Abstract
Objective:
To report the first 5-year overall survival results in patients with colorectal carcinoma metastatic to the liver who have undergone hepatic resection after staging with [18F] fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET).
Summary Background Data:
The 5-year overall survival after hepatic resection for colorectal cancer metastases without preoperative FDG-PET has been established in 19 studies (6070 patients). The median 5-year overall survival rate in these studies is 30% and has not improved over time. FDG-PET detects unsuspected tumor in 25% of patients considered to have resectable hepatic metastasis by conventional staging.
Methods:
From March 1995 to June 2002, all patients having hepatic resection for colorectal cancer metastases had preoperative FDG-PET. A prospective database was maintained.
Results:
One hundred patients (56 men, 44 women) were studied. Metastases were synchronous in 52, single in 63, unilateral in 78, and <5 cm in diameter in 60. Resections were major (>3 segments) in 75 and resection margins were ≥1 cm in 52. Median follow up was 31 months, with 12 actual greater than 5-year survivors. There was 1 postoperative death. The actuarial 5-year overall survival was 58% (95% confidence interval, 46–72%). Primary tumor grade was the only prognostic variable significantly correlated with overall survival.
Conclusions:
Screening by FDG-PET is associated with excellent postresection 5-year overall survival for patients undergoing resection of hepatic metastases from colorectal cancer. FDG-PET appears to define a new cohort of patients in whom tumor grade is a very important prognostic variable.
The 5-year survival of 100 patients treated by hepatic resection for colorectal cancer metastatic to the liver who were staged by FDG-PET was 58%. The median overall survival in 19 previous case series not staged by FDG-PET is 30%.
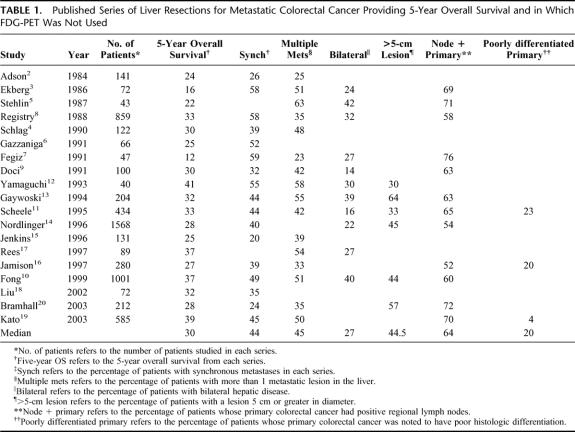
Hepatic resection is the most effective therapy for a subset of patients with colorectal carcinoma metastatic to the liver. Strict selection criteria are necessary because there is no survival benefit if residual disease remains after hepatectomy.1 Investigations are used to determine resectability and with uncommon exceptions, surgery is not performed when there is extrahepatic disease or when the extent of hepatic disease precludes complete eradication. At present, preoperative computed tomography (CT) of the abdomen and CT or radiography of the chest are standard radiologic staging investigations. Despite careful preoperative staging by these tests and colonoscopy, most patients have recurrence after liver resection. The 5-year overall survival after resection of colorectal liver metastases using current staging modalities has been established in 19 series encompassing 6070 patients2–20 (Table 1). The median 5-year overall survival rate is 30% with a range of 12% to 41%. Significantly, these results have not steadily improved over time. Accordingly, to reduce the frequency of futile hepatic resections, more effective staging tools are needed.
TABLE 1. Published Series of Liver Resections for Metastatic Colorectal Cancer Providing 5-Year Overall Survival and in Which FDG-PET Was Not Used
Positron emission tomography with the glucose analog [18F] fluoro-2-deoxy-D-glucose is a sensitive diagnostic test that images tumors based on the increased utilization of glucose by tumor cells. FDG-PET has been demonstrated to be more sensitive than CT in the detection of deposits of metastatic colorectal adenocarcinoma. Most large series report that approximately 25% of patients are discovered to have new tumors in the liver or extrahepatic sites on FDG-PET performed after standard imaging.21–30 Furthermore, we and others have found that FDG-PET frequently detects recurrent colorectal cancer in patients with normal CT scans but rising carcinoembryonic antigen (CEA) levels.21,25 One metaanalysis has reported an overall sensitivity of 97% and an overall specificity of 76% for FDG-PET in detecting recurrent colorectal cancer.26 FDG-PET seems even more sensitive than CT portography, a much more invasive test in the detection of intrahepatic and extrahepatic colorectal cancer recurrences.22
Despite the increased detection rate provided by FDG-PET, it has not yet been demonstrated that the use of this investigation for patient selection for surgery affects the standard measure of outcome in oncology—the 5-year overall survival rate. We previously reported that preoperative FDG-PET improved 3-year overall survival in a small group of 35 patients undergoing hepatic resection for colorectal metastases.31 The short-term follow-up period in this earlier study only permitted determination of the 3-year actuarial survival rate. Our findings at the 3-year follow up have now been confirmed by others.32 We have now gathered a larger series of patients screened by FDG-PET who have been followed for a longer time period. The study shows that FDG-PET has a dramatic effect on overall 5-year survival when compared with all available historical series in which FDG-PET was not used for preoperative cancer detection.
METHODS
From March 1995 through June 2002, 100 patients with colorectal carcinoma metastatic to the liver were evaluated and considered to have resectable disease after the completion of conventional staging. Conventional staging was abdominal CT and either chest radiography or CT. Magnetic resonance imaging of the abdomen was also performed in a few patients. In several instances, these investigations were performed at an outside institution and if the CT examination was not recent or of acceptable quality, CT was repeated before FDG-PET. FDG-PET was performed in all patients.
Most FDG-PET studies were performed at the Mallinckrodt Institute of Radiology, Washington University School of Medicine. When they were not performed at our institution, they were reviewed by our nuclear radiologists. If recent and deemed of adequate quality, they were used in operative planning; otherwise they were repeated. The protocol used for FDG-PET imaging has previously been described in detail.24 Patients fasted for a minimum of 4 hours before the study. To minimize interference from activity in the urinary tract, a urinary catheter was placed in the bladder, approximately 1500 mL of 0.9% saline was infused intravenously, and 20 mg furosemide was administered 20 minutes after FDG administration (except when contraindicated). Interpretation of all studies was performed in routine clinical fashion by an experienced nuclear radiologist. Subjective visual assessment was used in interpretation of FDG-PET images. An antecedent CT scan (or occasionally only the CT report) was available to the radiologist at the time of FDG-PET interpretation. All imaging results were correlated with the subsequent final histologic diagnosis, by findings at surgery, or by at least 6 months of clinical observation from the time of FDG-PET.
Laparotomy and abdominal exploration, including intraoperative ultrasonography of the liver, were performed in patients deemed to be operable after FDG-PET. Hepatic resection was then performed in patients still found to have operable disease. The hepatic resection was performed concurrently with resection of the primary colorectal carcinoma in 10 patients with synchronous metastases. No patients who required radiofrequency (RF) ablation in addition to resection to treat hepatic lesions are included in this series. Postoperative death was defined as any mortality within 30 days of surgery.10
Patients were evaluated postoperatively at regular intervals. Serial CEA measurements and CT scans were performed. FDG-PET was used in the postoperative period in many patients to search for residual tumor when this was suspected on the basis of other test results such as a rising CEA. Determination of recurrence was based on biopsy results, or unequivocally positive results on FDG-PET scan or CT. Suspicious results on follow-up FDG-PET were not taken as evidence of recurrence unless confirmed by a positive biopsy.
SAS version 8 software package (SAS Institute, Cary, NC) was used for the statistical analysis of data. Time to death was used as the end point in overall survival (OS) analysis, and patients who were alive were censored at the date of last clinical contact. For disease-free survival (DFS) analysis, time from the date of surgery to the date of recurrence or to the date of death (in patients in which death was not the result of colorectal cancer and recurrence had not been detected) were used as end points. To identify significant factors related to OS or DFS, Kaplan-Meier product limit estimators were calculated and compared by log rank tests. Multivariate Cox models were also fitted for OS and DFS while including only those factors showing P <0.50 in the univariate analysis. A P value under 0.05 was taken to indicate significance and all statistical tests were 2-sided. The terminology for liver anatomy and resections used in this article is the Brisbane 2000 terminology of the International Hepato-Pancreato-Biliary Association.33
RESULTS
From March 1995 through June 2002, 100 patients with hepatic metastases from a primary colorectal carcinoma underwent complete resection with curative intent after preoperative imaging with FDG-PET on the Hepatobiliary–Pancreatic Surgery service in our institution. A prospective database of all patients was maintained. The patient population consisted of 56 men and 44 women. Their median age was 61.1 years (range, 23–86 years). Patients were followed for a median of 31 months (range, 0.1–96 months).
The primary tumor was located in the colon in 66 patients (66%) and in the rectum in 31 patients (31%). In 3 patients, the only information obtainable was that the tumor originated in the large intestine. Information on stage and grade of primary tumor was obtainable in most patients. Fifty-seven of 96 (59%) had positive regional lymph nodes. Eighteen of 86 primary tumors were poorly differentiated; in 14 of 18 patients (77%) with poorly differentiated tumors, regional lymph nodes contained metastatic cancer.
The hepatic metastases were synchronous in 52 patients (52%). Synchronous lesions were defined as those discovered before or within 1 year of the resection of the primary tumor. Metachronous lesions were defined as those diagnosed greater than 1 year after the resection of the primary tumor. Metachronous metastases were diagnosed 1 to 7 years after resection of the primary tumor in 48 patients (48%). In 86 patients, serum CEA levels were available from specimens taken immediately before the hepatic resection. In 12 of 86 patients, CEA levels exceeded 100 ng/mL. CEA levels were greater than 200 ng/mL in 6 patients.
Hepatic resection was performed for 1 hepatic tumor in 63 patients and for more than 1 tumor in 37 patients. Twenty-four patients had 2 tumors, 6 patients had 3 tumors, and 7 patients had 4 or more intrahepatic tumors. Metastatic disease was confined to 1 hemiliver in 78 patients and was bilateral in 22 patients. In 60 patients, the largest lesion was less than 5 cm in greatest diameter, whereas in 40 patients, it was 5 cm or greater. The median size of the tumors was 4 cm (range, 0.9–20 cm); when there were multiple tumors, the size used for this calculation was that of the largest tumor.
Of the 100 hepatectomies, 98 were anatomic resections. In 75 patients, the resection involved 3 or more contiguous Couinaud segments, and in 25 patients, fewer than 3 contiguous segments were resected. In 14 patients, 2 or more discontinuous resections were performed. Only 2 patients underwent a wedge resection. In all cases, gross margins were negative. Resection margins were 1 cm or greater in 52 patients and less than 1 cm, but negative, in 45 patients. Microscopically positive margins were present in 3 specimens. In 3 patients, a hepatic artery infusion pump was placed for postoperative adjuvant regional chemotherapy. There was 1 perioperative death (1%).
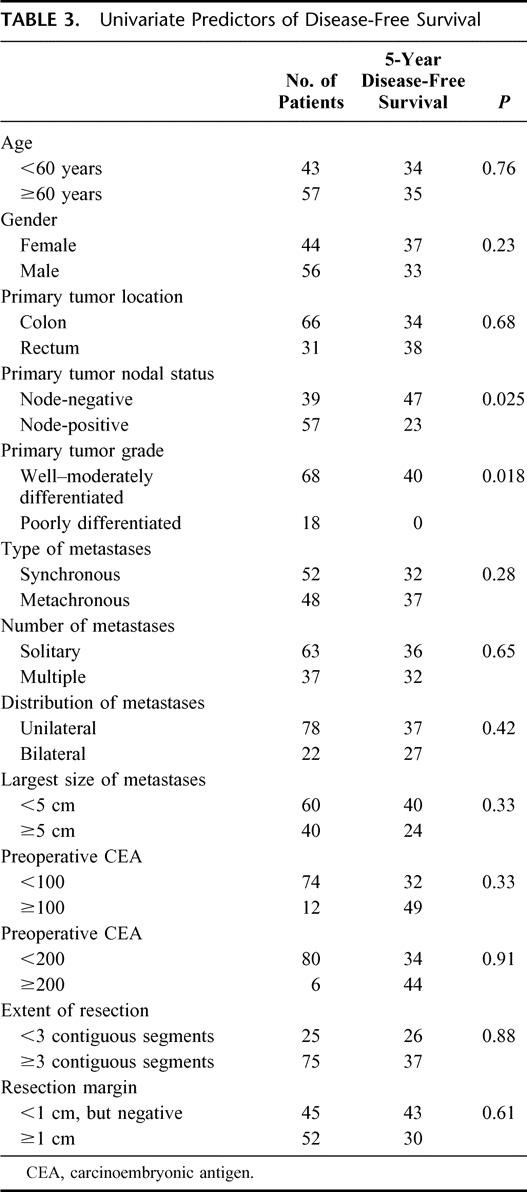
Kaplan-Meier curves for overall and disease-free survival are shown in Figure 1. Overall survival was 85.7% at 1 year, 66.0% at 3 years, and 58.6% at 5 year. The median overall survival was not reached. Disease-free survival was 63.7% at 1 year and 34.8% at both 3 and 5 years. The median disease-free survival was 22 months. There were 12 actual 5-year survivors in this series. The 95% confidence limits of the Kaplan-Meier estimate of 5-year survival were 45.6% to 71.6%. The lower confidence limit of 45.6% exceeded the Kaplan-Meier estimate of 5-year overall survival from any previously reported comparable case series not using preoperative FDG-PET (Table 1). The median of the Kaplan-Meier estimates of 5-year overall survival in those 19 series is 30% (range, 12–41%).2–20 Overall survival curves were available for 13 of these 19 studies.2,5,6,8–16,18 Figure 2 shows these curves superimposed alongside the overall survival curve from this series for comparison.
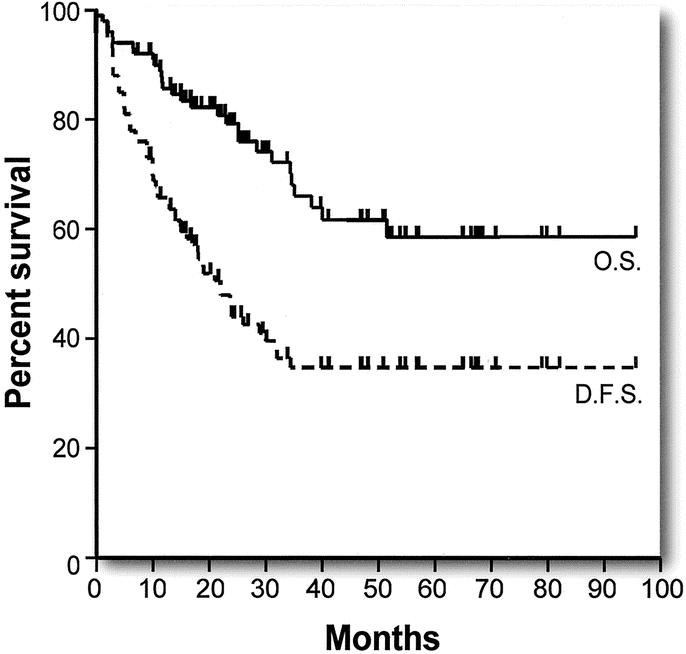
FIGURE 1. Actuarial overall survival (OS, solid line) and disease-free survival (DFS, broken line) after hepatic resection for colorectal liver metastases in patients staged with FDG-PET.
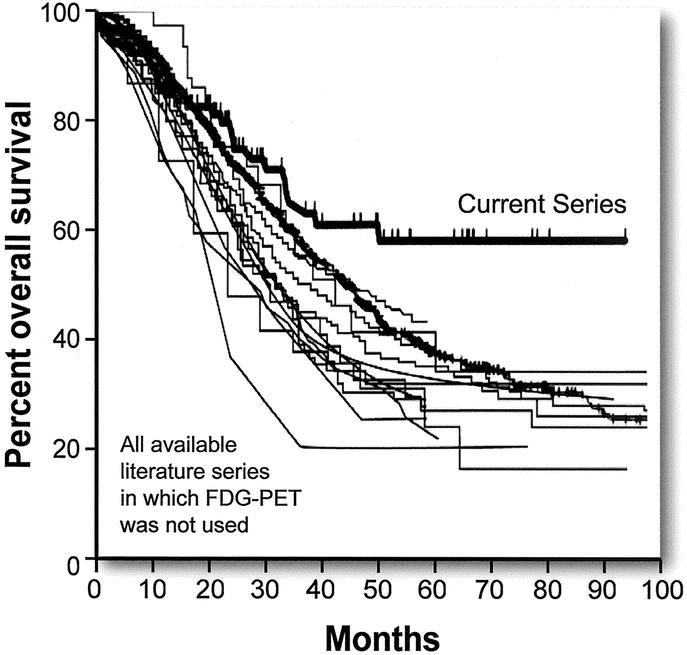
FIGURE 2. Actuarial overall survival for 13 published series not using FDG-PET staging in comparison to overall survival in this series.
Prognostic factors related to overall survival may be grouped into 4 categories: completeness of removal of the tumor(s), demographic factors, features of the primary tumor, and features of the metastatic tumor.34 Overall survival was not affected by the presence of previously described negative prognostic factors relating to features of the metastatic tumor but was highly correlated to histologic grade of the primary tumor.
The following features of the metastatic tumor were not related to overall survival: multiple lesions, bilateral disease, histologic margin <1 cm, synchronous lesions, extent of resection, and tumor size (Table 2). Disease-free survival was also not significantly impacted by any of these mentioned factors (Table 3).
TABLE 2. Univariate Predictors of Overall Survival
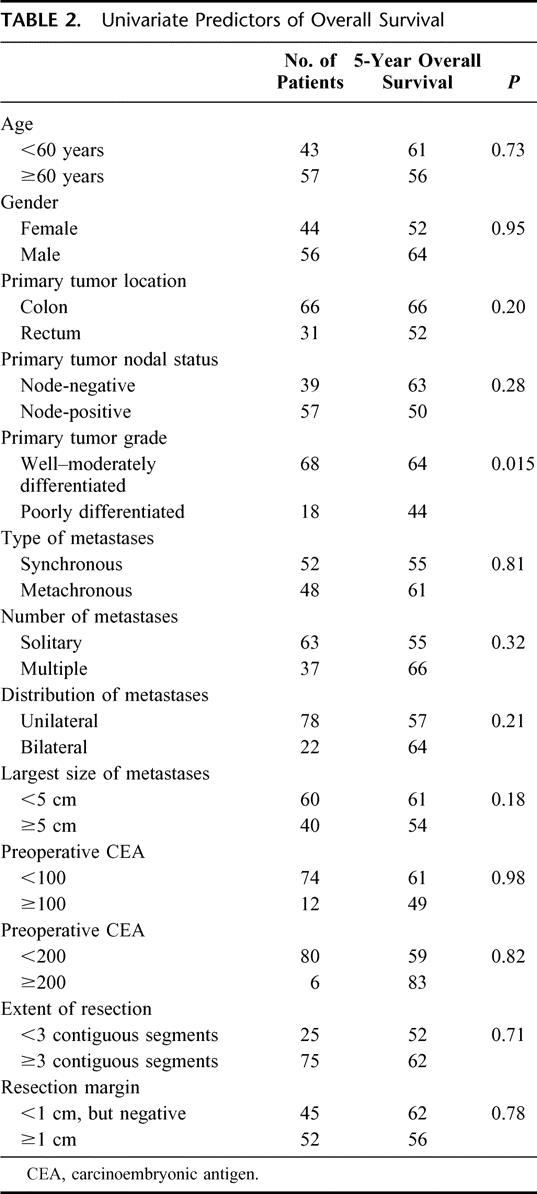
TABLE 3. Univariate Predictors of Disease-Free Survival
Overall survival and disease-free survival were significantly correlated with the grade of the primary tumor when poorly differentiated tumors were compared with moderately and well-differentiated tumors (Tables 2 and 3) (Fig. 3A, B). The disease-free survival rates are particularly noteworthy in that no patient with a poorly differentiated primary tumor has remained disease free for longer than 29 months. Of 18 patients, 14 have recurrence of disease within this period, whereas 4 of 18 patients remain disease-free at 11, 17, 24, and 26 months of follow up. Note also that the initial slope of the disease-free survival curve for moderate and well-differentiated tumors is only modestly better than that for poorly differentiated tumors but that this curve then flattens out and survival remains at 40% out to 5 years and beyond. Data for overall and disease-free survival in relation to lymph node status (positive or negative) at the time of resection of the primary tumor are shown in Figure 4A, B and Tables 2 and 3. Lymph node positivity was correlated with disease-free survival, but not with overall survival. The location of the primary in the large intestine was not significantly related to outcome (Tables 2 and 3).
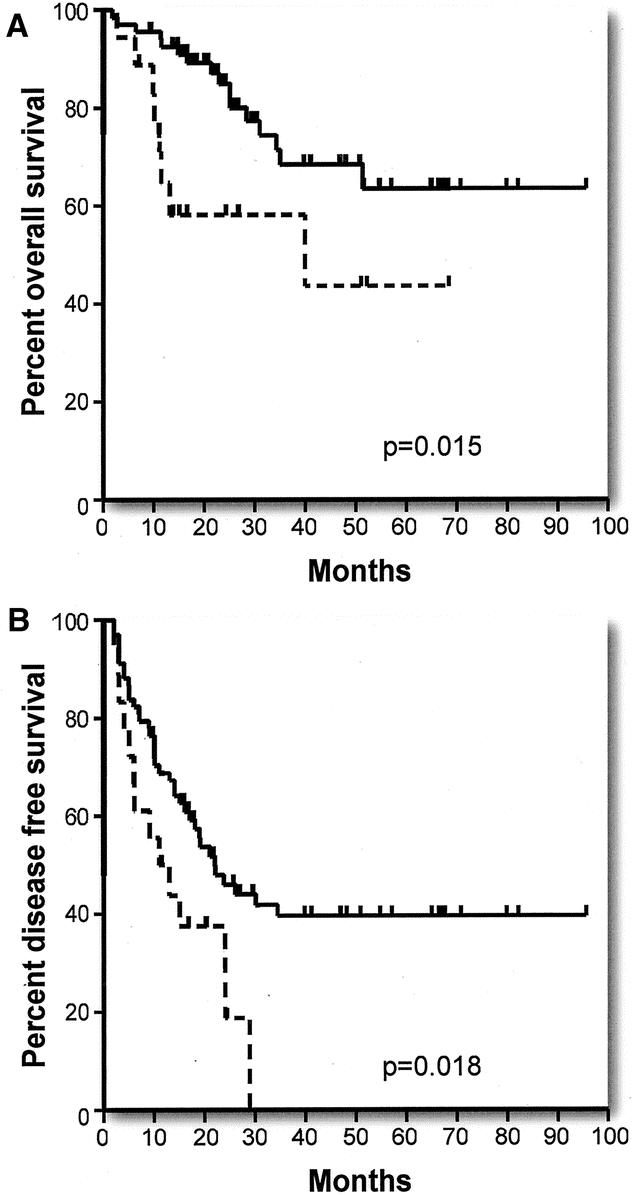
FIGURE 3. Effect of grade of primary tumor: (A) Actuarial overall survival in patients with well and moderately differentiated primary tumors (solid line) versus poorly differentiated primary tumors (broken line). (B) Actuarial disease-free survival in patients with well and moderately differentiated primary tumors (solid line) versus poorly differentiated primary tumors (broken line).
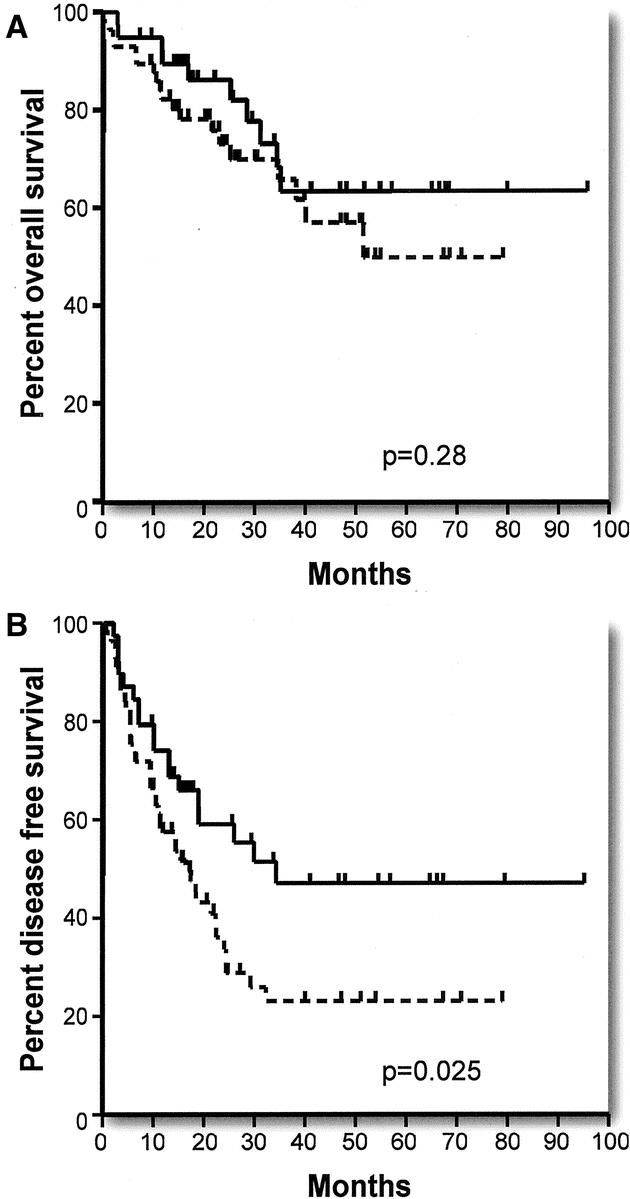
FIGURE 4. Effect of lymph node positivity in primary tumor specimen: (A) Actuarial overall survival in patients without (solid line) and with (broken line) positive lymph nodes. (B) Actuarial disease-free survival in patients without (solid line) and with (broken line) positive lymph nodes.
Outcome was not related to CEA level when the cut point chosen was 100 ng/mL or 200 ng/mL (Tables 2 and 3). Nor was outcome related to any of the usual demographic factors such as age and sex (Tables 2 and 3). The effect of completeness of removal of tumor could not be evaluated because only 3 of 100 patients had positive microscopic margins.
In the multivariate analysis, only grade of primary tumor was significantly related to overall survival (P = 0.008) or disease-free survival (P = 0.014) (Table 4). Nodal status, a significant predictor of disease-free survival in the univariate analysis, was not a significant predictor of disease-free survival in the multivariate analysis (Table 4).
TABLE 4. Multivariate Analysis of Predictors for Survival
In total, there were 63 recurrences in 52 patients. The median time to recurrence was 10 months (range, 1–32 months). Hepatic recurrences were observed in 46.2% of these patients and lung recurrences in 44.2%. Other sites of recurrence were brain, bone, abdominal lymph nodes and abdominal wall (all 5.8%), peritoneum (3.8%), and mediastinum and heart (each 1.9%). Twenty-eight patients with recurrence are alive at the time of writing. Their disease recurred in the liver (n = 11), lung (n = 14), retroperitoneal lymph nodes (n = 3), bone (n = 1), and abdominal wall (n = 1). Three patients had recurrence in both the liver and lung.
DISCUSSION
The major finding in this study is that the 5-year actuarial overall survival in patients screened with FDG-PET before hepatic resection for metastatic colorectal cancer was 58.6%. This is a substantial improvement in overall survival when compared with the results from a large number of historical series in which FDG-PET was not used. The 95% confidence limits of the 5-year survival allow us to provide an estimate of how well our results represent the true or population results of patients screened with FDG-PET. Using these values, it can be said that the true 5-year overall survival for our series of patients lies between 45.6% and 71.6%; 45.6% is the lower confidence limit. Determination of the 95% confidence limits allows better comparison between this series and other series. Unfortunately, 95% confidence limits were not available for the previously reported series. As shown in Table 1, the 5-year overall survival rates in case series from patients not screened with FDG-PET ranged from 12% to 41% with most results grouped around the median value of 30%. In 16 of the 19 series, 5-year survival ranged from 22% to 37%. The largest series of 1568 patients from Nordlinger et al14 reported a 5-year overall survival rate of 28%. Fong et al10 reported a 37% overall 5-year survival in 1001 patients from a single institution. The Registry series,8 with 859 patients, had a 33% 5-year survival rate. Statistically, it is likely that the largest series will be closest to the population mean and that the population mean will be close to the mean value of the multiple series. Therefore, the best estimate of the population mean of overall 5-year survival of patients who have liver resection without prior FDG-PET is approximately 33%. There was no relation between the 5-year survival results and year of publication (Table 1). This makes it unlikely that the comparisons between results in the current series and previously published series are biased by an improvement of results over time.
Preoperative screening with FDG-PET does not greatly increase the number of individuals who survive colorectal cancer metastases to the liver. A few individuals are found to have unknown liver secondaries without having extrahepatic disease detected at the same time,21–24,27,28,30,35 and for these patients, FDG-PET may enable a cure by liver resection that otherwise would not have occurred. However, the predominant effect of FDG-PET in this population is to detect occult metastatic disease and thereby reduce the number of futile operative procedures performed in patients with otherwise occult metastatic disease; most commonly, this impact is manifested by the detection of unsuspected extrahepatic disease.21–31 By changing the target population for surgery, the mean survival times of patients who do undergo surgery is improved. It has been estimated that 10,000 to 15,000 patients are candidates for resection of hepatic metastases from colorectal cancer in the United States each year based on conventional staging.34 Given that FDG-PET changes management in approximately 25% of patients,21–31 potentially 2500 to 3750 futile operative procedures, ie, hepatic resections or laparotomies at which the unsuspected disease is discovered, may be avoided per year, with attendant reduction in morbidity, mortality, and cost. Moreover, the use of FDG-PET provides the opportunity for appropriate treatments to be given to these patients at an earlier time; in some cases, this will be RF ablation or ablation combined with resection. Those are the main contributions of the test.
Several series have established indicators of a poor prognosis after resection of colorectal cancer metastases by use of multivariate analysis. With respect to the primary tumor, advanced stage,8–11,36–40 high grade,11,41 and location in the rectum36 have all been reported as prognostic of poor outcome. Variables related to the mode of presentation of the hepatic metastases that have been correlated with a poor prognosis include synchronous disease4,8,10,11,42 and increased CEA concentration.10,36,43 Finally, characteristics of the metastatic tumor in the liver that have been reported to be associated with a lessened prognosis include number of lesions3,8,10,36,38,39,42–45 and size of the metastasis.8–11,17,40,42 The presence of unilateral versus bilateral hepatic disease was not found to be a prognostic variable in the reviewed literature.8–11,17 In a large series, Fong et al10 and Iwatsuki et al46 have reported that the cumulative presence of certain poor prognostic features predicts a poor outcome.
In the present study, none of the established prognostic factors relating to the metastatic tumor in the liver were significant predictors of worse outcome. It is notable that the fraction of patients in this series with indicators of poor prognosis related to features of the liver tumors was less than that in studies in which FDG-PET was not used.2–20 For instance, the median value in previous reports for percentage of patients with multiple tumors is 45%2–5,7–13,15–17,19,20 versus 37% in this series. Furthermore, our previous report demonstrated that FDG-PET tended to preferentially exclude patients with metastases that were multiple, bilateral, or synchronous.31 Such patients were more likely to have extrahepatic disease detected by FDG-PET and therefore be eliminated from consideration for surgery. Presumably, this is the explanation for the relative reduction in the percentage of patients with indicators of poor prognosis, as judged by features of the hepatic tumors, in this study compared with those in which FDG-PET was not used. This shift in prognostic indicators induced by staging with FDG-PET could not be reevaluated in this study because in the short period between our 2 reports, FDG-PET has become widely available and we cannot identify those patients not referred because of positive findings, ie, this can no longer be evaluated on an institutional level. However, in essence, the target population for curative surgical therapy is being altered by FDG-PET to one with better tumor biology as measured by features of the hepatic metastases.
The effect of FDG-PET on prognostic variables in this disease is best understood if it is considered that FDG-PET may be defining a new population of patients with its own prognostic variables. In the population of patients staged by FDG-PET, the characteristics of the metastatic tumor become less important as prognostic variables, whereas the grade of the primary tumor becomes much more important. Stated otherwise, hepatic tumor number, hepatic tumor size, and synchronicity are good markers for outcome in patients not staged by FDG-PET because they are good surrogates for extrahepatic disease, but FDG-PET often detects those tumors for which they are good surrogates and seems to lessen their importance as prognostic markers. On the other hand, FDG-PET does not eliminate the prognostic power of primary tumor grade; rather, it appears to increase it. Presumably poor differentiation is a marker for micrometastatic or small-volume disease that is undetectable by FDG-PET. In previous studies in which it has been evaluated, tumor grade has been found not to be related to outcome,3,10,17,44,47,48 with the exception of studies from the Jena/Erlangen group.11,41 However, in many studies, it has not been evaluated as a prognostic variable. Our observations have been made in 100 patients, which is a suitable number for this type of analysis, but it is at the lower range of what is acceptable. Therefore, confirmation in a larger series of patients is required. If confirmed, current prognostic indices may require revision.10,46 However, even now, it is possible to say that patients with hepatic metastases that appear to be resectable by FDG-PET, but whose primary tumor was poorly differentiated, have a very high chance of recurrence and a very poor prognosis. This population should either not be treated by resection or studied in adjuvant therapy trials aimed at residual small-volume disease.
There were slightly more extrahepatic than intrahepatic recurrences of tumor in this series. However, the majority of extrahepatic recurrences were in the lungs. There were only 3 intraabdominal lymph node recurrences out of 63 recurrences (4.8%) in 52 patients (5.8%); 2 patients had peritoneal recurrences. In other studies not using FDG-PET for staging, the percentage of patients with intraabdominal lymph node recurrences seems to have been much higher, although exact comparisons are difficult because of differences in reporting.9,12,17 For instance, some reports use categories such as “widespread dissemination” or “liver and other” to describe recurrence. A lower rate of lymph node recurrence is in keeping with our experience and that of others that FDG-PET is particularly effective in finding undetected intraabdominal nodal disease.24,27,28,31 Also, hepatic recurrence in this study was 46% versus 55% to 68% in previous studies.7,9,12,17 The altered pattern of recurrence adds support to the point that the FDG-PET-staged population seems to represent a specific cohort with this disease.
There are excellent reasons for concluding that our superior results by comparison with those from studies in which FDG-PET was not used are attributable to the improved staging accuracy provided by FDG-PET. However, it is also appropriate to consider whether other influences such as the effect of newer forms of chemotherapy or the introduction of radiofrequency (RF) ablation might have contributed to the improved results.
Irinotecan and oxaliplatin are recently introduced agents that have activity against colorectal tumors. They have not been demonstrated to extend survival in resected patients, but it is possible that they may do so and thus could influence the results of studies such as this one. Oxaliplatin was not available to our patients before the closing date of this study; however, some patients who had liver resection in the last 1.5 years of the 7.5-year study period may have received irinotecan. Complete information on the number of patients receiving adjuvant chemotherapy and the schedule of chemotherapy is not available, because most patients were treated in local facilities at a distance from our institution, but to our knowledge, only 4 patients received irinotecan in the adjuvant setting. Note that neither agent was available at the time we reported that the 3-year overall survival for patients staged by FDG-PET was 77%,31 a result much superior to that previously reported and that has been confirmed by others.32 It is also possible that the 5-year overall survival might be improved by the use of these newer chemotherapeutic agents at the time of recurrence after resection, but this would not explain the relatively high disease-free survival rate of 35% in this series versus 20% in previous reports. In summary, we conclude that newer forms of chemotherapy are unlikely to have accounted for more than a fraction of the improved results observed in our patients.
RF ablation is another new modality now being used to treat patients with colorectal carcinoma metastases. The results of pure resection series could be affected if patients with poor prognostic factors were selected for treatment by RF ablation plus resection or by RF ablation alone. There would be a tendency to do just that in patients with multiple hepatic lesions. Because we have shown that patients with multiple tumors have a much higher chance of having PET-detectable extrahepatic disease, diverting such patients out of a resection group into a resection plus RF ablation group might lead to improved survival in the resection group. We have used RF ablation only in a small minority of patients with colorectal carcinoma metastases referred to our institution and have adhered to a strict policy of resection of all lesions whenever possible. A few patients have been treated by RF ablation or RF ablation plus resection when a patient with apparently resectable disease was found on FDG-PET to have additional hepatic lesions and no extrahepatic lesions. However, most patients found to have unresectable disease because of the number and position of hepatic lesions are also found to have extrahepatic disease on FDG-PET and thus are not eligible for any local therapy. Based on the foregoing, it would be predicted that many patients who are eligible for RF ablation because they have unresectable disease will be found to have extrahepatic disease on FDG-PET. It therefore appears that the rationale for staging of these patients by FDG-PET is even stronger than for the resectable group and predictable that the long-term outcome of such patients not staged by this modality would be rather poor. On the other hand, there may be subgroups within the resectable group, eg, those with single lesions less than 5 cm in diameter, in which the yield of FDG-PET may be low and perhaps too low for it to be recommended routinely; this will require further study.
In conclusion, FDG-PET is a valuable diagnostic tool that improves patient selection and, therefore, increases the survival rate of the population of patients undergoing resection of colorectal metastases to the liver. Despite these promising results, tumor recurrence remains a common phenomenon. However, routine preoperative staging with FDG-PET will frequently detect otherwise occult disease and thus prevent many futile laparotomies and hepatectomies or result in selection of more appropriate therapy such as that involving RF ablation.
ACKNOWLEDGMENTS
The authors thank Dr. Feng Gao for statistical assistance.
Discussions
Dr. Yuman Fong (New York, New York): I want to first congratulate Dr. Fernandez for his presentation and the entire group from Washington University under the leadership of Drs. Strasberg and Siegel for yet another important contribution that will guide our surgical therapies for liver malignancies.
Their paper clearly indicates that favorable outcomes can be achieved in well-selected patients who are well staged and who receive technically superb surgery. Their data suggest that PET scanning may contribute to this favorable outcome. What the paper has not completely defined for me are the details of when PET scanning should be used clinically.
My first question is, therefore, are the authors advocating the PET scan should be used for all patients before liver resection? In studies from my institution, we have found that the yield of FDG-PET scanning is directly related to the prognostic clinical risk score that we have devised. In patients that have 0–1 points, for example, the yield of PET was less than 10% and resectability was greater than 90%. So in my practice, in this low-yield group I don't require that a PET scan be done.
In a related question, what was the yield of PET in the authors’ study for the favorable patients, such as those with solitary, metachronous metastases from well differentiated cancers? Is there a group such as this where the authors would not advocate PET scanning?
My next comments are in regard to chemotherapy. There is no mention in the manuscript concerning the influence of chemotherapy. Other groups and we have found that patients who are on chemotherapy at the time of PET scanning have very poor yield for FDG-PET. In our own series, the detection of subcentimeter lesions in the liver was less than 5% in those patients that were on chemotherapy. How many of the patients in the present series were on chemotherapy and was there a difference in yield of FDG-PET? In fact, how many of the patients in these 100 actually had completely negative PET scans? Certainly, PET scanning cannot help clinically in those patients. How has chemotherapy contributed to the long-term outcomes that are seen here that are very favorable? In the last 5 years we have much better chemotherapies and biologic therapies that are available, such as CPT-11, oxaleplatin, and now avastin, and C225. How many patients in this series had adjuvant therapy after resection?
The final points are philosophic. The authors noted that the published series from the literature for resection of colorectal metastases have not improved over the last decade. I would contend that this does not mean we are not doing better with this disease. The explanation that I have offered for this is that as we get better at any operation, we tend to expand indications and offer more patients treatment.
Since the operative mortality is now less than 2% in most centers, surgeons are increasingly likely to operate on patients with more advanced disease. In most series now, resections of solitary liver metastases represents the minority of cases, and resecting 10 or more tumors is not uncommon. So I would contend that what looks like stagnant results will stay that way for the near future. Surgeons are willing to do cancer operations with a 1–2% mortality, if they are going to provide a third of the patients 5-year and long-term survival. The questions that are related are the following:
The authors seem to feel that PET scanning is most useful in patient selection and in eliminating patients from consideration for surgery. I tend to think that PET scanning is more useful in identifying more disease to increase the completeness of resection or ablative therapy. My question is, therefore, how many patients were eliminated by PET scanning from surgical therapy in the time period that these 100 patients were resected? How many patients had their surgical procedure altered by PET scanning that directed a larger operation or a combined resection and ablation? What were the results?
Thank you for providing me with the manuscript ahead of time—once again, congratulations on a nice study—and the privilege of discussing this paper.
Dr. Steven M. Strasberg (St. Louis, Missouri): Thank you, Dr. Fong, for reading the paper and for those perceptive questions.
All patients in our institution who present with colorectal metastases and who are being considered for liver resection are staged by FDG-PET scan. Dr. Fong stated that he had found subgroups, based on a low MSKCC risk score that have a very low rate of positivity for occult disease on FDG-PET. And he asked whether we have found such subgroups. We have not examined this question. It is doubtful that the number of patients in this series would allow us to define subgroups in this way with accuracy. Regarding the question on the use of chemotherapy, the referral pattern of our patients is such that the majority are sent by medical oncologists from outside the St. Louis metropolitan area. As a result postoperative chemotherapy is directed by a number of medical oncologists. Although most patients received adjuvant chemotherapy there was not a standard regimen.
Another question was in how many of the patients were the liver lesions not seen on FDG-PET scan. I do not have that data at hand but the number would have been very low. Every patient that I can recall that had a liver resection had FDG-PET positive lesions in the liver. However, we have also observed that patients who have had extensive chemotherapy can develop FDG-PET negativity while on treatment. Unfortunately, FDG-PET negativity does not necessarily mean complete tumor destruction, as injured but alive tumor cells may not accumulate FDG to the same extent as untreated cells. This is a shortcoming of FDG-PET as is the fact that mucinous tumors are also not well seen. We have presented only those patients who were considered resectable after FDG-PET. During this time period there were other patients who were treated with RF ablation or RF ablation plus resection because the extent in the liver precluded treatment by resection alone. We will report these patients separately.
Dr. Harold J. Wanebo (Providence, Rhode Island): Dr. Strasberg and his associates are to be congratulated on exploring a new paradigm for the surgical management of liver metastases from colorectal cancer. The achievement of a 58% survival rate is quite impressive.
My questions are as follows: What is the actual denominator here? What is the total number of screened patients that were actually reviewed and then the number that are actually screened? Approximately 25% is the number given, but what is the actual number? And what are the actual sites of the occult PET disease? Do you have some data on this? Were more of these extrahepatic?
You quote median survival in the historic series of about 30%. And the question is, what was the difference between these older series and your series, which was defined by PET? That is, did all the surgeons, including myself, miss these occult sites in routine intraoperative exams? For example, did these patients have lymph node or peritoneal metastases that were missed?
Finally, do you have a protocol consideration for patients that were screened out? Would any of these patients be candidates for some type of neoadjuvant approach and perhaps reoperation following that therapy?
Very impressive results. I enjoyed the opportunity to discuss this.
Dr. Steven M. Strasberg (St. Louis, Missouri): Thank you, Dr. Wanebo. As noted in the presentation, there are now about 10 series in the literature that have examined the effect on FDG-PET staging of colorectal cancer. They show that FDG-PET results in a change in management in about 25% of patients, usually as a result of detection of occult metastases. In many of these patients, the occult tumor is in an intra-abdominal extrahepatic location, often in retroperitoneal lymph nodes. We believe that the ability of FDG-PET to detect such deposits accounts for much of the improvement in survival rates that we have observed, because such metastases are quite difficult to diagnose at the time of liver surgery after only conventional staging. The fact that FDG-PET is efficient in detecting metastatic deposits in intra-abdominal extrahepatic locations is also reflected in the sites of recurrence of tumor in our series. Unlike other reports there were few recurrences in intra-abdominal extrahepatic locations, presumably because FDG-PET had eliminated patients with disease in these sites preoperatively. The bulk of the recurrences we observed were in the liver and lung.
It was also asked what happens to patients who are found to have FDG-PET positive lesions that exclude them from resection. When the disease is in an intra-abdominal extrahepatic site other than the site of the primary tumor we have not treated the patients surgically and they have been referred to a medical oncologist for treatment. We have performed liver resection with resection of a recurrence in the primary site in a few patients and liver and lung resection in some patients in this series in which the number of lesions has been small. But patients with extra-abdominal lesions other than lung did not have liver resection and were referred for chemotherapy. As mentioned previously patients who had disease confined to the liver, but were not treatable by resection alone, were treated by RF ablation or RF ablation and resection when that was appropriate. We are now beginning to do patients with extrahepatic disease confined to portal lymph nodes based on a recent report that this may be helpful.
In regard to the denominator—the total number of patients who received FDG-PET scans including those that were eliminated from resection—such a figure is not possible to obtain today on an institutional basis. In our previous study, we found that 25% of patients had occult tumors discovered by FDG-PET and most of these were eliminated from consideration for resection. However, now many institutions have PET scanners. Consequently, we have little information regarding patients who are not referred for surgery because of a FDG-PET scan performed at an outside institution. Getting such figures would now require statewide or nationwide study.
Dr. J. Michael Henderson (Cleveland, Ohio): My main question is about recurrence after resection. That wasn't dealt with in your presentation.
In reading your manuscript, what struck me is you had one of the lowest intrahepatic recurrence rates, around 45%, yet you had significant extrahepatic recurrence. With PET's advantage in directing extrahepatic disease, is that being seen primarily in your synchronous group of patients rather than the group with metachronous lesions? And are you really just detecting disease earlier? And what is your follow-up protocol in this group of patients? Are you PET scanning them after resection to pick up that extrahepatic disease?
Dr. Steven M. Strasberg (St. Louis, Missouri): Thank you, Dr. Henderson. Yes, we did have a significant number of extrahepatic recurrences, but as stated above most of these were in the lung. There were a few patients that had recurrence in other sites such as bone, but most extrahepatic recurrences were in the lung and there were few intra-abdominal extrahepatic recurrences.
Dr. C. Wright Pinson (Nashville, Tennessee): I very much enjoyed this excellent presentation by Dr. Fernandez and his colleagues.
We have for better than a decade also routinely used PET to evaluate patients with colorectal metastases of the liver. We have had similar experience finding additional disease in about 25% of the patients and influencing planned treatments in perhaps a third, mostly avoiding unnecessary laparotomies and futile resections. I agree that the main value of using PET is to define the population that will most benefit from hepatic resection. So my main comment is to agree with the authors.
However, I do want to point out that a very high proportion of their patients, 63%, had solitary tumors, and that probably contributed to some extent to their good results. I am interested in the fact that 75% of their patients had major resections and wonder if they believe that taking larger volumes of liver contributed to their good results. At the same time, I am puzzled by the fact that with a relatively small proportion of patients having multiple tumors and a large proportion having a major resection, why it is that perhaps half of these patients had margins of less than 1 centimeter?
You have indicated that you believe that PET scanning represents a surrogate for many of the previously identified prognostic variables. I would like to suggest that while that may partially be true, I think the small sample size may explain it as much as anything.
Dr. Steven M. Strasberg (St. Louis, Missouri): Thank you, Dr. Pinson. Unquestionably, patients in this series had better prognostic factors. But that is due to the fact that FDG-PET tends to eliminate patients with poor hepatic tumor prognostic factors, as we showed in our previous publication (reference 31). The reason seems to be that patients with poor hepatic prognostic factors such as multiple hepatic tumors also tend to have extrahepatic disease not detectable by standard imaging but which are detectable by FDG-PET. So in groups staged by FDG-PET there will be a shift toward having fewer patients with secondary hepatic tumor prognostic factors such as multiple tumors that are associated with worse outcome.
The question was asked why did 75% of patients have major liver resections or 3 or more Couinaud segments. I think there are probably 2 reasons. The first is that since our referral base is rather wide we believe that there is some streaming of patients requiring liver resections such that patients requiring more limited resection have surgery locally and patients requiring larger resection are referred. Also this series commenced in 1995 when segment oriented resections were not widely performed. We have moved to performing more local segment-based resections when possible. Also we are satisfied with margins under 1 cm. In fact, I think there is abundant literature, which indicates that a 1-cm margin is not necessary although it is ideal. What is important is that the margin be microscopically negative.
Regarding the question of whether prognostic factors found to be negative in this study would have been positive if the sample size had been larger, I agree that that is possible. For instance it is possible that some of the prognostic factors associated with secondary tumors in the liver might be positive if there were a much larger sample size. However, note that series of the size of our study but in patients who did not have FDG-PET scan have previously found factors such as hepatic tumor number to be significant, so at the very least the importance of these prognostic factors is reduced in the FDG-PET scanned population. Also a group of 100 patients as in this series is an appropriate number on which to perform a multivariate analysis.
Footnotes
Reprints: Steven M. Strasberg, MD, Box 8109, Suite 17308 West Pavilion Tower, 1 Barnes-Jewish Hospital Plaza, St. Louis, MO 63110. E-mail: strasbergs@msnotes.wustl.edu.
REFERENCES
- 1.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. [DOI] [PubMed] [Google Scholar]
- 2.Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119:647–651. [DOI] [PubMed] [Google Scholar]
- 3.Ekberg H, Tranberg KG, Andersson R, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. [DOI] [PubMed] [Google Scholar]
- 4.Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer—competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol. 1990;16:360–365. [PubMed] [Google Scholar]
- 5.Stehlin JS Jr, de Ipolyi PD, Greeff PJ, et al. Treatment of cancer of the liver. Twenty years’ experience with infusion and resection in 414 patients. Ann Surg. 1988;208:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazzaniga GM, Cogolo LA, Ciferri E, et al. Liver surgery for metastases: clinical results. J Surg Oncol Suppl. 1991;2:59–62. [DOI] [PubMed] [Google Scholar]
- 7.Fegiz G, Ramacciato G, D'Angelo F, et al. Patient selection and factors affecting results following resection for hepatic metastases from colorectal carcinoma. Int Surg. 1991;76:58–63. [PubMed] [Google Scholar]
- 8.Registry of Hepatic Metastases. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Surgery. 1988;103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 9.Doci R, Gennari L, Bignami P, et al. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg. 1991;78:797–801. [DOI] [PubMed] [Google Scholar]
- 10.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309–318; discussion 318–321. [DOI] [PMC free article] [PubMed]
- 11.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi A, Kurosaka Y, Kanno M, et al. Analysis of hepatic recurrence of colorectal cancer after resection of hepatic metastases. Int Surg. 1993;78:16–19. [PubMed] [Google Scholar]
- 13.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery 1994;116:703–710; discussion 710–711. [PMC free article] [PubMed]
- 14.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 15.Jenkins LT, Millikan KW, Bines SD, et al. Hepatic resection for metastatic colorectal cancer. Am Surg. 1997;63:605–610. [PubMed] [Google Scholar]
- 16.Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997;132:505–510; discussion 511. [DOI] [PubMed]
- 17.Rees M, Plant G, Bygrave S. Late results justify resection for multiple hepatic metastases from colorectal cancer. Br J Surg. 1997;84:1136–1140. [PubMed] [Google Scholar]
- 18.Liu CL, Fan ST, Lo CM, et al. Hepatic resection for colorectal liver metastases: prospective study. Hong Kong Med J. 2002;8:329–333. [PubMed] [Google Scholar]
- 19.Kato T, Yasui K, Hirai T, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46(suppl):S22–31. [DOI] [PubMed] [Google Scholar]
- 20.Bramhall SR, Gur U, Coldham C, et al. Liver resection for colorectal metastases. Ann R Coll Surg Engl. 2003;85:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valk PE, Abella-Columna E, Haseman MK, et al. Whole-body PET imaging with [18F]fluorodeoxyglucose in management of recurrent colorectal cancer. Arch Surg. 1999;134:503–511; discussion 511–513. [DOI] [PubMed]
- 22.Delbeke D, Vitola JV, Sandler MP, et al. Staging recurrent metastatic colorectal carcinoma with PET. J Nucl Med. 1997;38:1196–1201. [PubMed] [Google Scholar]
- 23.Abdel-Nabi H, Doerr RJ, Lamonica DM, et al. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose whole-body PET: correlation with histopathologic and CT findings. Radiology. 1998;206:755–760. [DOI] [PubMed] [Google Scholar]
- 24.Ogunbiyi OA, Flanagan FL, Dehdashti F, et al. Detection of recurrent and metastatic colorectal cancer: comparison of positron emission tomography and computed tomography. Ann Surg Oncol. 1997;4:613–620. [DOI] [PubMed] [Google Scholar]
- 25.Flanagan FL, Dehdashti F, Ogunbiyi OA, et al. Utility of FDG-PET for investigating unexplained plasma CEA elevation in patients with colorectal cancer. Ann Surg. 1998;227:319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huebner RH, Park KC, Shepherd JE, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med. 2000;41:1177–1189. [PubMed] [Google Scholar]
- 27.Fong Y, Saldinger PF, Akhurst T, et al. Utility of 18F-FDG positron emission tomography scanning on selection of patients for resection of hepatic colorectal metastases. Am J Surg. 1999;178:282–287. [DOI] [PubMed] [Google Scholar]
- 28.Lai DT, Fulham M, Stephen MS, et al. The role of whole-body positron emission tomography with [18F]fluorodeoxyglucose in identifying operable colorectal cancer metastases to the liver. Arch Surg. 1996;131:703–707. [DOI] [PubMed] [Google Scholar]
- 29.Vitola JV, Delbeke D, Sandler MP, et al. Positron emission tomography to stage suspected metastatic colorectal carcinoma to the liver. Am J Surg. 1996;171:21–26. [DOI] [PubMed] [Google Scholar]
- 30.Flamen P, Stroobants S, Van Cutsem E, et al. Additional value of whole-body positron emission tomography with fluorine-18-2-fluoro-2-deoxy-D-glucose in recurrent colorectal cancer. J Clin Oncol. 1999;17:894–901. [DOI] [PubMed] [Google Scholar]
- 31.Strasberg SM, Dehdashti F, Siegel BA, et al. Survival of patients evaluated by FDG-PET before hepatic resection for metastatic colorectal carcinoma: a prospective database study. Ann Surg. 2001;233:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonneux M, Reffad A, Detry R, et al. FDG-PET improves the staging and selection of patients with recurrent colorectal cancer. Eur J Nucl Med Molec Imag. 2002;29:915–921. [DOI] [PubMed] [Google Scholar]
- 33.Terminology Committee of the International Hepato-Pancreato-Biliary Association. HPB. 2000;2:233–239. [Google Scholar]
- 34.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin North Am. 2003;12:165–192, xi. [DOI] [PubMed] [Google Scholar]
- 35.Beets G, Penninckx F, Schiepers C, et al. Clinical value of whole-body positron emission tomography with [18F]fluorodeoxyglucose in recurrent colorectal cancer. Br J Surg. 1994;81:1666–1670. [DOI] [PubMed] [Google Scholar]
- 36.Younes RN, Rogatko A, Brennan MF. The influence of intraoperative hypotension and perioperative blood transfusion on disease-free survival in patients with complete resection of colorectal liver metastases. Ann Surg. 1991;214:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortner JG, Silva JS, Golbey RB, et al. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. I. Treatment by hepatic resection. Ann Surg. 1984;199:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ambiru S, Miyazaki M, Isono T, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999;42:632–639. [DOI] [PubMed] [Google Scholar]
- 39.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lise M, Bacchetti S, Da Pian P, et al. Patterns of recurrence after resection of colorectal liver metastases: prediction by models of outcome analysis. World J Surg. 2001;25:638–644. [DOI] [PubMed] [Google Scholar]
- 41.Scheele J, Altendorf-Hofmann A, Grube T, et al. [Resection of colorectal liver metastases. What prognostic factors determine patient selection?] Chirurgie. 2001;72:547–560. [DOI] [PubMed] [Google Scholar]
- 42.Kokudo N, Tada K, Seki M, et al. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg. 2001;181:153–159. [DOI] [PubMed] [Google Scholar]
- 43.Cady B, Stone MD, McDermott WV Jr, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992;127:561–568; discussion 568–569. [DOI] [PubMed]
- 44.Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–504; discussion 504–505. [DOI] [PMC free article] [PubMed]
- 45.van Ooijen B, Wiggers T, Meijer S, et al. Hepatic resections for colorectal metastases in The Netherlands. A multiinstitutional 10-year study. Cancer. 1992;70:28–34. [DOI] [PubMed] [Google Scholar]
- 46.Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holm A, Bradley E, Aldrete JS. Hepatic resection of metastasis from colorectal carcinoma. Morbidity, mortality, and pattern of recurrence. Ann Surg. 1989;209:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doci R, Bignami P, Montalto F, et al. Prognostic factors for survival and disease free survival in hepatic metastases from colorectal cancer treated by resection. Tumori. 1995;81:143–146. [PubMed] [Google Scholar]