar in both groups. The average blood loss during hepatectomy was 437.5 mL in the TF group and 558.6 mL in the TE group. The mean duration for the surgery was 6.2 hours in the TF group and 6.8 hours in the TE group. None of the donors for the patients in the TF or the TE group needed any transfusion of packed red cells, fresh frozen plasma, or platelets.
The mean ICU stay and total hospital stay were 5 ± 2.3 days (range, 2–9 days) and 17.5 ± 7.3 days (range, 8–27 days) in the TF group, 5.8 ± 4.1 days (range, 2–18 days) and 19.5 ± 13.3 days (range, 8–71 days) in the TE group, respectively (Table 4). Two patients (25%) were reoperated in the TF group for completion hepatectomy/partial splenectomy (n = 1) and bile leak (n = 1). Nine patients (30%) underwent reoperation in the TE group for early bile leak (n = 3), late biliary stricture (n = 1), bleeding (n = 2), infection (n = 2), and hepatic artery thrombosis (n = 1).
TABLE 4. Patient Outcomes

Mean postoperative follow-up was 817 ± 325 days and 672 ± 224 days in the TF and TE patients, respectively. Overall patient survival is 100% in the TF patients and 90 in the TE patients 9 (P = 1.00).
DISCUSSION
Few would dispute that liver transplantation is among the most technically challenging operations in current practice. End-stage liver disease has inherent features that create an environment conducive to coagulopathy and hemorrhage and therefore the likelihood of transfusion. Over the years, however, refinements in surgical technique, coagulation management, intraoperative monitoring, and medical judgment have contributed to widespread success in hepatic transplantation and have even allowed a certain element of predictability. Packaged pricing of the transplant operation by managed care companies has been the result of predictability in lengths of hospital stay, resource consumption, and outcomes.
Likewise, operative predictability, rather than uncertainty, exists for many liver transplant operations. High risk factors for operative bleeding include prior operations, bacterial peritonitis, portal vein thrombosis, and severity of portal hypertension and coagulopathy. Exclusion of patients with these risk factors gives the reassurance of predictability to the procedure. Such discrimination means that TF-LDLT patients must be transplanted before the onset of severe coagulopathy and irreversible anemia. The advent of LDLT permits elective operation before the final stages of liver disease.20–23
At the inception of our TF-LDLT program, we made several assumptions. The first is that there would be an inevitable loss of blood in the TF patients comparable to that of TE patients since the surgical technique of the operation is identical in both groups. Second, the blood loss from the operation should be relatively predictable from the preoperative status of the patient and that it must not be prohibitive. Whether or not an operation is prohibitive is determined by the cushion that can be provided against blood loss in the operating room. This cushion is a balance between the technical difficulty of the recipient on the one hand, and the preoperative hematocrit and the tolerance of ANH on the other. When the cushion exceeds the predicted blood loss, then the decision to proceed is sound. Commonly, a straightforward liver transplant requires a 3- to 5-unit transfusion, similar to the amount provided by ANH in our patients. Finally, the operation had to have several safety valves where the procedure could be aborted before the commencement of the donor operation and before a death in the recipient. The donor and recipient operations would have been aborted if 1) augmentation failed in the presence of anemia, 2) ANH had been poorly tolerated, 3) recipient anatomic factors were restrictive, or 4) blood loss exceeded ANH and ICS replacement capabilities.
Although the recipient surgery was started first to assess the feasibility of performing a TF hepatectomy, this did not impact in anyway the course of the donor surgery. The same principles of TF surgery were used for the donors. As to be expected, the donor safety was of the utmost concern and there was no form of time constraints or pressure on the surgeon performing the donor hepatectomy. As indicated, no blood products were used in any of the donors in this group. Since then we have now performed a total of 94 donor hepatectomies. Only one donor of the 94 received 1 unit of packed red cells for precipitous intraoperative hypotension from bleeding, which was rapidly controlled.24 More recently, we have developed a novel technique of decompressing the portal circulation through a left portohepatic shunt, which allows us to perform the recipient hepatectomy even prior to the completion of the donor hepatectomy if the case permits. This shunt not only decompresses the portal circulation but is used as a vent during portal anastomosis and graft reperfusion allowing the tailoring of the portal flow to the graft size to avoid any liver congestion. In addition, it adds to the comfort of the surgeon doing the transplant.25 This shunt was not used in this patient population.
Several points are clear from the results. Blood augmentation can be successful despite the presence of severe liver disease (average CPT score 8). Blood loss in TF-LDLT is comparable to TE-LDLT, and in some cases was substantial. Importantly, however, a buffer to loss of red cell mass was created by the use of preoperative blood augmentation. This translates into whole blood available as an ANH reinfusion reservoir during the operation supplementing not just the red cell mass but also platelets and coagulation factors. ANH was well tolerated hemodynamically and its use did not interfere with the coagulation parameters.
Analysis of the data of liver transplantation in JW patients reported from the Thomas E Starzl Transplantation Institute showed that less than 2 patients per 1000 would be candidates for TF cadaveric transplantation using their preoperative inclusion criteria and a similar organ allocation policy.26 Such challenges make cadaveric liver transplantation rare for JW patients.27–29
We feel that the management strategies of TF medicine and the surgical technology of LDLT provide the capability to withstand predictable blood losses for typical patients that undergo liver transplantation and that many JW candidates could be transplanted with our TF-LDLT strategy. The JW population accepts extracted fractions, which are distinct from whole blood implying red blood cells, white blood cells, platelets, and fresh frozen plasma.30 The ability to be able to use some of these human derived products such as cryoprecipitate and albumin provides a small cushion of safety. More newly developed products such as factor VII A, which activates the conversion of factor X subsequently converting prothrombin to thrombin and causes platelet activation, may play a significant role in controlling bleeding secondary to coagulopathy.31 This may have significant application in the management of bleeding in surgical patients with coagulopathy.
Several studies point to the benefits and safety of a transfusion-avoidance strategy in surgery. Moderate anemia is well tolerated during operation in patients without underlying cardiovascular disease.32–34 Indeed, the beneficial effect of blood as an oxygen carrier is often overestimated since blood is also an excellent volume expander. Transfused blood has been shown to have a significant effect on the posttransplant patient course, graft function, and risk of infection35–38
Cell salvage destroys platelets and removes clotting factors during processing while ANH preserves all blood components for later reinfusion.14,15 Therefore, both techniques should be used simultaneously in transfusion-avoidance strategies to avoid dilutional coagulopathies when large amounts of salvage blood are reinfused. These concepts are the basis for the ANH described in this report.
Historically, surgical disciplines have been relatively passive regarding blood transfusion. Perception of blood as a safe, necessary, and cheap product may explain this attitude. Chronic blood shortages, highly publicized risks of transfusion-related transmission of hepatitis C and HIV, exclusion of blood donors from France and England where Mad Cow disease is endemic, and recent West Nile virus scares have ignited interest in transfusion risks and cost.39–42
Recent U.S. blood banking data show an estimated 13 million units of blood transfused annually,40,41 over half in surgical patients, most of whom are older than 60 years. This segment of the population is expected to double over the next 30 years, while the expected blood donor pool contraction will lead to severe shortages. Transfusion costs are also underestimated as they include neither the costs of early side effects nor long-term care costs of acquired diseases. A recent study revealed that as many as 1.8% of the population in the United States is probably infected with hepatitis C,43 many as a result of blood transfusions. A significant percentage of these will ultimately come to hepatic transplantation.
JWs uncategorically refuse blood products at risk for death. Consequently, issues regarding blood transfusion and JW patients center around legal, ethical, and belief debates, and have suffered from lack of medical focus.44–47 Many medical practitioners view the JW creed as a fringe belief and, as such, relegate transfusion refusal/avoidance to similar status. By doing so, the medical community is somewhat blinded from real issues regarding transfusion practice and refrains from wider scientific investment in the innovation, understanding and development of techniques in transfusion-free medicine and surgery.
The implications of the consistent performance of TF-LDLT are quite broad. If preoperative medical and anesthetic preparation can pave the way for a safe technical operation of such difficulty, then certainly lesser elective surgical procedures should be targeted. The benefits of a TF approach are manifold. It is likely that the practice of ANH and cell saver even without preoperative augmentation could eliminate the need for transfusion in most general surgical patients with normal red cell mass and low cardiovascular risk.
In the context of a TF practice, there should be consideration of a hematic system that is horizontally integrated across several other organ systems. The assessment of this system depends on an evaluation of cellular elements, hormonal factors, physical pressures, hemodynamics, preexisting anatomic conditions, and coagulation factors. When the system functions properly, then adequate perfusion, local and systemic hemostasis, and homeostasis will be attained. The integrity of this system is evaluated prior to operation, just as with any other system. If the hematic system appears to lack the capacity to undergo predictable surgical stresses, then our strategy is to manipulate certain components of the system. Whenever these manipulations fail to achieve a certain threshold standard for that particular operation, then the operation should not be performed as a TF procedure.
CONCLUSION
Timely living-related liver transplantation can be done successfully without blood or blood product transfusion in selected recipients. Adequate preparation with preoperative transjugular intrahepatic portal-systemic shunt and bone marrow stimulation, the use of acute normovolemic hemodilution and intraoperative cell salvage during surgery, is essential. These techniques, however, are not limited to Jehovah's Witnesses and may be widely applied to other operations to limit transfusion. Surgeons are the largest consumers of blood products and should go beyond the ethical and legal issues that surround major surgery in Jehovah's Witness patients to become the driving force in understanding, improving, and developing a transfusion-free technology.
Footnotes
Reprints: Nicolas Jabbour, MD, Department of Surgery, USC/Keck School of Medicine, 1510 San Pablo Street, #430, Los Angeles, CA 90033. E-mail: njabbour@surgery.usc.edu.
REFERENCES
- 1.Starzl TE, Demetriis AJ, Van Thiel D. Liver transplantation (1). N Engl J Med. 1989;321:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetriis AJ, Van Thiel D. Liver transplantation (2). N Engl J Med. 1989;321:1092–1099. [DOI] [PubMed] [Google Scholar]
- 3.Starzl TE, Demetriis AJ. Liver transplantation: a 31-year perspective. Part I. Curr Probl Surg. 1990;27:49–116. [DOI] [PubMed] [Google Scholar]
- 4.Starzl TE, Demetriis AJ. Liver transplantation: a 31-year perspective. Part II. Curr Probl Surg. 1990;27:117–178. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Demetriis AJ. Liver transplantation: a 31-year perspective. Part III. Curr Probl Surg. 1990;27:181–240. [DOI] [PubMed] [Google Scholar]
- 6.United Network of Organ Sharing. 2000 Annual Report of the Scientific Registry of Transplant Recipients and the Organ Procurement and Transplantation Network: Transplant Data 1989– 1998. Richmond, VA: United Network of Organ Sharing, February 2001.
- 7.Cohen B, Wight C. A European perspective on organ procurement: breaking down the barriers to organ donation. Transplantation. 1999;68:985–990. [DOI] [PubMed] [Google Scholar]
- 8.Adamson J. Erythropoietin, iron metabolism, and red blood cell production. Semin Hematol. 1996;33(suppl 2):5–9. [PubMed] [Google Scholar]
- 9.Goldberg MA. Erythropoiesis, erythropoietin, and iron metabolism in elective surgery: preoperative strategies for avoiding allogeneic blood exposure. Am J Surg. 1995;170(suppl):37–43. [DOI] [PubMed] [Google Scholar]
- 10.Jabbour N, Zajko AB, et al. Transjugular intrahepatic portosystemic shunt in patients with end-stage liver disease: results in 85 patients. Liver Transplant Surg. 1996;2:139–147. [DOI] [PubMed] [Google Scholar]
- 11.Monk TG, Goodnough LT. Acute normovolemic hemodilution. Clin Orthop. 1998;357:74–81. [DOI] [PubMed] [Google Scholar]
- 12.Matot I, Scheinin O, Jurim O, et al. Effectiveness of acute normovolemic hemodilution to minimize allogeneic blood transfusion in major liver resections. Anesthesiology. 2002;97:794–800. [DOI] [PubMed] [Google Scholar]
- 13.Bak Z, Abildgård L, Lisander B, et al. Transesophageal echocardiographic hemodynamic monitoring during preoperative acute normovolemic hemodilution. Anesthesiology. 2000;92:1250–1256. [DOI] [PubMed] [Google Scholar]
- 14.Williamson KR, Taswell HF. Intraoperative blood salvage: a review. Transfusion. 1991;31:662–675. [DOI] [PubMed] [Google Scholar]
- 15.Spain DA, Miller FB, et al. Quality assessment of intraoperative blood salvage and autotransfusion. Am Surgeon. 1997;63:1059–1063. [PubMed] [Google Scholar]
- 16.Kang YG, Martin DJ, Marquez J. Intraoperative changes in blood coagulation and thromboelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriks H, Meijer K, de Wolf JTM, et al. Reduced transfusion requirements by recombinant factor VIIa in orthotopic liver transplantation: a pilot study. Transplantation. 2001;71:402–405. [DOI] [PubMed] [Google Scholar]
- 18.Porte RJ, Molenaar IQ, Beligiomini B, et al. Aprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomized double-blind study. Lancet. 2000;355:1303–1309. [DOI] [PubMed] [Google Scholar]
- 19.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:L649–L652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcos A. Right lobe live donor liver transplantation: a review. Liver Transplantation. 2000;6:3–20. [DOI] [PubMed] [Google Scholar]
- 21.Trotter JF, Wachs ME, Gregory T, et al. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. [DOI] [PubMed] [Google Scholar]
- 22.Cronin DC II, Millis JM, Siegler M. Transplantation of liver grafts from living donors into adults: too much, too soon. N Engl J Med. 2001;344:1633–1637. [DOI] [PubMed] [Google Scholar]
- 23.Surman OS. The ethics of partial-liver donation. N Engl J Med. 2002;346:1038. [DOI] [PubMed] [Google Scholar]
- 24.Gagandeep S, Jabbour N, Genyk Y, et al. Restricting fresh frozen plasma in hepatic resections. J Am Coll Surg. 2003;197:339–340. [DOI] [PubMed] [Google Scholar]
- 25.Jabbour N, Gagandeep S, Mateo R, et al. Left portohepatic shunt: decreasing excessive portal venous inflow during live donor liver transplantation. J Am Coll Surg. 2003;197:1056–1057. [DOI] [PubMed] [Google Scholar]
- 26.Ramos HC, Todo S, et al. Liver transplantation without the use of blood products. Arch Surg. 1994;129:528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snook NJ, O'Beirne HA, et al. Use of recombinant human erythropoietin to facilitate liver transplantation in a Jehovah's Witness. Br J Anaesth. 1996;76:740–743. [DOI] [PubMed] [Google Scholar]
- 28.Seu P, Neelankanta G, Csete M, et al. Liver transplantation for fulminant hepatic failure in a Jehovah's Witness. Clin Transplant. 1996;10:404–407. [PubMed] [Google Scholar]
- 29.Detry O, Honore P, Delwaide J, et al. Liver transplantation in a Jehovah's Witness. Lancet. 1999;353:1680. [PubMed] [Google Scholar]
- 30.Jehovah's Witnesses. Perfect life: not just a dream (official publication of the Jehovah's Witnesses). Watchtower. 2000:29–31. [Google Scholar]
- 31.Schreiber MA, Holcomb JB, Hedner U, et al. The effect of recombinant factor VIIa on noncoagulopathic pigs with grade V liver injuries. J Am Coll Surg. 2003;196:691–697. [DOI] [PubMed] [Google Scholar]
- 32.Hebert PC, Wells G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. [DOI] [PubMed] [Google Scholar]
- 33.Weiskopf RB, Viele MK, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221. [DOI] [PubMed] [Google Scholar]
- 34.Carson JL, Duff A, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. [DOI] [PubMed] [Google Scholar]
- 35.Palomo Sanchez JC, Jimenez C, Moreno G, et al. Effects of intraoperative blood transfusion on post operative complications and survival after orthotopic liver transplantation. Hepatogastroenterology. 1998;45:1026–1033. [PubMed] [Google Scholar]
- 36.Miki C, Iriyama K, Gunson BK, et al. Influence of intraoperative blood loss on plasma levels of cytokines and endotoxin and subsequent graft liver function. Arch Surg. 1997;132:136–141. [DOI] [PubMed] [Google Scholar]
- 37.Schroeder RA, Johnson LB, et al. Total blood transfusion and mortality after orthotopic liver transplantation. Anesthesiology. 1999;91:329–330. [DOI] [PubMed] [Google Scholar]
- 38.Cacciarelli TV, Keefe EB, et al. Effect of intraoperative blood transfusion on patient outcome in hepatic transplantation. Arch Surg. 1999;134:25–29. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber GB, Busch T, Michael P, et al. The risk of transfusion-transmitted viral infections. N Engl J Med. 1996;334:1685–1690. [DOI] [PubMed] [Google Scholar]
- 40.Goodnaugh LT, Brecher ME, et al. Transfusion medicine. N Engl J Med. 1999;340:438–447. [DOI] [PubMed] [Google Scholar]
- 41.Goodnaugh LT, Brecher ME, et al. Medical progress: transfusion medicine (second of 2 parts): blood conservation. N Engl J Med. 1999;340:525–533. [DOI] [PubMed] [Google Scholar]
- 42.Sloand EM, Pitt E, et al. Safety of the blood supply. JAMA. 1995;274:1368–1373. [PubMed] [Google Scholar]
- 43.Alter MJ, Druszon MD, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988–1994. N Engl J Med. 1999;431:556–562. [DOI] [PubMed] [Google Scholar]
- 44.Finfer S, Howell S, Miller J, et al. Managing patients who refuse blood transfusions: an ethical dilemma. Major trauma in two patients refusing blood transfusion. Br Med J. 1994;308:1423–1424. [PMC free article] [PubMed] [Google Scholar]
- 45.Sacks DA, Koppes RH. Caring for the female Jehovah's Witness: balancing medicine, ethics, and the First Amendment. Am J Obstet Gynecol. 1994;170:452–455. [DOI] [PubMed] [Google Scholar]
- 46.Muramoto O. Jehovah's Witnesses and blood transfusions. Lancet. 1998;352:824. [DOI] [PubMed] [Google Scholar]
- 47.Wilcox P. Jehovah's Witnesses and blood transfusion. Lancet. 1999;353:757–758. [DOI] [PubMed] [Google Scholar]