Abstract
Objective:
To review a single-institution 6-year experience with laparoscopic live donor nephrectomy detailing the technical modifications, clinical results, as well as the trends in donor and recipient morbidity.
Summary Background Data:
Since 1995, laparoscopic donor nephrectomy has had a significant impact on the field of renal transplantation, resulting in decreased donor morbidity, without jeopardizing procurement of a high-quality renal allograft. This technique has become the preferred method of allograft procurement for many transplantation centers worldwide but still remains technically challenging with a steep learning curve.
Methods:
Records from 381 consecutive laparoscopic donor nephrectomies were reviewed with evaluation of both donor and recipient outcomes. Trends in donor and recipient complications were assessed over time by comparing the outcomes between four equally divided groups.
Results:
All 381 kidneys were procured and transplanted successfully with only 8 (2.1%) open conversions. Mean operative time was 252.9 ± 55.7 minutes, estimated blood loss 344.2 ± 690.3 mL, warm ischemia time 4.9 ± 3.4 minutes, and donor length of stay was 3.3 ± 4.5 days. There was a significant decline in total donor complications, allograft loss, and rate of vascular thrombosis with experience. The rate of ureteral complications declined significantly when comparing our early (Group A) versus later (Groups B–D) experience.
Conclusion:
Laparoscopic donor nephrectomy has remained a safe, less invasive, and effective technique for renal allograft procurement. Over our 6-year experience and with specific refinements in surgical technique, we have observed a decline in both donor and recipient morbidity following laparoscopic live donor nephrectomy.
Laparoscopic live donor nephrectomy has reduced donor morbidity while providing a high quality renal allograft for the recipient, and has thus become the preferred method of allograft procurement at many transplantation centers worldwide. This study reviews the trends in both donor and recipient morbidity over a 6-year, single-institution experience.
Over the past decade, the annual supply of renal allografts has continued to fall short of the increasing numbers of patients seeking renal transplantation. This widening gap between the supply and demand for renal allografts in conjunction with the profound advantages of live versus cadaveric renal transplantation has prompted efforts to increase live renal donation. Laparoscopic live donor nephrectomy was introduced in 1995 by Ratner et al as a less invasive alternative to kidney procurement in hopes of decreasing the disincentives to live renal donation.1 Since then, laparoscopic donor nephrectomy has emerged as the preferred technique at many institutions, resulting in less postoperative pain and shorter hospital stays and postoperative convalescence for the donor patient, while maintaining equivalent recipient outcomes as compared with results from conventional open donor nephrectomy.2–8
Despite the advantages of laparoscopic live donor nephrectomy, this technique still remains challenging even for the most experienced laparoscopist. With time and greater experience, as well as with specific refinements in surgical technique, we have witnessed a reduction in both donor and recipient morbidity. Herein we review our 6-year experience with laparoscopic live donor nephrectomy evaluating our overall clinical outcomes as well as trends in donor and recipient morbidity.
MATERIALS AND METHODS
Patient Data and Clinical Parameters
Clinical and operative records from 381 consecutive laparoscopic live donor nephrectomy cases performed by four surgeons (L.R.K., L.E.R., R.A.M., and T.W.J.) between February 8, 1995 and November 20, 2001 were reviewed. Intraoperative and postoperative variables for both donor and recipient patients including complications, reoperations, and transfusions were tabulated. Immediate allograft function was assessed by measurement of daily serum creatinine following transplantation. Long-term allograft function was assessed by calculated creatinine clearances (Cockroft-Gault method). Comparisons in short- and long-term renal allograft function were made between the laparoscopic group and 48 open donor nephrectomy cases performed at our institution between January 12, 1995 through March 31, 1997 during the time when the laparoscopic technique was first introduced. To determine the trends in both donor and recipient morbidity, the laparoscopic series was divided into four groups (groups A−D) of 95 patients each to compare our clinical outcomes over time. Group D was comprised of 96 patients.
Statistical Analysis
Student t test was used for comparison of immediate and long-term allograft function between laparoscopic and open groups. Analysis of trends in donor morbidity was assessed by Mantel-Haenszel χ2 analysis and Fisher exact test. Comparison of mean operative time, estimated blood loss, and warm ischemia time between groups was performed using linear regression analysis with SAS software (Cary, NC). A P value of <0.05 was considered statistically significant.
Operative Technique
The detailed steps of a laparoscopic donor nephrectomy have been described previously by our group.9,10 Because the majority of our procedures were left-sided donor nephrectomies, specific modifications to our left-sided technique are briefly mentioned herein. Modifications to our right-sided technique with special emphasis on techniques for optimizing length of the anatomically shorter right renal vein have been previously described by Mandal et al11
Modification 1: Retraction of Bowels and Exposure of the Renal Hilum
After incising the line of Toldt along the ipsilateral colon, a 12-mm trocar is first placed through a 1-cm horizontal incision made in the lower midline along the planned Pfannenstiel extraction site. The trocar is removed and the fascial tract bluntly dilated with the surgeon's index finger so as to allow a 15-mm Endocatch device (United States Surgical Corporation, Norwalk, CT) to fit snugly within the tract when inserted, thus preventing loss of pneumoperitoneum. With the bag closed, this device is useful as a blunt retractor to facilitate gentle medial reflection of the colon and to provide exposure of the renal hilum. When using the Endocatch device as a retractor, great care must be taken to minimize inadvertent forceful movement of the device as the distal end of the metal sheath can potentially cause abrasion injury to the spleen or surrounding bowel. In lieu of the Endocatch device, a 10-mm paddle retractor can also be used. At the end of the operation, the Endocatch bag is deployed, and the kidney is entrapped and delivered through the Pfannenstiel incision.
Modification 2: Preservation of Ureteral Blood Supply
Blunt dissection is carried out medial to the gonadal vein, keeping this structure and the mesoureter along the entire length of the ureter down to the pelvic inlet. Use of electrocautery is minimized and dissecting between the proximal ureter and lower pole of the kidney is avoided so as not to compromise the sole remaining blood supply to the ureter arising from branches of the renal artery.
Modification 3: Preservation of Lateral, Posterior, and Inferior Renal Attachments During Dissection of the Renal Hilum
During dissection of the renal artery and vein, the lateral, posterior, and inferior (ie, ureteral) attachments to the kidney are maintained creating a three-point fixation to the retroperitoneum. These attachments are preserved until the hilum is completely dissected to limit mobility of the kidney and prevent torsion of the kidney about its vascular pedicle.
RESULTS
Donor Outcomes
Of the 381 consecutive cases, 362 (95%) were left-sided and 19 (5%) were right-sided laparoscopic donor nephrectomies. All 381 kidneys were procured and transplanted successfully with adequate renal artery and renal vein length to perform the recipient operation using standard techniques. Mean operative time was 253 ± 55.7 minutes, estimated blood loss 334 ± 690.3 mL, and warm ischemia time 4.9 ± 3.4 minutes. Mean length of donor hospital stay was 3.3 ± 4.5 days.
Donor complications following laparoscopic live donor nephrectomy are listed in Table 1. Total complication rate was 16.5% (63 patients) for the series with 29 (7.6%) major complications and 34 (8.9%) minor complications. The open conversion rate was 2.1% (8 patients), reoperation rate was 1.8% (7 patients), and the transfusion rate was 3.4% (13 patients). Of the 8 patients who required open conversion, 6 were emergent due to renal artery (3 patients) and renal vein (3 patient) injuries. There were 2 elective open conversions: one due to small bowel distention and lack of working space and the other due to dense intraperitoneal adhesions. There were 7 patients who required reoperation with the following causes: epigastric artery injury requiring open ligation (1), incisional hernia at allograft delivery site requiring prosthetic mesh repair (1), ischemia of the left testicle requiring orchidopexy (1), postoperative bleeding requiring exploratory laparotomy (3), and duodenal injury requiring duodenojejunostomy (1). There were four other bowel injuries noted in the series. One patient sustained a small bowel serosal injury that was repaired laparoscopically. Two other small serosal injuries occurred: one to the small bowel and another to the colon, which were repaired through the extraction site following delivery of the kidney. The last patient sustained a small bowel enterotomy while creating the extraction site for delivery of the kidney requiring a 2-inch bowel resection. There were no donor mortalities.
TABLE 1. Donor Complications
Recipient Outcomes
Recipient hospital stays averaged 8.5 ± 9.0 days. Immediate renal allograft function between the laparoscopic and open group was similar with mean serum creatinine of 2.6 ± 2.3 versus 2.0 ± 1.8 mg/dL (P = 0.13), respectively, by postoperative day 4. Long-term allograft function up to 5 years postoperatively was likewise similar between the laparoscopic and open groups (65.5 ± 25.8 vs. 63.7 ± 28.9 mL/min at 5 years following transplantation respectively, P = 0.88).
In terms of recipient complications, 24 (6.3%) patients developed ureteral complication, which included any ureteral stenosis or leak requiring further (percutaneous or operative) intervention. There were a total of 8 (2.1%) patients who developed vascular thrombosis following transplantation resulting in loss of the renal allograft. Renal vein thrombosis occurred in 5 cases. In 3 of these patients, the kidney was procured from the right side, of which two kidneys had duplicate, short renal veins. The remaining causes of vascular thrombosis included cholesterol emboli (1) and renal artery thrombosis (2). In addition to these cases, there were 22 other patients who sustained a loss of their renal allograft (30 patients total, 7.9%) with the following causes: severe cell-mediated rejection (16 patients), humoral rejection (1 patient), noncompliance with medications (3 patient), recurrent focal segmental glomerulosclerosis (1 patient), and hemorrhage from the renal artery anastomosis (1 patient). Ninety-one patients (23.9%) experienced acute allograft rejection within the first 3 months following surgery with delayed graft function occurring in 17 (4.5%) patients. There were 23 (6%) recipient mortalities: 6 due to sepsis, 8 from cardiovascular complications, 1 respiratory arrest, and 8 due to other causes. Only one death occurred in the immediate postoperative period (first postoperative day) and was due to hemorrhage from the renal artery anastomosis with subsequent cardiac arrest. The remainder of the deaths occurred ≥ 1 month following renal transplantation.
Trends in Donor and Recipient Morbidity
Mean operative times for groups A, B, C, and D were 234 ± 49.7, 280.2 ± 56.8, 271.5 ± 45.6, and 226.4 ± 51.4 minutes, respectively. The mean estimated blood loss was 261.3 ± 173.1, 335 ± 410.3, 441.9 ± 1270.4, and 343.4 ± 349.1 mL, respectively, and the mean warm ischemia time was 4.6 ± 1.3, 4.8 ± 1, 5.4 ± 6.9, and 4.8 ± 1.2 minutes, respectively. There was no significant difference noted in the mean operative time, estimated blood loss, and warm ischemia times between groups based on linear regression analysis. The trends in both donor and recipient complications over our 6-year experience are depicted in Figure 1. There was a significant decline in the rate of total donor complications with experience (P = 0.03). There was a trend toward a decline in ureteral complications over time. Although this trend did not achieve statistical significance, there was a significant decline (P = 0.05) noted when comparing our initial experience (group A) to our later experience (groups B−D combined). Lastly, there was a significant decline in renovascular thrombosis (P = 0.01) and the loss of renal allografts (P = 0.001) over our series.
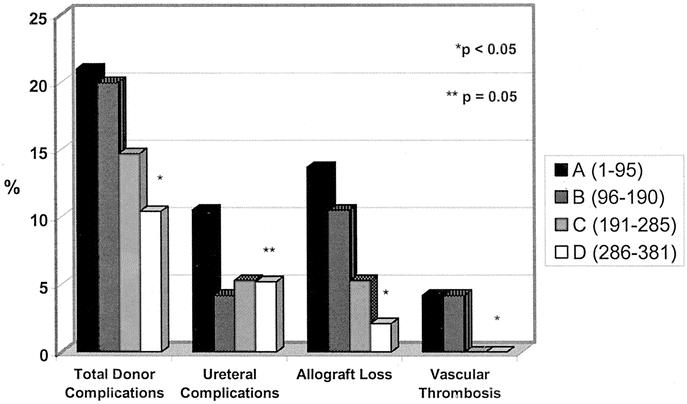
FIGURE 1. Trends in donor and recipient morbidity over 381 consecutive laparoscopic live donor nephrectomies divided into four groups (A−D). *Significant decline (P < 0.05) when evaluating overall trend between groups A through D. **Significant decline (P = 0.05) between early experience (group A) as compared with later experience (groups B−D combined).
DISCUSSION
Laparoscopic live donor nephrectomy was introduced in 1995 as method of reducing the disincentives to live kidney donation by reducing the impact of the open nephrectomy operation on the donor patient. Although clear benefits to the donor patient were realized very early using this less invasive technique, including a reduction in postoperative pain, shorter hospitalization, and shorter convalescence, this was tempered by the steep learning curve and initially high donor morbidity. This is exemplified by the 21% total donor complication rate encountered during the first 95 cases in our series (group A). With greater experience and with specific refinements in our surgical technique, we have witnessed a significant reduction in donor complications with a 10.4% total complication rate (5.2% major complications) in our last 96 cases (group D). This is comparable to the 8% to 20% total complication (0.2%−8.1% major) rate reported in contemporary open donor nephrectomy series.12–18 The types and incidences of complications differ between our laparoscopic series as compared with the reported complications in these open series. The occurrence of major vascular complications requiring conversion to open surgery is unique to the laparoscopic approach as such complications are more readily managed during the open surgical approach and may not always be reported. Although mostly minor, bowel complications occurred in our series with an incidence of 1.3% as compared with a reported incidence of 0% with open surgery. Postoperative pneumothorax appears to be more common with open surgery and occurs in 1% to 7% of cases as compared with 0.3% in our series. The incidence of perioperative reoperation (1.8%) was more common in our laparoscopic series and negligible with open surgery. However, late complications requiring reoperation have been reported following open donor nephrectomy with one series noting a 2% incidence of bowel obstruction, all requiring reexploration with lysis of adhesions.18 In this series, all procedures were performed thorough a transperitoneal, midline abdominal approach. The incidence of reoperation for bowel obstruction is likely lower following the more commonly performed extraperitoneal flank approach. In our series following transperitoneal laparoscopic donor nephrectomy, no small bowel obstructions have been observed to date.
The most serious major complications that occurred during laparoscopic donor nephrectomy included iatrogenic renovascular and bowel injuries. In our series, there were three renal artery and three renal vein injuries requiring open conversion. To minimize iatrogenic vascular injuries, the authors have modified their technique of dissection of the renal hilum. First, three-dimensional CT angiography is routinely performed on all donor candidates preoperatively to help identify any subtleties in the renal vascular anatomy, including the presence of duplicate renal vessels, and lumbar vessels. Second, the Endocatch device is used as a blunt device for medial retraction of the ipsilateral colon and small bowel, thus optimizing exposure of the renal vessels during dissection around the hilum. Third, sharp dissection is minimized around the renal vessels to avoid accidental laceration or transection of the major renal vessels or their branches. Use of the endoscopic GIA stapler (United States Surgical Corporation) to divide the renal artery and vein obviates the need to introduce scissors into the operative field during this critical part of the operation, when inadvertent vascular injury can occur due to the requirement for complex movements to be executed in rapid succession. Lastly, the use of hemostatic clips is minimized near the base of the renal artery and vein as they may interfere with proper placement and firing of the endoscopic GIA stapler during transection of the renal vessels. Early in our series, there were 3 cases of incomplete transection of the renal artery due to misfiring of the endoscopic GIA stapling device, 2 of which required conversion to open surgery.
Bowel injuries occurred in 5 patients in our series, 3 of which involved minor serosal injuries that were recognized during the operation that were subsequently oversewn with no sequelae. One patient sustained a duodenal injury that was thought to be due to retraction of the duodenum with a 3-mm laparoscopic retractor. The last patient sustained a small bowel enterotomy during preparation of the kidney extraction site. This patient had a history of a previous laparotomy and had developed extensive adhesions to the anterior abdominal wall. To minimize the risk of bowel injuries during laparoscopic dissection, direct manipulation of the bowel should be minimized and blunt instrumentation should be used for retraction. The authors prefer the use of the 15-mm Endocatch device (without the bag deployed) or a laparoscopic paddle retractor for this purpose.
Recipient complications have also declined with experience. During our early experience, we noted a high rate of ureteral complications (10.5% in the first 95 cases) likely due to dissecting too close to the ureter and compromising its delicate blood supply. In our current technique, blunt dissection is used around the ureter, dissection is performed medial to the gonadal vein, and a generous amount of mesoureter is maintained surrounding the ureter. When comparing our first 95 cases (group A) to the remaining 286 patients (groups B−D), the ureteral complication rate declined significantly (P = 0.05). In the last 96 cases, the ureteral complication rate was 5.2%. The current modification in ureteral dissection may not be the only variable responsible for the decline in ureteral complications; however, it adheres to the principal of preserving vascular supply to the ureter and therefore its beneficial effect is at the very least suggestive. Others have noted a decline in ureteral complications with similar modifications in ureteral dissection.19,20 Of note, ureteral complications during open donor nephrectomy have also been reported and occur in 1.6% to 6.3% of cases.15,21,22
Technical-related vascular thrombosis occurred in 8 patients in our series (2.1%), each resulting in loss of the renal allograft. In 3 of these cases, the harvested kidney was from the right side, 2 of which had short duplicate renal veins. Because of the anatomically shorter right renal vein, the authors have since modified their technique to right-sided laparoscopic donor nephrectomy to optimize the length of the right renal vein.11 Repetitive kinking and torsion of the kidney about its vascular pedicle may compromise the integrity of the renal vasculature. Maintaining the lateral, posterior, and inferior attachments of the kidney until the renal vessels are completely dissected can minimize such an event. These attachments provide a three-point fixation of the kidney, thus preventing the kidney from falling medially and obscuring the renal hilum during dissection of the renal vein and artery. With experience, we have witnessed a significant decline in technical-related vascular thrombosis (P = 0.01) with no further events occurring in the last 200 cases. In addition, the incidence of allograft loss has declined significantly over time (P = 0.001) with only a 2.1% incidence in the last group of 96 patients.
Interestingly, the mean operative time, estimated blood loss, and warm ischemia time during laparoscopic donor nephrectomy did not decline over our experience. The most likely explanation for these observations is that these results reflect the experience of not one, but four, operating surgeons with different levels of laparoscopic expertise. In addition, as there continues to be a constant influx of new residents and fellows that are exposed to this technique at our academic teaching institution, this has led to an invaluable intraoperative teaching experience at the expense of perhaps slightly longer operative times. Lastly, this finding may simply be a reflection of the technical complexity inherent within this operation. Nevertheless, over our 6-year experience with laparoscopic live donor nephrectomy and with specific refinements in our surgical technique, we have demonstrated a continued reduction of both donor and recipient morbidity while maintaining excellent short- and long-term renal allograft function comparable to that of open surgery.
CONCLUSION
Laparoscopic live donor nephrectomy has had a substantial impact on the donor operation by providing a less invasive approach to kidney procurement as compared with open surgery. This has resulted in less morbidity for the donor patient while maintaining a high quality allograft for the recipient. Over our 6-year experience, specific refinements in surgical technique have led to a significant reduction in total donor complications, as well as a decline in the rate of recipient complications including ureteral complications, graft losses, and incidence of vascular thrombosis. Despite these improvements, laparoscopic live donor nephrectomy remains a technically challenging operation with little to no margin for error and continues to have a steep learning curve. By providing an insight into the evolution of our technique of laparoscopic live donor nephrectomy, we hope to provide others with valuable information to help reduce the learning curve for this technically demanding operation.
Footnotes
Reprints: Li-Ming Su, MD, Johns Hopkins Bayview Medical Center, Department of Urology, A-345, 4940 Eastern Avenue, East Baltimore, MD 21224. E-mail: LSU11@jhmi.edu.
REFERENCES
- 1.Ratner LE, Ciseck LJ, Moore RG, et al. Laparoscopic live donor nephrectomy. Transplantation. 1995;60:1047–1049. [PubMed] [Google Scholar]
- 2.Ratner LE, Kavoussi LR, Sroka M, et al. Laparoscopic assisted live donor nephrectomy: a comparison with the open approach. Transplantation. 1997;63:229–233. [DOI] [PubMed] [Google Scholar]
- 3.Lee BR, Chow GK, Ratner LE, et al. Laparoscopic live donor nephrectomy: outcomes equivalent to open surgery. J Endourol. 2000;14:811–819. [DOI] [PubMed] [Google Scholar]
- 4.Ratner LE, Montgomery RA, Kavoussi LR. Laparoscopic live donor nephrectomy: the four year Johns Hopkins University experience. Nephrol Dial Transplant. 1999;14:2090–2093. [DOI] [PubMed] [Google Scholar]
- 5.Sosa JA, Albini TA, Powe NR, et al. Laparoscopic vs. open live nephrectomy: a multivariate patient outcome analysis. Transplantation. 1998;65(suppl):85. [Google Scholar]
- 6.Flowers JL, Jacobs S, Cho E, et al. Comparison of open and laparoscopic live donor nephrectomy. Ann Surg. 1997;226:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London E, Rudich S, McVicar J, et al. Equivalent renal allograft function with laparoscopic versus open live donor nephrectomies. Transplant Proc. 1999;31:258–260. [DOI] [PubMed] [Google Scholar]
- 8.Odland MD, Ney AL, Jacobs DM, et al. Initial experience with laparoscopic live donor nephrectomy. Surgery. 1999;126:603–607. [PubMed] [Google Scholar]
- 9.Fabrizio MD, Ratner LE, Montgomery RA, et al. Laparoscopic live donor nephrectomy. Urol Clin North Am. 1999;26:247–256. [DOI] [PubMed] [Google Scholar]
- 10.Ratner LE, Fabrizio M, Chavin K, et al. Technical considerations in the delivery of the kidney during laparoscopic live-donor nephrectomy. J Am Coll Surg. 1999;189:427–430. [DOI] [PubMed] [Google Scholar]
- 11.Mandal AK, Cohen C, Montgomery RA, et al. Should the indications for laparoscopic live donor nephrectomy of the right kidney be the same as for the open procedure? Anomalous left renal vasculature is not a contraindication to laparoscopic left donor nephrectomy. Transplantation. 2001;71:660–664. [DOI] [PubMed] [Google Scholar]
- 12.D'Alessandro AM, Sollinger HW, Knechtle SJ, et al. Living related and unrelated donors for kidney transplantation: a 28-year experience. Ann Surg. 1995;222:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson EM, Remucal MJ, Gilligham KJ, et al. Complications and risks of living donor nephrectomy. Transplantation. 1997;64:1124–1128. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer D, Sahyoun AI, Madras PN, et al. Two hundred one consecutive living-donor nephrectomies. Arch Surg. 1998;133:426–431. [DOI] [PubMed] [Google Scholar]
- 15.Streem SB, Novick AC, Steinmuller DR, et al. Flank donor nephrectomy: efficacy in the donor and recipient. J Urol. 1989;141:1099–1101. [DOI] [PubMed] [Google Scholar]
- 16.Ottelin MC, Bueschen AJ, Lloyd LK, et al. Review of 333 living donor nephrectomies. South Med J. 1994;87:61–64. [DOI] [PubMed] [Google Scholar]
- 17.Waples MJ, Belzer FO, Uehling DT. Living donor nephrectomy: a 20-year experience. Urology. 1995;45:207–210. [DOI] [PubMed] [Google Scholar]
- 18.Dunn JF, Nylander WA Jr, Richie RE, et al. Living related kidney donors: a 14-year experience. Ann Surg. 1986;203:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philosophe B, Kuo PC, Schweitzer EJ, et al. Laparoscopic versus open donor nephrectomy: comparing ureteral complications in the recipients and improving the laparoscopic technique. Transplantation. 1999;68:497–502. [DOI] [PubMed] [Google Scholar]
- 20.Dunkin BJ, Johnson LB, Kuo PC. A technical modification eliminates early ureteral complications after laparoscopic donor nephrectomy. J Am Coll Surg. 2000;190:96–97. [DOI] [PubMed] [Google Scholar]
- 21.Kuo PC, Cho ES, Flowers JL, et al. Laparoscopic living donor nephrectomy and multiple renal arteries. Am J Surg. 1998;176:559–563. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery RA, Kavoussi LR, Su LM, et al. Improved recipient results after 5 years of performing laparoscopic donor nephrectomy. Transplant Proc. 2001;33:1108–1110. [DOI] [PubMed] [Google Scholar]



