Abstract
The relationship between chromatin remodeling and histone acetylation at the yeast CUP1 gene was addressed. CUP1 encodes a metallothionein required for cell growth at high copper concentrations. Induction of CUP1 with copper resulted in targeted acetylation of both H3 and H4 at the CUP1 promoter. Nucleosomes containing upstream activating sequences and sequences farther upstream were the targets for H3 acetylation. Targeted acetylation of H3 and H4 required the transcriptional activator (Ace1p) and the TATA boxes, suggesting that targeted acetylation occurs when TATA-binding protein binds to the TATA box or at a later stage in initiation. We have shown previously that induction results in nucleosome repositioning over the entire CUP1 gene, which requires Ace1p but not the TATA boxes. Therefore, the movement of nucleosomes occurring on CUP1 induction is independent of targeted acetylation. Targeted acetylation of both H3 and H4 also required the product of the SPT10 gene, which encodes a putative histone acetylase implicated in regulation at core promoters. Disruption of SPT10 was lethal at high copper concentrations and correlated with slower induction and reduced maximum levels of CUP1 mRNA. These observations constitute evidence for a novel mechanism of chromatin activation at CUP1, with a major role for the TATA box.
Eukaryotic DNA is packaged into the cell nucleus in the form of chromatin. The basic structural repeat unit of chromatin is the nucleosome, in which 147 bp of DNA are wrapped in nearly two superhelical turns around a central core histone octamer that is composed of two molecules each of the four core histones H2A, H2B, H3, and H4. A molecule of histone H1 is bound to the nucleosome core and to the linker DNA and directs the folding of the chromatin fiber. The assembly of DNA into nucleosomes represses transcription in vitro, raising the question of how the cell copes with chromatin. It is now clear that chromatin structure is more than just a DNA packaging system: it is intimately connected with events in gene regulation. This is evident from the identification of two classes of chromatin-modifying activities (17, 43, 48): (i) remodeling complexes, which use the energy of ATP hydrolysis to effect changes in chromatin structure, and (ii) histone modifying complexes, which modify histones posttranslationally (notably, histone acetyltransferases [HATs] and deacetylases).
Histone acetylation occurs on specific lysine residues in the tail domains of all four core histones. The tail domains are not required for maintenance of the nucleosome core structure (see reference 53 for a detailed discussion). They bind to DNA on the outside of the core and to linker DNA (1). Acetylation reduces their affinity for DNA by reducing their positive charge. Thus, the histone tails can reduce access to DNA on the surface of the nucleosome, and acetylation, by relieving this block, can facilitate the binding of transcription factors (25, 35, 47).
The correlation between histone acetylation and gene activity has been known for a very long time, but we are only now beginning to understand the molecular mechanisms involved. The breakthrough came with the identification of a yeast coactivator, Gcn5p, as a histone acetylase (5). There are now many examples of the specific recruitment of acetylases to promoters and other regulatory elements on gene activation, thus targeting specific nucleosomes for acetylation. Similarly, recruitment of deacetylases appears to be an important repressive mechanism (54). More recently it has been proposed that acetylation does not just represent a mechanism for facilitating the binding of transcription factors but that the acetylation pattern of particular nucleosomes constitutes part of a code that is recognized by various factors, perhaps including remodeling complexes (44).
We have adopted the yeast CUP1 gene as a model for understanding the role of chromatin structure in gene regulation. It encodes a metallothionein responsible for protecting cells from the toxic effects of excess copper ions (7, 18). Its regulation is relatively simple and well understood: the N-terminal domain of a transcriptional activator, Ace1p (also called Cup2p), exhibits copper-dependent DNA binding at upstream activating sequences (UASs) in the CUP1 promoter (6, 15). Ace1p activates transcription through its C-terminal acidic activation domain. The CUP1 promoter contains two consensus TATA boxes (10) and an initiation element (24). CUP1 is also induced relatively weakly by heat shock, mediated via the binding of heat shock factor to sites in the CUP1 promoter (29).
Although much work has been done on defining acetylases, deacetylases and their targets, there is much less information on the connections between remodeling complexes and acetylation. For such a study, it is necessary to have a detailed description of the chromatin structure of the gene of interest and of how it is remodeled on induction, as well as details of changes in acetylation. In budding yeast, this is true for the PHO5 (45) and PHO8 (39) genes, and a detailed model for their regulation has been developed, based on the roles of the Swi/Snf remodeling complex and SAGA, a Gcn5p-containing acetylase complex (16), in their activation. CUP1 is a Swi/Snf- and Gcn5p-independent gene (19) and so might be expected to exhibit a different remodeling mechanism. Indeed, a recent study of the chromatin structure of CUP1 and how it responds to induction (42) suggests that the mechanism of chromatin remodeling at CUP1 is different. For PHO5 and PHO8, substantial changes in chromatin structure were limited to the promoter. In contrast, a gene-wide redistribution of nucleosomes was observed on induction of CUP1 (Fig. 1B). This redistribution of nucleosomes required the activator, Ace1p, but not the TATA boxes.
FIG. 1.
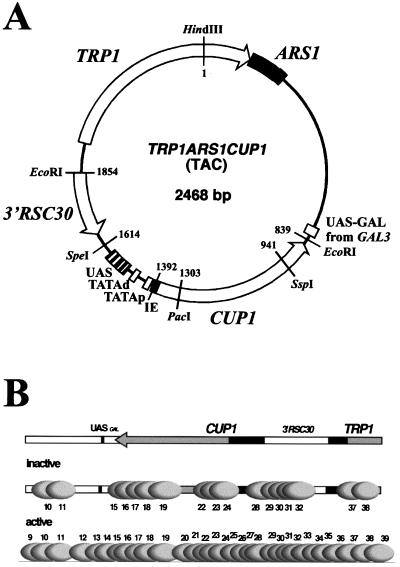
Chromatin structure of CUP1. A map of the TAC minichromosome. TAC is based on the yeast plasmid TRP1ARS1; TRP1 is a selection marker, and ARS1 is the origin of replication. TRP1ARS1 also contains the upstream region of neighboring GAL3, including a binding site for Gal4p (UASGAL). CUP1 was inserted at the EcoRI site, together with the 3′ flanking region of RSC30, the gene neighboring CUP1 in the genome. The SpeI site marks the limit of the CUP1 promoter, which contains two TATA boxes, five Ace1p binding sites (UAS), and an initiation element (IE). (B) Positioned nucleosomes on CUP1 in TAC minichromosomes purified from copper-induced WT cells and ace1Δ cells (42). The CUP1 and TRP1 promoters are shown as dark boxes. Grey ovals indicate positioned nucleosomes drawn to scale, numbered relative to the HindIII site in TRP1 (not all of TAC is shown; see panel A). TAC from ace1Δ cells (inactive CUP1) is organized into clusters of overlapping positions separated by linkers of various lengths. In copper-induced TAC (active CUP1), nucleosomes occupy additional positions in the linkers, implying that induction results in the movement of nucleosomes over the entire CUP1 gene, including flanking regions. This movement does not require the TATA boxes. It was proposed that nucleosome movement reflects the creation of a dynamic chromatin structure via the Ace1p-dependent recruitment of a nucleosome repositioning activity. Note: nucleosome positions on CUP1 are determined primarily by DNA sequence (41).
Here, we have examined the role of histone acetylation in CUP1 induction. We observed targeted acetylation of both H3 and H4 in nucleosomes on the CUP1 promoter after induction. Targeted acetylation required the presence of the activator, Ace1p. Surprisingly, it also required the TATA boxes. Since the TATA boxes are not required for the movement of nucleosomes (42), it may be concluded that the putative nucleosome repositioning activity does not require targeted histone acetylation to function. Thus, targeted histone acetylation at the CUP1 promoter is likely to be a relatively late event during activation, occurring at the moment of recruitment of TATA-binding protein (TBP) and its associated proteins or at a later stage in initiation.
In a search for HAT activities acting at CUP1, we discovered that the SPT10 gene is required for targeted acetylation of both H3 and H4. Spt10p contains a domain homologous to the HAT domain of Gcn5p (34). SPT10 was originally identified as one of a set of SPT genes, mutations in which suppress insertion of the yeast transposable element Ty (14). The SPT genes include a large number of proteins important in transcription, including TBP itself and subunits of SAGA (51). SPT10 is not an essential gene, but the null allele is associated with slow growth and defects in transcriptional regulation (13, 32, 33). SPT10 has been identified in a number of mutant screens as a global regulator of core promoter activity, acting at or near the TATA box (12, 37, 55). Taken together, these observations indicate that CUP1 is regulated via a novel chromatin remodeling mechanism involving major roles for Spt10p and the TATA boxes.
MATERIALS AND METHODS
Yeast strains and plasmids.
YDCcup1Δ2::TAC, YRAace1Δ::TAC, and YDCcup1Δ2::TACdubR (= TAC-TATAΔ) have been described (42). YDCcup1Δ2::TACpR (TAC with mutations in the proximal TATA box) and YDCcup1Δ2::TACdR (TAC with mutations in the distal TATA box) were constructed by transformation of YDCcup1Δ2 with circularized TAC containing the mutations, derived from derivatives of pGEM-TAC constructed using a PCR-based method (42). BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0; ATCC 4040002) and an spt10Δ mutant derived from BY4741 (ATCC 4001298) were obtained from American Type Culture Collection (4). YDCspt10Δ::TAC was derived from YDCcup1Δ2::TAC by transformation to a Ura+ phenotype with a PacI-KpnI digest of pNEB-SPT10ΔURA3A; the disruption was confirmed by Southern blotting. SPT10 was cloned as a 2,312-bp BamHI fragment by PCR from genomic DNA, from the second codon to the 3′ flanking region, inserted at the BamHI site of pNEB193 (New England Biolabs), with the 3′ end of SPT10 closest to the SacI site in the polylinker to obtain pNEB-SPT10B. The insert was sequenced. For pNEB-SPT10ΔURA3A, the 1,686-bp BstZ17I-MscI SPT10 fragment in pNEB-SPT10B was replaced with the 1,162-bp SmaI-PmeI URA3 fragment from pNEB-URA3 (42), such that URA3 and SPT10 were transcribed in the same direction. Plasmids pTAC1 to pTAC7 are subclones of fragments from the TAC insert in pGEM-TAC(+) (42), constructed as follows: pTAC1, the 423-bp HindIII-NheI fragment from the 3′ end of TRP1 inserted into pNEB193 cut with HindIII/XbaI. pTAC2, the 416-bp NheI-EcoRI fragment containing the 5′ GAL3 region inserted into pNEB193 cut with XbaI/EcoRI. pTAC3, the 469-bp EcoRI-PacI fragment containing the CUP1 open reading frame (ORF), inserted at the same sites in pNEB193. pTAC4, the 306-bp PacI-SpeI CUP1 promoter fragment inserted into pNEB193 cut with XbaI/PacI. pTAC5, the 3′ RSC30 region inserted as a 240-bp SpeI-EcoRI fragment into pNEB193 cut with EcoRI/XbaI. pTAC6, the 242-bp EcoRI-MfeI TRP1 promoter fragment inserted into pNEB193 cut with EcoRI. pTAC7, the 372-bp MfeI-HindIII fragment from the TRP1 ORF, inserted into pNEB193 cut with EcoRI/HindIII. pTAC4dubR, the 306-bp PacI-SpeI CUP1 promoter fragment with the TATA box mutations, obtained from pGEM-TACdubR (42) inserted into pNEB193 cut with XbaI/PacI.
Mung bean nuclease mapping of transcripts.
Yeast cells were grown at 30°C and shaken at 300 rpm to an absorbance of 0.5 at 600 nm. Copper(II) sulfate was added (1 mM) to one of two 50-ml aliquots of cells. After 15 min, the cells were harvested, washed in ice-cold water, resuspended in a solution containing 400 μl of 10 mM Tris-HCl (pH 8.0), 10 mM EDTA, and 0.5% sodium dodecyl sulfate and extracted with 400 μl of unbuffered phenol. The lysate was vortexed for 2 min and incubated at 65°C for 2 h. The aqueous phase was recovered and reextracted with phenol and chloroform. Sodium acetate was added to a 0.3 M concentration, and the RNA was precipitated with isopropanol. RNA concentrations were determined by absorbance at 260 nm, and RNA integrity was checked in formaldehyde-agarose gels. For mung bean nuclease mapping (3), the CUP1 probe was the 327-bp BspHI-SpeI promoter fragment labeled at the BspHI end with T4 kinase. The PGK1 probe was an internal 72-bp AccI-Fnu4HI fragment labeled at the AccI end. Eighty micrograms of RNA was precipitated with isopropanol and dissolved in a solution containing 30 μl of 80% formamide-0.4 M NaCl-40 mM piperazine N,N′-bis(2-ethanesulfonic acid)(pH 6.6). Labeled probes were added (2-μl volume). The solution was heated to 94°C for 30 min, hybridized overnight at 56°C, adjusted to 300 μl with 50 mM sodium acetate-1 mM zinc acetate, and digested with mung bean nuclease (3 μl at 10 U/μl; New England Biolabs) at 30°C for 1 h. The digestion products were purified and analyzed in a 6% acrylamide-Tris-borate-EDTA-8 M urea gel.
Chromatin immunoprecipitation.
TAC minichromosomes were purified to the stage prior to electroelution and digested to core particles using MNase exactly as described previously (42), except that 1.5 μM trichostatin A (WAKO) (added as a 10 mM solution in ethanol) was included in all buffers from the cell lysis step onwards. Typically, 45 ng of TAC minichromosomes in 100 μl of 10 mM Tris-HCl (pH 8.0)-35 mM NaCl-2.5 mM CaCl2 was digested with 2.5 U of MNase (Worthington) for 2 min at 30°C. EDTA was added to a 5 mM concentration, and the tubes were placed on ice. The samples were diluted with an equal volume of buffer: 10 mM Tris-HCl (pH 8.0), 5 mM Na-EDTA, 0.565 M NaCl, 0.2 mg of bovine serum albumin/ml, 5 μg of leupeptin/ml, 0.1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 15 μg of pepstatin A/ml, and 1.5 μM trichostatin A. The core particles were incubated with 5 μl of antibody overnight at 4°C with rotation (mock incubations were identical except for the absence of antibody). The antibodies used were directed against diacetylated H3 (lysines 9 and 14; Upstate 06-599) and hyperacetylated H4 (Upstate 06-866). One hundred microliters of a 1:1 slurry of protein A Sepharose (Amersham) in 0.3 M NaCl-1 mM Tris-HCl (pH 8.0)-0.01 mM Na-EDTA was added to the core particles and incubated for 1.5 h at 4°C with rotation. The resin was collected with a brief spin, and the supernatant was removed and retained. The resin was washed three times with 0.5 ml of 0.5 M NaCl-20 mM Tris-HCl (pH 8.0)-0.05% Triton X-100. The DNA bound to the resin was eluted by resuspension in 500 μl of 1% sodium dodecyl sulfate in 1 mM Tris-HCl (pH 8.0)-0.01 mM Na-EDTA. Potassium acetate was added to a 1 M concentration, and the supernatant was extracted with phenol-chloroform (1:1) and precipitated with ethanol in the presence of 20 μg of glycogen. Core particle DNA was purified from a 3% agarose gel (140 to 160 bp) and end labeled with T4 kinase as described previously (42). For Southern blots, 0.5 μg each of pTAC1-7 and pNEB193, all linearized with ScaI (which has a unique site in the vector), was electrophoresed in 3% agarose gels and transferred to GeneScreen Plus membranes. Hybridizations with core particle DNA were performed at 60°C. Monomer extension was as described previously (42). Northern blots were performed using formaldehyde-agarose gels (26).
RESULTS
We have examined the histone acetylation status of CUP1 in various yeast strains: wild type (WT), a strain lacking the Ace1p activator (ace1Δ), and a strain in which the TATA boxes in the CUP1 promoter were mutated (TATAΔ). These strains lacked chromosomal copies of CUP1 and instead contained TRP1ARS1CUP1 (TAC), a 2,468-bp yeast plasmid based on TRP1ARS1 with CUP1 inserted (Fig. 1A). TRP1 was used as a selection marker to maintain the plasmid, and ARS1 is a replication origin.
Inactivation of CUP1 by TATA box mutations.
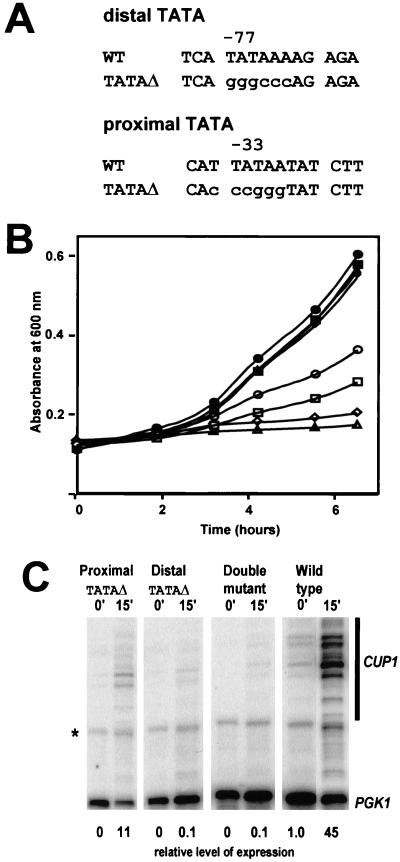
CUP1 contains two consensus TATA boxes, proximal and distal, located at −33 and −77 relative to the major upstream start site (18) (Fig. 2A). The distal TATA box is at the usual location for TATA boxes found in the genes of budding yeast; the proximal TATA box is relatively close to the start site, where TATA boxes are usually located in genes from other eukaryotes. A set of strains containing TAC with mutations in the proximal TATA box, the distal TATA box, or both TATA boxes was constructed; the TATA boxes were converted to G/C-rich sequences marked by restriction sites (Fig. 2A) (less radical mutations were less effective [data not shown]). The physiological effects of these mutations were ascertained by growth of cells in the presence of a high concentration of copper ions (1 mM) (Fig. 2B). In the absence of copper, all strains grew well and at the same rate. In the presence of copper, the WT grew more slowly, as reported previously (42). Mutation of the distal TATA box had a much more severe effect on growth in copper than mutation of the proximal TATA box, but the cells still grew, albeit very slowly. However, the combined mutations (the double mutant) were lethal. Thus, the TATA mutations effectively disabled CUP1 transcription.
FIG. 2.
Mutation of both TATA boxes inactivates the CUP1 gene. (A) Sequences of the proximal and distal TATA boxes in the CUP1 promoter, with mutations (TATAΔ) shown in lowercase. Nucleotide positions are reported with respect to the major upstream start site (18). (B) Effect of copper on growth of yeast cells in synthetic complete medium lacking tryptophan. Filled symbols, no copper; open symbols, +1 mM CuSO4; circles, WT TAC; squares, TAC (proximal TATAΔ);diamonds, TAC (distal TATAΔ); triangles, TAC-TATAΔ (double mutant). (C) Mung bean nuclease mapping of CUP1 transcripts. RNA was prepared from cells before and after induction with 1 mM CuSO4 for 15 min. A phosphorimager scan is shown (all lanes are from the same gel). Multiple closely spaced start sites were observed. CUP1 transcripts were quantified relative to PGK1. The level of expression relative to uninduced WT cells (set at 1.0) is shown at bottom. The band marked with an asterisk is derived from PGK1.
This conclusion was confirmed by quantification of CUP1 transcripts by mung bean nuclease mapping (Fig. 2C). In this case, transcripts were assayed 15 min after addition of copper and quantitatively compared using the gene for the glycolytic enzyme phosphoglycerokinase, PGK1, as internal control. In WT cells, CUP1 was rapidly induced by about 45-fold. The proximal TATA mutation significantly reduced both basal and copper-activated transcription, with an 11-fold increase in expression relative to uninduced WT cells. The distal TATA and the double TATA mutations were much more effective: CUP1 transcripts were undetectable in the absence of copper, and induction was extremely weak, corresponding to less than 1% of the transcripts synthesized in induced WT cells. The double TATA mutant (TAC-TATAΔ) was used in the experiments described below.
Targeted acetylation of H3 and H4 at the CUP1 promoter requires Ace1p and the TATA boxes.
We exploited our ability to purify TAC minichromosomes as native chromatin from yeast cells to identify nucleosomes targeted for acetylation using high-resolution mapping techniques. Briefly, nuclei were prepared from spheroplasts which had been incubated with or without copper for induction of CUP1. Nuclei were lysed, and the supernatant, containing TAC chromatin, was layered on a sucrose cushion and subjected to a high-speed spin. TAC chromatin in the cushion was washed thoroughly using a centrifugal filter. The result was a preparation of TAC chromatin containing some much larger genomic chromatin fragments but free of ribosomes (42). The effect of copper induction was assessed by comparing TAC from copper-induced WT cells with TAC from ace1Δ cells, rather than with TAC from uninduced WT cells, because the process of spheroplasting results in a partial derepression of CUP1; this is not observed in ace1Δ cells (42).
TAC minichromosomes were purified from copper-induced WT cells, uninduced and copper-induced ace1Δ cells, and copper-induced cells carrying TAC-TATAΔ, as described previously (42). Trichostatin A, a histone deacetylase inhibitor, was added to prevent deacetylation during isolation. TAC chromatin was digested to nucleosome core particles using micrococcal nuclease (MNase). The core particles were incubated with an antibody raised against diacetylated H3 (acetylated on lysine residues 9 and 14) or against hyperacetylated H4. Core particles bound by the antibody were separated from the unbound particles using protein A Sepharose followed by a series of washes. The bound core particles were eluted, and the DNA was extracted, purified from a gel (140 to 160 bp), and end labeled with T4 kinase (Fig. 3A). The fraction of core particles in the various immunoprecipitates ranged from 5 to 15% of the input core particles.
FIG. 3.
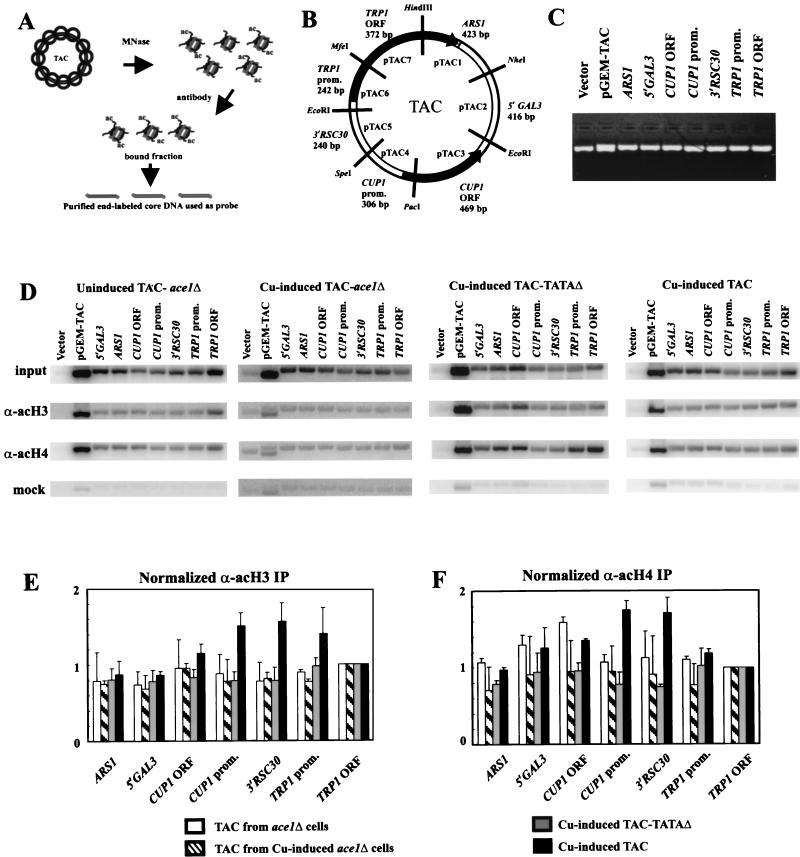
Targeted acetylation of H3 and H4 at the CUP1 promoter requires Ace1p and the TATA boxes. (A) Schematic of the TAC IP experiment. (B) Map of the TAC episome indicating the seven subcloned regions, defined by the restriction sites indicated: (i) ARS1 (also containing the 3′ end of TRP1); (ii) 5′GAL3, the upstream region of GAL3; (iii) CUP1 ORF; (iv) CUP1 promoter; (v) 3′ RSC30; (vi) TRP1 promoter; and (vii) TRP1 ORF. The size of each fragment is indicated. (C) Example of a gel used for Southern blotting, stained with ethidium bromide. Vector, plasmid with no insert. pGEM-TAC, a HindIII digest of this plasmid (bands corresponding to the TAC insert and the vector). Equal amounts of linearized plasmid were loaded. (D) Hybridization of labeled core particle DNA from purified TAC minichromosomes to Southern blots of gels like that shown in panel C. TAC minichromosomes were purified from ace1Δ cells (with or without copper induction) and copper-induced WT cells; TAC-TATAΔ minichromosomes were purified from copper-induced cells. TAC chromatin was digested to core particles for use in IP experiments. Labeled core DNAs derived from input core particles, core particles immunoprecipitated with the anti-acetylated H3 antibody or the anti-hyperacetylated H4 antibody, and mock immunoprecipitates (no antibody) were used as probes. Phosphorimages are shown. For TAC-TATAΔ blots, pTAC4dubR was used instead of pTAC4 (pTAC4dubR is the same as pTAC4, except that it carries the TATA box mutations). (E and F) Quantitative analysis of IP data in panel D. Panel E shows acetylated H3; panel F shows hyperacetylated H4. TAC from uninduced (white bars) and copper-induced (striped bars) ace1Δ cells, TAC-TATAΔ from copper-induced cells (grey bars), and TAC from copper-induced WT cells (black bars) are analyzed. For each data set, the bands were quantified by a phosphorimager and normalized to the TRP1 ORF, which was set equal to 1. These numbers were then divided by the input values normalized identically. Error bars represent the standard error for at least three independent experiments for H3 and two independent experiments for H4.
The distribution of acetylated nucleosomes in TAC chromatin was determined using the method of Clark and Felsenfeld (8). Labeled core particle DNA derived from the input or the immunoprecipitate (IP) was used as a probe of Southern blots of various plasmids containing different regions of TAC and quantified using a phosphorimager. A set of seven subclones of biologically relevant regions of TAC was used (pTAC1-7) (Fig. 3B); these were the following: ARS1 plus the 3′ end of TRP1, the upstream region from GAL3, the CUP1 ORF, the CUP1 promoter, the 3′ flank of RSC30 (the gene neighboring CUP1 in the yeast genome [2]), the TRP1 promoter, and most of the ORF of TRP1. Subcloned plasmids were used instead of restriction digests of TAC to avoid problems with size-dependent binding of DNA to the membrane. An example of a gel used for blotting is shown (Fig. 3C). Also included were a vector control for specific hybridization and a HindIII digest of pGEM-TAC (the parent plasmid for TAC), which yields a vector band and a complete TAC band, to give a total signal.
The input signals for the seven regions of TAC differed from region to region (Fig. 3D). This was mostly because they were of different lengths (ranging from 240 to 469 bp), as the signals were much more similar after normalization to insert size, ranging from 80 to 120% of the signal for the TRP1 ORF (not shown). This indicated that nucleosomes were quite evenly distributed in TAC chromatin. The input signals for different TAC preparations were similar but not identical (Fig. 3D), indicating some minor differences in nucleosome density in TAC chromatin from different sources. To compare different TAC samples, each data set was normalized to the signal for the coding region of TRP1 (pTAC7). This assumed that the acetylation status of the TRP1 ORF was invariant for TAC in all strains (TRP1 was used as a selection marker and was active in all strains). The distributions of diacetylated H3 (Fig. 3E) and hyperacetylated H4 (Fig. 3F) in TAC were determined by dividing the normalized IP signals by the normalized input signals for the same sample. This corrected for insert size and nucleosome density, allowing a direct comparison of the relative acetylation of H3 and H4 in nucleosomes in different regions of TAC.
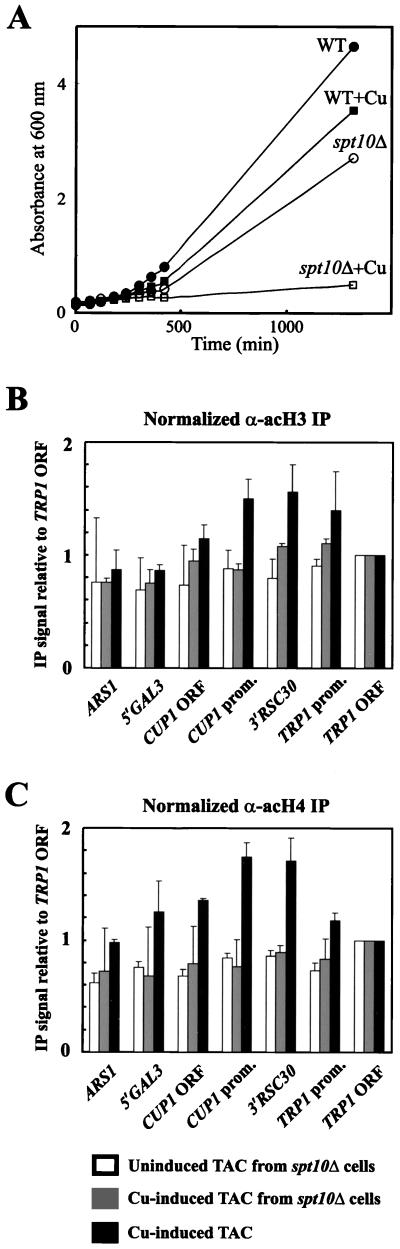
Strong signals (relative to the no-antibody mock incubations) were obtained for all seven regions of TAC, indicating that all regions were significantly acetylated on H3; this background represents global acetylation (49). However, the signals for the copper-induced CUP1 promoter and the 3′ RSC30 region were enhanced relative to the signals from the rest of TAC (Fig. 3E), indicating that H3 acetylation was targeted to the CUP1 promoter and the neighboring 3′ RSC30 region. The increases in acetylation on the CUP1 ORF and the TRP1 promoter were not significant compared with TAC from ace1Δ cells. Targeted acetylation was not observed in TAC chromatin from ace1Δ cells or in TAC-TATAΔ chromatin, indicating that both Ace1p and the TATA boxes were required for targeted acetylation. Activation of CUP1 was accompanied by an approximately twofold increase in H3 acetylation at both the CUP1 promoter and the region immediately upstream (3′ RSC30), relative to inactive TAC chromatin (TAC-TATAΔ or TAC from ace1Δ cells). The 3′ flank of RSC30 is not required for CUP1 function.
The results were essentially the same for hyperacetylated H4: induction resulted in targeted acetylation of H4 in nucleosomes on the CUP1 promoter and in the 3′ RSC30 region. Intriguingly, unlike H3, H4 on the CUP1 ORF was more acetylated in copper-induced TAC and uninduced TAC from ace1Δ cells, although not in TAC-TATAΔ; the significance of this is unclear. It is concluded that targeted acetylation of H3 and H4 required the presence of the Ace1p activator and the CUP1 TATA boxes.
The acetylated H3 IP is enriched in nucleosomes containing CUP1 upstream activating sequences and the 3′ flank of RSC30.
The region of CUP1 that displayed increased H3 and H4 acetylation was confined to the CUP1 promoter (306 bp) and the 3′ RSC30 region (another 240 bp, totaling 546 bp). It did not spread significantly into the CUP1 ORF. We proceeded to determine exactly which positioned nucleosomes were targeted for acetylation, using the monomer extension method to map the positions of nucleosomes in the acetylated H3 IP.
The monomer extension technique (56) was discussed in detail previously (42). Briefly, the chromatin is completely digested with MNase to nucleosome core particles (exactly as in the IP experiment above). Core particle DNA (140 to 160 bp) is purified from a gel, end labeled with kinase, and used as primer in a primer extension reaction using Klenow enzyme and single-stranded TAC DNA as template. Single-stranded DNA is used to ensure that only one of the two strands of core DNA can prime. The extension products are cleaved with a restriction enzyme having a unique site in TAC, and the resulting molecules are resolved in a sequencing gel. The length of the extension product is equal to the distance of the farthest nucleosome border to the restriction site. Thus, complex chromatin structures can be mapped without ambiguity. A complication in the analysis is that the extent of exonucleolytic trimming of core particles by MNase is variable (yielding particles containing 147 to 160 bp); therefore, clusters of bands within about 20 bp of one another are assumed to derive from the same nucleosome. A summary of monomer extension analysis of TAC chromatin is shown in Fig. 1B.
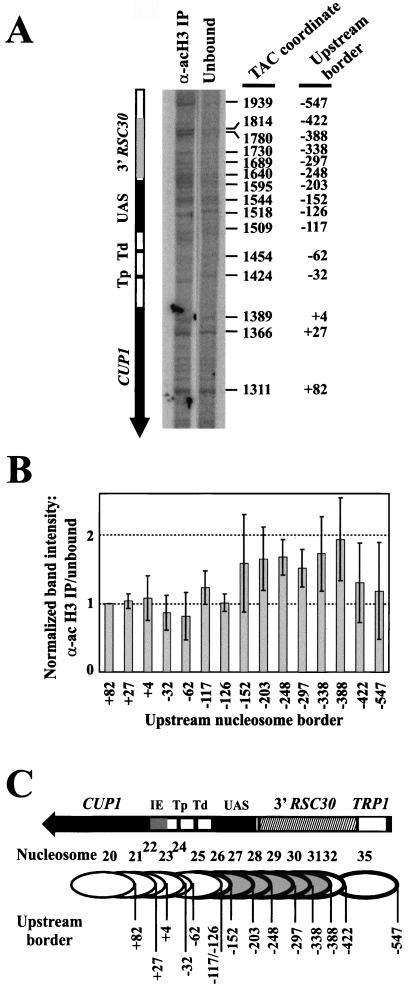
Nucleosome core particles were sorted into unbound and bound fractions using the anti-acetylated H3 antibody, and labeled core particle DNA was prepared. The amounts of core DNA recovered from the IP were small but sufficient. Monomer extension reactions were designed to resolve the chromatin structure of the CUP1 promoter region (using SspI, which has a unique site near the 3′ end of CUP1) (Fig. 1A). For quantitative comparison of the signals from immunoprecipitated and unbound core particles (Fig. 4A ), it was necessary to normalize to a control band. For this, the band at +82 was chosen, corresponding to a nucleosome on the CUP1 ORF and including only about 10 bp of the CUP1 promoter region, as defined in Fig. 3B. There were some clear quantitative differences between the IP and the unbound fraction (Fig. 4A); some nucleosomes were enriched in the IP. A quantitative analysis of four independent experiments is presented in Fig. 4B. All major bands in each track were normalized to the band at +82. The IP and the unbound fraction were compared by calculating the ratio of each normalized IP band to its counterpart in the unbound fraction. A ratio of 1.0 indicated no enrichment in the IP. Nucleosomes with upstream borders at +27, +4, −32, −62, −117, and −126 showed no enrichment in the IP. However, nucleosomes at −203, −248, −297, −338, and −388 were all significantly enriched in the IP, by about 1.5-fold. It was unclear whether the nucleosomes at −152, −422, and −547 were enriched as the standard errors were too large, reflecting significant variability for these three nucleosomes from experiment to experiment. In this regard, it is worth noting that these nucleosomes are located at the boundaries of the targeted region (Fig. 4C). The enrichments were modest but consistent with the signals observed in the quantitative Southern blot experiment (Fig. 3).
FIG. 4.
Nucleosomes containing CUP1 UASs and sequences farther upstream are the targets for H3 acetylation. (A) Identification of positioned nucleosomes enriched in the acetylated H3 IP obtained with copper-induced TAC from WT cells: monomer extension of nucleosomes in the IP with those in the unbound fraction. A phosphorimage is shown (the exposures of the two lanes are slightly different tofacilitate comparison). The size of each band equals the distance of the farthest border of a positioned nucleosome from the unique SspI site. The coordinates of each border with respect to the HindIII site in TRP1 are given at right (42), and the coordinates with respect to the major upstream start site of CUP1 (+1) are given at far right. Tp, proximal TATA box; Td, distal TATA box. (B) Quantitative analysis of nucleosomes in the acetylated H3 IP (data in panel A). The intensities of the bands in each monomer extension track were normalized to the band at +82 (in the CUP1 ORF), and the ratio of the normalized band in the IP to the normalized band in the unbound fraction was calculated for each nucleosome. (A ratio of 1.0 indicates that this nucleosome is not enriched in the IP.) Error bars represent the standard error for data from four independent experiments. Coordinates are given with respect to the major upstream start site (+1). (C) Schematic showing nucleosomes targeted for H3 acetylation. Nucleosomes are numbered as in Fig. 1B; upstream borders are indicated. Nucleosomes enriched in the IP are shown shaded. The bands at −117 and −126 both derive from nucleosome 25. Nucleosomes 33 and 34 were quantitatively minor and are not included in the analysis.
These observations are summarized in Fig. 4C. The most downstream nucleosome enriched in the IP has an upstream border at −203, corresponding to a downstream border at about −56, including the distal TATA box but not the proximal one (nucleosome 27). The most upstream nucleosome enriched in the IP had an upstream border at −388, corresponding to a downstream border at about −241, and was entirely within the 3′ RSC30 region (nucleosome 31). These borders for the acetylated region are consistent with the observations presented in Fig. 3, indicating that the CUP1 promoter and the 3′ RSC30 are the target regions.
Inspection of the distribution of nucleosomes targeted for H3 acetylation (Fig. 4C) revealed the following: (i) Nucleosomes containing the initiation element were not targeted. (ii) Of the nucleosomes covering one or both TATA boxes (nucleosomes 23 to 27), only nucleosome 27 was clearly targeted (it contains the distal TATA box and four of the five Ace1p binding sites). (iii) Of the nucleosomes covering Ace1p binding sites (located between −108 and −220; nucleosomes 25 to 30), most were targets for H3 acetylation (nucleosomes 27 to 30). In conclusion, the region targeted for H3 acetylation extended from about −56 to about −388 and appears to be focused on the Ace1p binding sites and the region just upstream (the 3′ flank of RSC30).
Targeted acetylation at the CUP1 promoter requires the SPT10 gene product.
Our initial attempts to identify HAT activities acting at CUP1 involved construction of null mutants in known yeast HAT genes, which were tested for growth defects in the presence of copper. Strains carrying gcn5Δ, sas2Δ, or sas3Δ mutations did not exhibit growth defects in copper (not shown). There is evidence in the literature that the HATs yTAF130/145 (27, 31), Gcn5p (19), and Esa1p (38) do not regulate CUP1. Therefore, null strains for less-well-characterized HATs were obtained from the yeast deletion project (4) and tested for growth defects in copper. One of the strains tested was null for SPT10, a gene encoding a putative HAT, identified by homology with the HAT domain of Gcn5p (34). The WT strain (BY4741) was highly resistant to copper, with just a small reduction in the growth rate in 1 mM copper (Fig. 5A). The degree of copper resistance is strain dependent and is determined by the number of CUP1 genes present (the chromosomal CUP1 locus is amplified in highly resistant strains [18]). We confirmed that BY4741 and its spt10Δ mutant contained the same number of chromosomal copies of CUP1 by Southern blot (not shown). The growth of the spt10Δ mutant in the absence of copper was much slower than that of the WT, as reported previously (32). In the presence of 1 mM copper, spt10Δ cells grew extremely slowly (Fig. 5A). A detailed analysis of the copper sensitivity of spt10Δ cells indicated that 1.25 mM copper was lethal (not shown, but see Fig. 5B).
FIG. 5.
SPT10 is required for maximal induction of CUP1 and survival at high copper concentrations. (A) Effect of copper on growth of WT (filled symbols) and spt10Δ (open symbols) BY4741 cells in synthetic complete medium with (squares) or without (circles) 1 mM CuSO4. Growth was measured by absorbance at 600 nm. (B) Time course of copper induction: effect of copper on growth of WT (filled squares) and spt10Δ (open squares) BY4741 cells in synthetic complete medium inoculated with 1.25 mM CuSO4 at time zero. (C) Induction of CUP1 mRNA during growth shown in panel B. Phosphorimages of Northern blots of RNA purified from WT and spt10Δ cells probed for CUP1 or ACT1 mRNA. Total RNA loadings were compared by staining of an agarose gel with ethidium bromide. (D) Quantification of Northern blots in panel C: filled squares indicate CUP1 mRNA in WT cells and open squares indicate CUP1 mRNA in spt10Δ cells after normalization to total RNA.
The effect of the spt10Δ mutation on induction of CUP1 mRNA was determined by Northern blot analysis (Fig. 5). WT and spt10Δ cells were grown to an optical density at 600 nm of about 0.3, and then copper was added to a concentration of 1.25 mM; this halted the growth of the spt10Δ cells quite rapidly (Fig. 5B). RNA was prepared at various times after induction, and Northern blots were probed for CUP1 and ACT1 mRNAs (Fig. 5C). In spt10Δ cells, CUP1 mRNA was induced by copper but accumulated relatively slowly, reaching a maximum level equal to only about half that in WT cells (Fig. 5D). (ACT1 mRNA in spt10Δ cells slowly decreased with time, consistent with cell death, and so could not be used for normalization; total RNA was used instead.) Thus, SPT10 was required for rapid and maximal induction of CUP1. Loss of SPT10 function was lethal at high copper concentrations, presumably because insufficient CUP1 mRNA was produced to direct synthesis of enough metallothionein to chelate all the copper ions entering the cell. The spt10Δ mutation is phenotypically equivalent to a reduction in CUP1 gene dosage (since strains with fewer copies of CUP1 are more sensitive to copper [18]).
To determine whether targeted acetylation at the CUP1 promoter was affected in spt10Δ cells, a TAC strain carrying the spt10Δ mutation was constructed. This strain also showed a severe growth defect in 1 mM copper (Fig. 6A ). TAC chromatin was purified from uninduced and copper-induced spt10Δ cells and subjected to IP analysis (Fig. 6B), as described above (Fig. 3). Targeted acetylation of H3 and H4 was not observed in TAC chromatin purified from uninduced or copper-induced spt10Δ cells (Fig. 6B and C). It is concluded that targeted acetylation of both H3 and H4 at the CUP1 promoter required the product of the SPT10 gene.
FIG. 6.
SPT10 is required for targeted acetylation of both H3 and H4 at the CUP1 promoter. (A) Effect of copper on growth of WT (filled symbols) and spt10Δ (open symbols) cells containing TAC in synthetic complete medium lacking tryptophan, with (squares) or without (circles) 1 mM CuSO4. Growth was measured by absorbance at 600 nm. Quantitative analysis of IP data is shown for acetylated H3 (B) and hyperacetylated H4 (C). Data for TAC from uninduced (open bars) and copper-induced (grey bars) spt10Δ cells are compared with datafrom Fig. 3 for TAC from copper-induced cells (black bars). Error bars for spt10Δ data represent the standard error for two (uninduced) or three (induced) independent experiments.
DISCUSSION
Targeted acetylation of H3 and H4 at the CUP1 promoter.
We have demonstrated that targeted acetylation of H3 and H4 occurs at the CUP1 promoter in response to copper induction. The increase in acetylation observed was modest, as is usually the case for genes in budding yeast (e.g., see references 19 and 22), probably because the average nucleosome in yeast is already 50% acetylated (50). It is important to note that acetylation of the rest of TAC chromatin was significant and that the targeted acetylation observed at the CUP1 promoter was over and above background or “global” acetylation (49). Targeted acetylation of H3 occurred primarily in nucleosomes containing binding sites for Ace1p (UASs) and the region just upstream of the CUP1 promoter, the 3′ flank of RSC30. Most of the nucleosomes positioned over one or both of the TATA boxes were not targeted for H3 acetylation. Targeted acetylation of H3 and H4 required the presence of the transcriptional activator, Ace1p, the TATA boxes, and the product of the SPT10 gene.
Targeted acetylation was preserved during purification of induced CUP1 in the form of TAC minichromosomes, enabling us to map the targeted nucleosomes at very high resolution. However, the acetylation pattern might have been altered during purification despite the presence of trichostatin A. If occurring, this is unlikely to affect the basic observation, because Krebs et al. (19) also observed increased acetylation of H3 on induction of CUP1, using formaldehyde cross-linked cells. However, Deckert and Struhl (11) observed no change for H3 and a decrease in acetylation of H4 using the formaldehyde method. We do not understand why the results of Deckert and Struhl differ from those reported here and by Krebs et al. (19). Perhaps the dynamics of histone acetylation during CUP1 induction are important: CUP1 mRNA is rapidly induced and then slowly decreases as cells adapt to copper (36). If the acetylated state is considered as a transient chromatin intermediate (as for the PHO8 gene [39]), the fraction of acetylated nucleosomes at the promoter might vary considerably during the induction period.
Targeted acetylation at CUP1 requires the product of the SPT10 gene.
To identify the acetylase acting at the CUP1 promoter, a number of mutants in known HAT genes were screened for growth defects in copper. Of these, only the spt10Δ mutant showed a severe defect. At high copper concentrations, when CUP1 is presumably maximally expressed, spt10Δ cells exhibited slower induction of CUP1 and produced only about half as much CUP1 mRNA. The result was cell death, presumably because insufficient metallothionein could be produced to bind all the copper ions entering the cell. These observations demonstrate that Spt10p is required for full induction of CUP1.
SPT10 has been isolated independently in screens for mutations which activate core promoters (i.e., promoters lacking UAS sequences) for transcription (12, 37, 55). In one study (55), the CUP1 core promoter was tested. These studies suggested that Spt10p is a global transcriptional regulator which plays a negative role at or near the TATA box. This fits well with our observation that the TATA boxes are required for acetylation at CUP1. However, we have demonstrated an activating role for Spt10p at CUP1. Perhaps Spt10p cannot activate in the absence of a UAS. In any case, an activating as well as a repressing regulatory role for SPT10 is indicated by the observation (32) that spt10 mutations resulted in increased expression of some genes under repressing conditions (which we did not observe for CUP1) but decreased levels of expression under fully induced conditions (which we did observe for CUP1).
Remodeling of CUP1 chromatin: nucleosome repositioning and targeted acetylation.
Induction of CUP1 results in a gene-wide repositioning of nucleosomes (42) (Fig. 1B). This repositioning extends to upstream and downstream flanking regions and requires Ace1p but not the TATA boxes. Since acetylation of H3 and H4 is local (i.e., restricted to the CUP1 promoter region) and does require the TATA boxes, it may be concluded that repositioning is independent of targeted acetylation. Thus, the creation of a dynamic chromatin structure for CUP1, in which nucleosomes are moved around, can occur in the absence of targeted acetylation. The fact that targeted acetylation at CUP1 is dependent on the TATA boxes suggests that it is a relatively late event in gene activation, occurring at the moment when TBP binds to the TATA box during the formation of a preinitiation complex or at a later stage. (It is possible that TBP is recruited to the promoter even in the absence of the TATA boxes and that acetylation is triggered only when it binds to the TATA boxes.) This has important implications for the role of targeted acetylation in initiation at CUP1, because many of the key events in initiation have already occurred: binding of the activator, movement of nucleosomes, and, possibly, the formation of the preinitiation complex.
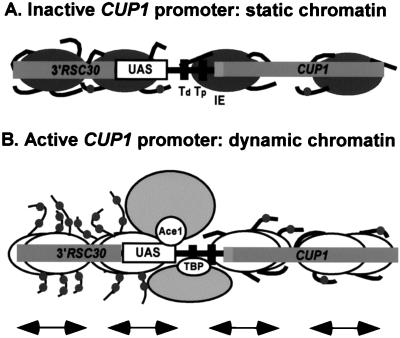
Our observations are summarized in the model shown in Fig. 7. Four immobile nucleosomes are shown on the inactive CUP1 promoter with nucleosomes acetylated at background levels (Fig. 7A). The first step in activation is the binding of copper-activated Ace1p. There are five Ace1p binding sites in the CUP1 promoter, which might or might not be present in a nucleosome, depending on which position is occupied (only one major position blocks all five Ace1p binding sites). Sites in the linker might also be blocked by the binding of core histone tail domains (1). Most transcription factors bind poorly to nucleosomes, although there are some exceptions (52). We have evidence that the binding of Ace1p to nucleosomal sites is strongly inhibited (unpublished data). Another factor is the possible cooperative binding of multiple molecules of Ace1p, which might be sufficient to overwhelm the nucleosome, as shown in vitro for other factors (46).
FIG. 7.
A model for chromatin remodeling events occurring during induction of CUP1. Only the CUP1 promoter region of TAC is shown, with nucleosomes drawn approximately to scale. Nucleosomes can occupy any of several possible positions in vivo (Fig. 1B); four are shown here. (A) Inactive CUP1: the nucleosomes are immobile and the chromatin structure is static. The histone tail domains are mostly bound to nucleosomal DNA or linker DNA and are acetylated at background (global) levels. (B) Active CUP1: copper-activated Ace1p locates a binding site in the UAS region. A remodeling activity is recruited, and nucleosomes begin to slide back and forth, creating a dynamic chromatin structure over the entire gene. TBP with its associated factors binds to one of the two TATA boxes at a moment when it is nucleosome free. A HAT activity (probably Spt10p) is activated and acetylates H3 and H4 in nucleosomes upstream of the TATA box.
After the binding of Ace1p, or concomitant with it, it is proposed that a remodeling complex recruited by Ace1p creates a dynamic chromatin structure for the entire gene, in which nucleosomes are moved continuously between positions (Fig. 7B) (42). This process should render the underlying DNA sequence essentially transparent to binding factors. Since it has been shown by chromatin IP that TBP is detectable at the CUP1 promoter only after addition of copper (23, 28), it is likely that the next step is the binding of TBP with its associated factors to one of the TATA boxes at a moment when it is nucleosome free. Finally, the HAT activity (presumably Spt10p), which might be associated with TBP or might be recruited at an even later stage in initiation, acetylates nucleosomes upstream of the TATA boxes.
What might be the function of targeted acetylation at CUP1, given that it is focused on nucleosomes containing UASs? Although the initial binding of Ace1p occurs in the absence of targeted acetylation, the function of acetylation might still be to facilitate binding of Ace1p to nucleosomal sites, if Ace1p undergoes a cycle of dissociation and rebinding. The first round of transcription would be relatively slow because the histone tails inhibit binding of Ace1p, but subsequent rounds would be faster due to the removal of this impediment. Some transcription factors exhibit enhanced binding to hyperacetylated nucleosomes in vitro (25, 35, 47), as does TBP (40). We have evidence that this is also true of Ace1p (unpublished data). Alternatively, the acetylated histone tails might constitute a signal for the recruitment of additional proteins required for transcription (44).
Multiple mechanisms for remodeling chromatin.
It is instructive to contrast events occurring at CUP1 with those reported for other genes. Detailed information is available on both chromatin structural changes and histone acetylation for relatively few genes, notably PHO5 (45), PHO8 (39), and the β-interferon gene (30), which are dependent to various extents on Gcn5p and Swi/Snf activities. Induction of both PHO5 and PHO8 involves the disruption or displacement of four positioned nucleosomes on the promoter. In the case of PHO8, this process requires the activator, which recruits the Gcn5p-containing SAGA complex, resulting in transient, targeted acetylation of H3 and H4. The Swi/Snf complex is then recruited and completes the remodeling of promoter chromatin (39). Events at the β-interferon promoter occur in a similar order (30). However, at the HO gene, Swi/Snf is recruited first and then SAGA (9), suggesting that different remodeling mechanisms are possible even with the same acetylase and remodeling complex.
Acetylation at the PHO8 promoter is targeted to the four nucleosomes that undergo remodeling (39). Targeted acetylation at HIS3 extends upstream from the promoter into the neighboring PET56 promoter (21). Thus, for both CUP1 and HIS3, nucleosomes over upstream sequences having no known role in regulation are targeted for acetylation. Unlike CUP1, though, targeted acetylation of H3 at the HIS3 promoter does not require the TATA box (21). The boundaries of targeted acetylation at the HO promoter coincide with its unusually extensive control region (about 1 kb); the entire region is targeted for acetylation in a cell cycle-dependent manner (20). Thus, CUP1 is an example of a novel chromatin remodeling mechanism, since, unlike HIS3, targeted acetylation requires the TATA boxes and, unlike PHO8, remodeling is not confined to the promoter region and occurs in the absence of targeted acetylation. This probably reflects the involvement of a different acetylase and a different remodeling complex at CUP1.
Acknowledgments
We thank Bryan Turner and Jayne Lavender for providing us with antibodies in the early part of this work. We thank Ann Dean, Jurrien Dean, Rohinton Kamakaka, Vasily Studitsky, and Fred Winston for valuable comments on the manuscript.
REFERENCES
- 1.Angelov, D., J. M. Vitolo, V. Mutskov, S. Dimitrov, and J. J. Hayes. 2001. Preferential interaction of the core histone tail domains with linker DNA. Proc. Natl. Acad. Sci. USA 98:6599-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus-Hill, M. L., A. Schlichter, D. Roberts, H. Erdjument-Bromage, P. Tempst, and B. R. Cairns. 2001. A Rsc3/Rsc30 zinc cluster dimer reveals novel roles for the chromatin remodeler RSC in gene expression and cell cycle control. Mol. Cell 7:741-751. [DOI] [PubMed] [Google Scholar]
- 3.Berk, A. J., and P. A. Sharp. 1977. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease digest hybrids. Cell 12:721-732. [DOI] [PubMed] [Google Scholar]
- 4.Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115-132. [DOI] [PubMed] [Google Scholar]
- 5.Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843-851. [DOI] [PubMed] [Google Scholar]
- 6.Buchman, C., P. Skroch, J. Welch, S. Fogel, and M. Karin. 1989. The CUP2 gene product, regulator of yeast metallothionein expression, is a copper-activated DNA-binding protein. Mol. Cell. Biol. 9:4091-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butt, T. R., E. J. Sternberg, J. A. Gorman, P. Clark, D. Hamer, M. Rosenberg, and S. T. Crooke. 1984. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc. Natl. Acad. Sci. USA 81:3332-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, D. J., and G. Felsenfeld. 1992. A nucleosome core is transferred out of the path of a transcribing polymerase. Cell 71:11-22. [DOI] [PubMed] [Google Scholar]
- 9.Cosma, M. P., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97:299-311. [DOI] [PubMed] [Google Scholar]
- 10.Culotta, V. C., T. Hsu, S. Hu, P. Fürst, and D. Hamer. 1989. Copper and the ACE1 regulatory protein reversibly induce yeast metallothionein transcription in a mouse extract. Proc. Natl. Acad. Sci. USA 86:8377-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deckert, J., and K. Struhl. 2001. Histone acetylation at promoters is differentially affected by different activators and repressors. Mol. Cell. Biol. 21:2726-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denis, C. L., and T. Malvar. 1990. The CCR4 gene from Saccharomyces cerevisiae is required for both nonfermentative and spt-mediated gene expression. Genetics 124:283-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dollard, C., S. L. Ricupero-Hovasse, G. Natsoulis, J. D. Boeke, and F. Winston. 1994. SPT10 and SPT21 are required for transcription of particular histone genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 14:5223-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fassler, J. S., and F. Winston. 1988. Isolation and analysis of a novel class of suppressor of Ty insertion mutations in Saccharomyces cerevisiae. Genetics 118:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fürst, P., S. Hu, R. Hackett, and D. Hamer. 1988. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell 55:705-717. [DOI] [PubMed] [Google Scholar]
- 16.Grant, P. A., D. Schieltz, M. G. Pray-Grant, D. J. Steger, J. C. Reese, J. R. Yates, and J. L. Workman. 1998. A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell 94:45-53. [DOI] [PubMed] [Google Scholar]
- 17.Gregory, P. D., K. Wagner, and W. Hörz. 2001. Histone acetylation and chromatin remodeling. Exp. Cell Res. 265:195-202. [DOI] [PubMed] [Google Scholar]
- 18.Karin, M., R. Najarian, A. Haslinger, A. P. Valenzuela, J. Welch, and S. Fogel. 1984. Primary structure and transcription of an amplified genetic locus: the CUP1 locus of yeast. Proc. Natl. Acad. Sci. USA 81:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs, J. E., C. J. Fry, M. L. Samuels, and C. L. Peterson. 2000. Global role for chromatin remodeling enzymes in mitotic gene expression. Cell 102:587-598. [DOI] [PubMed] [Google Scholar]
- 20.Krebs, J. E., M.-H. Kuo, C. D. Allis, and C. L. Peterson. 1999. Cell cycle-regulated histone acetylation required for expression of the yeast HO gene. Genes Dev. 13:1412-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo, M., E. vom Bauer, K. Struhl, and C. D. Allis. 2000. Gcn4 activator targets Gcn5 histone acetyltransferase to specific promoters independently of transcription. Mol. Cell 6:1309-1320. [DOI] [PubMed] [Google Scholar]
- 22.Kuo, M., J. Zhou, P. Jambeck, M. E. A. Churchill, and C. D. Allis. 1998. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 12:627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuras, L., and K. Struhl. 1999. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399:609-613. [DOI] [PubMed] [Google Scholar]
- 24.Leblanc, B. P., C. J. Benham, and D. J. Clark. 2000. An initiation element in the yeast CUP1 promoter is recognized by RNA polymerase II in the absence of TATA box-binding protein if the DNA is negatively supercoiled. Proc. Natl. Acad. Sci. USA 97:10745-10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, D. Y., J. J. Hayes, D. Pruss, and A. P. Wolffe. 1993. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell 72:73-84. [DOI] [PubMed] [Google Scholar]
- 26.Lehrach, H., D. Diamond, J. M. Wozney, and H. Boedtker. 1977. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry 16:4743-4751. [DOI] [PubMed] [Google Scholar]
- 27.Li, X., S. R. Bhaumik, and M. R. Green. 2000. Distinct classes of yeast promoters revealed by differential TAF recruitment. Science 288:1242-1244. [DOI] [PubMed] [Google Scholar]
- 28.Li, X., A. Virbasius, X. Zhu, and M. R. Green. 1999. Enhancement of TBP binding by activators and general transcription factors. Nature 399:605-609. [DOI] [PubMed] [Google Scholar]
- 29.Liu, X., and D. J. Thiele. 1996. Oxidative stress induces heat shock factor phosphorylation and HSF-dependent activation of yeast metallothionein gene transcription. Genes Dev. 10:592-603. [DOI] [PubMed] [Google Scholar]
- 30.Lomvardas, S., and D. Thanos. 2001. Nucleosome sliding via TBP DNA binding in vivo. Cell 106:685-696. [DOI] [PubMed] [Google Scholar]
- 31.Moqtaderi, Z., Y. Bai, D. Poon, P. A. Weil, and K. Struhl. 1996. TBP-associated factors are not generally required for transcriptional activation in yeast. Nature 383:188-191. [DOI] [PubMed] [Google Scholar]
- 32.Natsoulis, G., C. Dollard, F. Winston, and J. D. Boeke. 1991. The products of the SPT10 and SPT21 genes of Saccharomyces cerevisiae increase the amplitude of transcriptional regulation at a large number of unlinked loci. New Biol. 3:1249-1259. [PubMed] [Google Scholar]
- 33.Natsoulis, G., F. Winston, and J. D. Boeke. 1994. The SPT10 and SPT21 genes of Saccharomyces cerevisiae. Genetics 136:93-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuwald, A. F., and D. Landsman. 1997. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 22:154-155. [DOI] [PubMed] [Google Scholar]
- 35.Ng, K. W., P. Ridgway, D. R. Cohen, and D. J. Tremethick. 1997. The binding of a Fos/Jun heterodimer can completely disrupt the structure of a nucleosome. EMBO J. 16:2072-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peña, M. M. O., K. A. Koch, and D. J. Thiele. 1998. Dynamic regulation of copper uptake and detoxification genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:2514-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prelich, G., and F. Winston. 1993. Mutations that suppress the deletion of an upstream activating sequence in yeast: involvement of a protein kinase and histone H3 in repressing transcription in vivo. Genetics 135:665-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid, J. L., V. R. Iyer, P. O. Brown, and K. Struhl. 2000. Coordinate regulation of yeast ribosomal protein genes is associated with targeted recruitment of Esa1 histone acetylase. Mol. Cell 6:1297-1307. [DOI] [PubMed] [Google Scholar]
- 39.Reinke, H., P. D. Gregory, and W. Hörz. 2001. A transient histone hyperacetylation signal marks nucleosomes for remodeling at the PHO8 promoter in vivo. Mol. Cell 7:529-538. [DOI] [PubMed] [Google Scholar]
- 40.Sewack, G. F., T. W. Ellis, and U. Hansen. 2001. Binding of TATA binding protein to a naturally positioned nucleosome is facilitated by histone acetylation. Mol. Cell. Biol. 21:1404-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen, C.-H., and D. J. Clark. 2001. DNA sequence plays a major role in determining nucleosome positions in yeast CUP1 chromatin. J. Biol. Chem. 276:35209-35216. [DOI] [PubMed] [Google Scholar]
- 42.Shen, C.-H., B. P. Leblanc, J. A. Alfieri, and D. J. Clark. 2001. Remodeling of yeast CUP1 chromatin involves activator-dependent repositioning of nucleosomes over the entire gene and flanking sequences. Mol. Cell. Biol. 21:534-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 45.Svaren, J., and W. Hörz. 1997. Transcription factors vs. nucleosomes: regulation of the PHO5 promoter in yeast. Trends Biochem. Sci. 22:93-97. [DOI] [PubMed] [Google Scholar]
- 46.Utley, R. T., J. Côté, T. Owen-Hughes, and J. L. Workman. 1997. SWI/SNF stimulates the formation of disparate activator-nucleosome complexes but is partially redundant with cooperative binding. J. Biol. Chem. 272:12642-12649. [DOI] [PubMed] [Google Scholar]
- 47.Vettese-Dadey, M., P. A. Grant, T. R. Hebbes, C. Crane-Robinson, C. D. Allis, and J. L. Workman. 1996. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. EMBO J. 15:2508-2518. [PMC free article] [PubMed] [Google Scholar]
- 48.Vignali, M., A. H. Hassan, K. E. Neely, and J. L. Workman. 2000. ATP-dependent chromatin remodeling complexes. Mol. Cell. Biol. 20:1899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogelauer, M., J. Wu, N. Suka, and M. Grunstein. 2000. Global histone acetylation and deacetylation in yeast. Nature 408:495-498. [DOI] [PubMed] [Google Scholar]
- 50.Waterborg, J. H. 2000. Steady-state levels of histone acetylation in Saccharomyces cerevisiae. J. Biol. Chem. 275:13007-13011. [DOI] [PubMed] [Google Scholar]
- 51.Winston, F., and P. Sudarsanam. 1998. The SAGA of Spt proteins and transcriptional analysis in yeast: past, present and future. Cold Spring Harb. Symp. Quant. Biol. 63:553-561. [DOI] [PubMed] [Google Scholar]
- 52.Wolffe, A. P. 1997. Sinful repression. Nature 387:16-17. [DOI] [PubMed] [Google Scholar]
- 53.Wolffe, A. P. 1998. Chromatin: structure and function, 3rd ed. Academic Press, London, United Kingdom.
- 54.Wu, J., and M. Grunstein. 2000. 25 years after the nucleosome model: chromatin modifications. Trends Biochem. Sci. 25:619-623. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita, I. 1993. Isolation and characterization of the SUD1 gene, which encodes a global repressor of core promoter activity in Saccharomyces cerevisiae. Mol. Gen. Genet. 241:616-626. [DOI] [PubMed] [Google Scholar]
- 56.Yenidunya, A., C. Davey, D. J Clark, G. Felsenfeld, and J. Allan. 1994. Nucleosome positioning on chicken and human globin gene promoters in vitro. Novel mapping techniques. J. Mol. Biol. 237:401-414. [DOI] [PubMed] [Google Scholar]