Abstract
Objective:
We sought to determine the outcome of living donor liver transplantation (LDLTx) in 316 adult patients with hepatocellular carcinoma (HCC).
Summary Background Data:
LDLTx has increasingly been performed worldwide, but the impact of the procedure on HCC has not been evaluated in a large series.
Methods:
Between October 1989 and December 2003, 1389 adults underwent LDLTx at 49 centers in Japan. In 316 (22.8%) who received LDLTx for HCC (70 females, 22%, median age 57 years; and 246 males, 88%, median age, 54 years), we analyzed pretransplant clinical status, imaging diagnosis, transplant procedure, pathologic study of explanted liver, and outcome. In 232 patients (73.4%), various surgical and nonsurgical therapies had been employed prior to LDLTx. The median follow-up period was 16 months (range, 2.5–72.0)
Results:
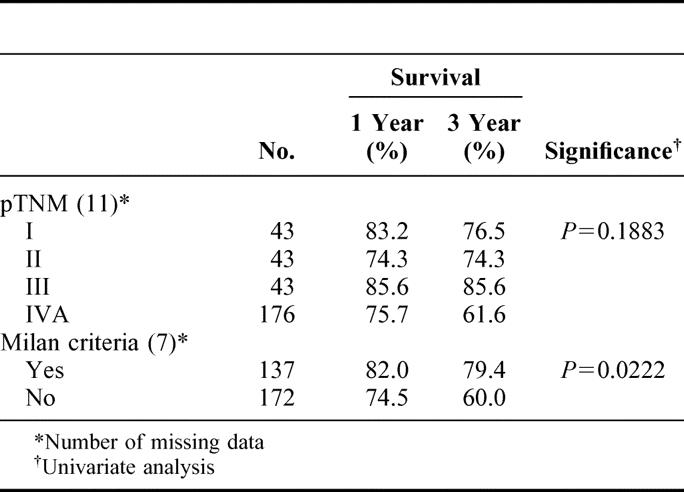
Currently, 236 (74.7%) of the patients are living. One- and 3-year patient survivals were 78.1% and 69.0%, respectively. Model end-stage liver disease score and preoperative serum alpha-fetoprotein level were independent risk factors for patient survival. Forty patients (12.7%) developed HCC recurrence. Alpha-fetoprotein level, tumor size, vascular invasion, and bilobar distribution were independent risk factors for HCC recurrence. Grade of histologic differentiation of HCC showed close correlation with tumor characteristics and recurrence. One- and 3-year recurrence-free survivals were 72.7% and 64.7%, respectively. When the Milan criteria were applied, patient survival and disease-free survival at 3 years were 78.7% and 79.1%, respectively, in patients who met the criteria, and 60.4% and 52.6%, respectively, in those who did not.
Conclusion:
LDLTx can achieve acceptable survival in HCC patients, even when liver function is markedly impaired, or HCC is uncontrollable by conventional antitumor treatments.
Living donor liver transplantation in 316 adult patients with hepatocellular carcinoma yielded 3-year patient and recurrence-free survival of 69.0% and 64.7%, respectively.
Liver transplantation (LTx) is theoretically an ultimate cure for hepatocellular carcinoma (HCC), achieving total removal of cancerous lesions and underlying liver cirrhosis.1 Through most of the 1980s, however, results of LTx for HCC were discouraging, with high recurrence rates and patient survival of only 30% at 3 years.2 In the early 1990s, Iwatsuki et al3 and Bisumuth et al4 determined clinicopathologic risk factors responsible for recurrence, which led to the development of the Milan criteria by Mazzafero et al.5 According to these criteria, LTx for HCC was limited to patients with solitary tumors up to 5 cm in diameter or with no more than 3 nodules, each 3 cm or less in diameter. Patients with extrahepatic spread and/or macrovascular invasion were ineligible. When the Milan criteria were instituted, the recurrence rate fell to 8%, and tumor-free patient survival at 4 years was 83%. With worldwide adoption of these criteria, 5-year survival rates rose to between 60% and 80%.6–8
The severe disparity between the demand for transplants and the supply of organs from deceased donors (DD), however, has meant that many HCC patients who might benefit from transplantation either die before an organ becomes available or must drop off the waiting list as the result of tumor progression.9–11 The number of annual deaths by HCC is estimated at >20,000 in the United States and at >30,000 in Japan.12,13
Living donor liver transplantation (LDLTx), initially invented for pediatric recipients,14,15 has been used recently in many adults with satisfactory results.16–21 In Japan, where use of DD organs is rare (only 24 deceased donor liver transplantations [DDLTx] have been performed in the last 6 years), LDLTx has become a common practice in the treatment of HCC. Reported herein are the results of this approach in 316 adult cases nationwide.
METHODS
From October 1989 until December 2003, a total of 2669 LDLTx were performed at 49 centers in Japan (1280 in children; 1389 in adults). HCC was the primary indication in 329 patients (12.3%) at 29 centers. Of these, 316 were included in this study; the others were excluded because they had no cancerous lesion in their liver (n = 5), had complete necrosis of HCC with preoperative therapy (n = 2), were lost to follow-up (n = 1), or were children (n = 5).
A national registry of transplant centers performing LDLTx for HCC was established in March 2000. Two questionnaires were sent each year to each center. One questionnaire inquired about the timing of patient selection, graft type, and graft volume. The other inquired about the clinical course of each case, including pretransplant liver function, imaging studies, operation, histopathological findings of the explant, and follow-up. Entry data included age, sex, viral type, Child-Pugh classification, model end-stage liver disease (MELD) score, and serum α-fetoprotein (AFP) level (ng/mL). The MELD score was calculated using an equation22 with total bilirubin level (mg/dL), creatine level (mg/dL), and international normalized ratio. Imaging studies included ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and/or CT and MRI angiogram, within 2 to 3 months prior to LDLTx. Pathologic data collected during study of the explants included number and distribution of tumors, size of largest tumor(s), severity of vascular invasion, and histologic differentiation of cancer cells occupying major part of the nodule(s). HCC invasion into the portal vein was classified as Vp0 (none), Vp1 (microscopic), Vp2 (into the segmental branch), and Vp3 (into the lobar vein or the main trunk). Data on survival, mortality, cause of death, and date and location of HCC recurrence also were collected.
Given the limits of this kind of study, definitions of respective categories could not be well standardized and controlled. All of the participating centers are experienced and well equipped, however, and all patients were managed by qualified hepatologists, radiologists, pathologists, and surgeons.
Of the 316 patients, 246 were male (77.8%) with a median age of 54 years (range, 25–70); 70 were female (22.2%) with a median age of 57 years (range, 21–70). Cirrhosis was due to viral infection in 288 patients (91.1%; HCV, n = 182 [57.6%]; HBV, n = 99 [31.3%]; both HCV and HBV, n = 7 [2.2%]) and nonviral causes (eg, alcohol, cryptogenic cirrhosis) in 28 (8.9%). More than half the patients suffered from advanced liver failure (Child's class C); approximately 10% had preserved liver function (Child's class A). Eighty-four patients (26.6%) received no treatment before transplantation. In 232 patients, anticancer therapies had been attempted, including partial or anatomic resection (n = 36, 11.4%), cytoablation by radiofrequency ablation (RFA) or microwave coagulation therapy (n = 35, 11.1%), transarterial chemoembolization (TACE; n = 139, 44%), percutaneous ethanol injection (n = 19, 6%). and systemic chemotherapy (n = 3, 0.9%).
Three of the participating centers transplanted only left lobes from living donors, for the sake of donor safety. At the other centers, either the left or the right lobe was used for LDLTx depending upon careful determination of a suitable graft volume for the recipient, Graft volume versus standard volume (GV/SV) ratio >40% or Graft volume versus body weight (GV/BW) ratio >0.8 and an acceptable remnant liver volume (>30–40% of the total volume) in the donor. Right lobes were used in 233 patients (73.7%) and left lobes in 80 (25%); 2 patients received left lateral segments and 1 received the posterior sector of the right lobe. Surgical techniques and postoperative management as currently practiced in Japan have been described elsewhere. Patients were followed until March 15, 2004, with a median follow-up period of 16 month, ranging from 2.5 to 72 months.
Statistical Analysis
Values are expressed as median, range, or percentage. Data was analyzed with χ2, t test, and analysis of variance. Survival curves were estimated by the Kaplan-Meier method and compared with log-rank test. Stepwise Cox proportional hazards regression analysis was used for multivariate analysis. The SPSS software (Release 9.0.1, Microsoft Corp., Redmond, WA) was used for statistical analysis. The number of missing data for each analysis is shown in Table 1.
TABLE 1. Univariate Analysis of Patient Survival

TABLE 1. continued.
RESULTS
Center Characteristics
The decision to perform LDLTx for HCC at 29 centers was based on 2 factors: the degree of liver failure and the extension of HCC. More than 80% of the programs considered Child's class C to be an absolute indication for LDLTx. More than 90% denied LDLTx as a first-line treatment of Child's class A or B patients, but patients with worsening liver function were re-evaluated for the procedure. Although all centers considered extrahepatic spread and major vascular invasion to be contraindications, the Milan criteria were adopted as strict criteria at only one-third of the programs. At the other centers, patients who exceeded the Milan criteria were considered for LDLTx on a case-by-case basis. Conventional treatments with medical, radiologic or surgical interventions were applied first for the treatment of HCC at 90% of the centers. In general, LDLTx was offered only when liver function was severely impaired, or HCC became uncontrollable by these modalities.
Survival
Of the 316 recipients, 236 (74.7%) are alive, with recurrent tumor in 18. One- and 3-year patient survivals were 78.1% and 69.0%, respectively; 1- and 3-year recurrence-free survivals were 72.7% and 64.7%, respectively (Fig. 1). Eighty patients (25.3%) died, 35 within 3 months after transplantation. Early deaths were from sepsis caused by various medical and surgical complications. Among pretransplant determinations, the MELD score, but not the Child's-Pugh class, was an independent risk factor for early mortality. Mortality at each MELD score was 6.9% (<10), 9.6% (10–20), 20.6% (20–30), and 50% (>30; Fig. 2). None of the pretransplant treatments increased early and late mortality (Table 1). Among the 45 deaths that occurred more than 3 months after LDLTx, 22 were caused by tumor recurrence, 15 by sepsis, and 8 by miscellaneous causes.
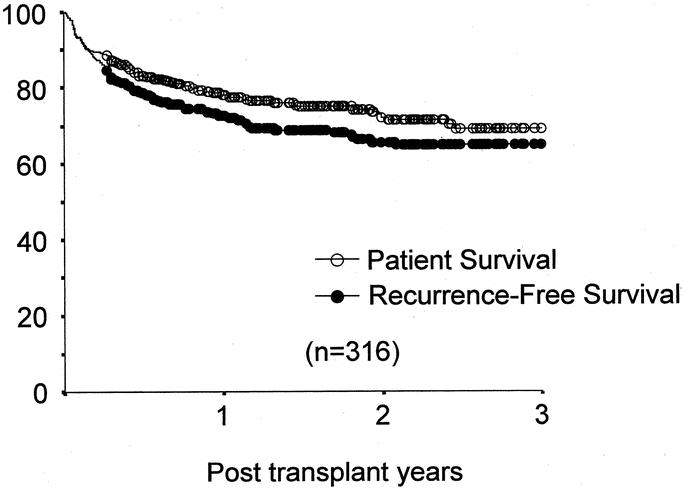
FIGURE 1. Patient- and recurrence-free survival after LDLTx.

FIGURE 2. Patient survival by MDLD score.
On univariate analysis, pretransplant serum AFP level, MELD score, and tumor characteristics (eg, size, vascular invasion and differentiation) were significantly associated with 3-year patient survival (Table 1). Pretransplant serum AFP level and the MELD score were independent risk factors for 3-year patient survival by multivariate analysis (Table 2).
TABLE 2. Multivariate Analysis

HCC Recurrence
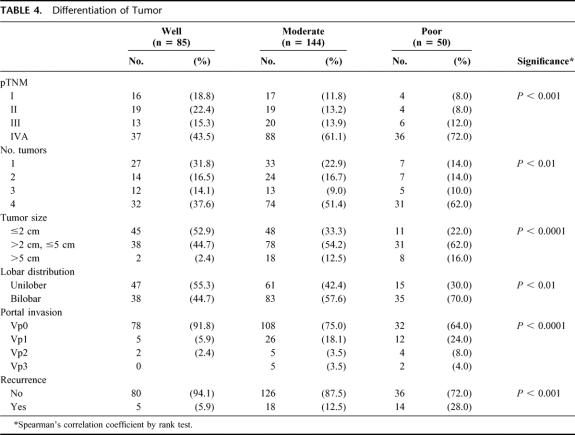
Forty patients (13%) developed HCC recurrence; 18 are alive. The cumulative incidences of tumor recurrence after LDLTx were 10 (25%) by 3 months, 17 (42.5%) by 6 months, 30 (75%) by 1 year, 37 (92.5%) by 2 years, and 40 (100%) by 3 years. The initial presentation of recurrence was confined to the liver in 6 patients (15%), to the lung in 8 (20%), and to the bone in 9 (22.5%); recurrence presented in multiple sites in 17 patients (42.5%). Pretransplant serum AFP levels and all of the pathologic tumor characteristics (ie, number, size, lobar distribution, vascular invasion and differentiation) showed significant corrections with HCC recurrence (Table 3). On multivariate analysis, pretransplant serum AFP levels, tumor distribution, and vascular invasion were independent risk factors for recurrence (Table 2). The AFP level showed close correlation with multiple tumor, larger size, macroscopic invasion, and poor differentiation. Three-year recurrence rates at each AFP level were 5% (AFP <20 ng/mL), 9.4% (20–200 ng/mL), 18.8% (200–1000 ng/mL), and 46.9% (>1000 ng/ml). Histologic grade was also correlated with number, size, vascular invasion, distribution, and recurrence rate (Table 4). Poorly differentiated HCC were associated with higher recurrence rates than well or moderately differentiated cancers.
TABLE 3. Univariate Analysis of Tumor Recurrence Factor
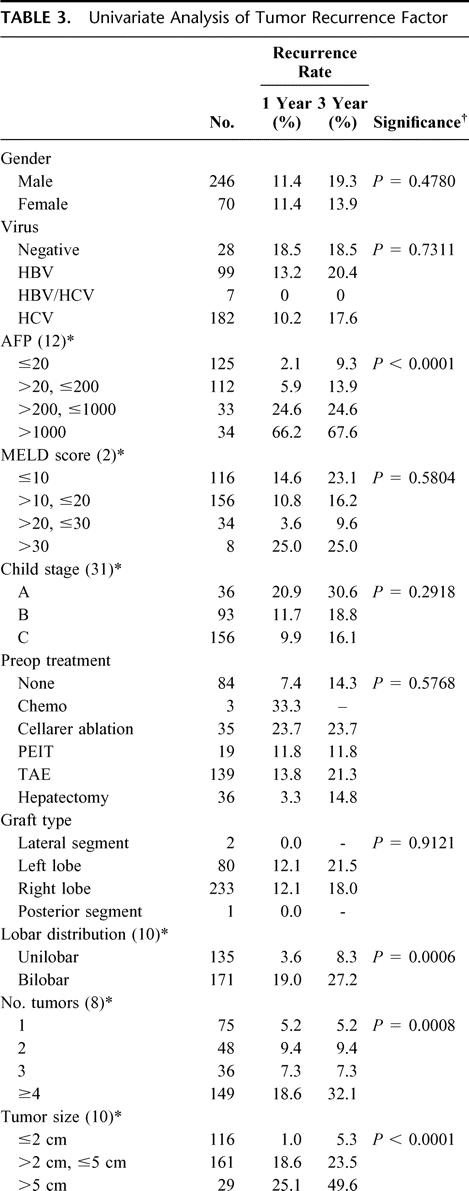
TABLE 3. continued.

TABLE 4. Differentiation of Tumor
Milan Criteria
Of the 316 recipients, 138 (43.7%) met the Milan criteria, 171 (54.1%) did not, and 7 (2.2%) were unclassified due to incomplete data. Patient survival among those who met the criteria was 81.2% at 1 year and 78.7% at 3 years, versus 75.0% at 1 year and 60.4% at 3 years among those who did not (Fig. 3). One- and 3-year recurrence-free survival were 81.6% and 79.1%, respectively, among patients who met the Milan criteria, compared with 64.9% and 52.6%, respectively, among those who did not. Among patients who met the Milan criteria, only 2 (1.4%) had tumor recurrence, while 38 (22.2%) of those who exceeded the criteria had recurrence. Cumulative HCC recurrence in patients meeting or exceeding the Milan criteria was 2.5% and 19.6% at 1 year, and 2.5% and 36.6% at 3 years, respectively (Fig. 4).
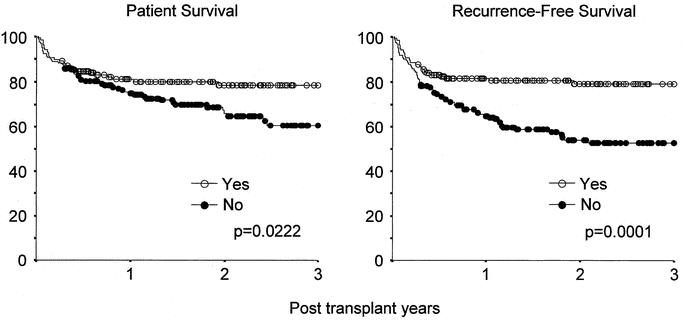
FIGURE 3. Patient- and recurrence-free survival by the Milan criteria.

FIGURE 4. Cumulative recurrence rate by the Milan criteria.
Association of the Milan criteria, as determined by pretransplant imaging studies, with the results of pathologic analyses of the explanted liver, was analyzed in the 268 cases that had complete sets of both information. Diagnosis of the tumor staging was identical in 79.5% between both analyses, whereas 14.2% were underestimated and 6.3% were overestimated by imaging studies.
DISCUSSION
Results of the present study suggest that LDLTx offers an acceptable chance and duration of survival to patients who would otherwise die soon from liver failure or HCC that cannot be controlled by conventional treatments. Pretransplant MELD scores were independent risk factors for early mortality. Various surgical and nonsurgical treatments prior to LDLTx did not augment the early and late mortality. AFP level, tumor size, vascular invasion, and bilobar distribution were independent risk factors for cancer recurrence. Histologic grade of tumor was closely correlated with tumor characteristics and recurrence. Finally, although the Milan criteria were reliable in predicting patient and disease free survival, 60% of patients who exceeded the criteria were able to live for at least 3 years after transplant.
LDLTx is being performed at many centers worldwide, but the risks and benefits to both recipient and donor must be thoroughly considered in each case.23 Donor safety is the most important concern in LDLTx. Among approximately 5000 LDLTx performed worldwide, at least 8 donors have died or required salvage with DDLTx.24,25 Recently, 1 donor developed liver failure and died in Japan, which was the first donor death after nearly 2000 consecutive cases. Furthermore, according to a recent report,26 228 (12.4%) of 1841 living donors developed various complications, such as biliary leakage, gastric stasis, pulmonary embolism, surgical infection, and others. The morbidity rate following right lobectomy in living donors was 19%, compared with 12% and 8.2% with left lobectomy and left lateral segmentectomy, respectively. In general, more than 50% of donors are thought to suffer from various minor and major complications.27 Finally, it is not yet clear to what extent medical, psychologic, and socioeconomic problems may occur in long run, particularly when the donor becomes ill or the recipient dies.
From the recipient's standpoint, on the other hand, 15% to 20% of the patients have been lost or required retransplantation of liver graft from various complications at early period after LDLTx.17,18,21,28 When liver function is severely impaired (MELD score >30), early mortality was 50% in the present study. In addition, recurrence of HCC and viral liver disease are persistent threats to long-survivors. Because of such risks, liver transplant centers in Japan reserve LDLTx as a rescue treatment and early-stage HCC is managed initially by various conventional methods (eg, microwave coagulation therapy, RFA, TACE, or resection).
Unexpectedly, the present study demonstrated that LDLTx could offer 60% 3-year survival to patients who do not meet the Milan criteria. Because 73.4% of the recipients received adjuvant and/or surgical treatments prior to LDLTx, such modalities appear to give additional benefit to the recipients in reducing HCC recurrence, or patients with higher potential for recurrence would have been eliminated before LDLTx could be attempted.
In contrast to the Japanese experience with LDLTx for HCC, outside of Japan DDLTx has been performed as a first-line treatment of patients with early HCC.9–11 In addition, in the United States, the current MELD allocation system upgrades HCC patients to avoid a drop-off of candidates during the wait for transplantation.11 Although the MELD system has been shown not to alter the mortality of waiting candidates and early survival after DDLTx in individual disease categories, more HCC patients are being transplanted since the implementation of MELD than previously. In addition, Hayashi et al29 and Freeman et al30 reported that no HCC was found in the explants in 20–30% of recipients who received DDLTx under the MELD allocation system. On the other hand, many advocate an application of various adjuvant treatments as a bridge to LTx,31–34 as practiced in Japan. Because offering DDLTx to all HCC patients is unrealistic, the Barcelona group35 suggest that, if cadaver organs are not available, patients with early stage HCC and preserved liver function should be treated by hepatic resection or RFA, and those with impaired liver function by TACE. Several studies, while not randomized, have reported favorable outcomes with TACE followed by DDLTx, particularly when large advanced HCC nodules responded well to the treatment.33,34 With regard to hepatic resection, Poon et al36 reported the possibility of salvage by LTx in 79% of the surviving patients after a median follow-up of 48 months. Morbidity and mortality after the primary LTx for HCC or the secondary LTx after resection of HCC remain controversial.37,38
The present study confirmed the importance of the Milan criteria in predicting outcome after LDLTx. Among the 138 patients who met the criteria, recurrence-free survival was 75% at 3 years; only 2 of the 138 had HCC recurrence. These results are comparable with those achieved in non-HCC recipients after DDLTx. More importantly, however, the study showed that 60% of recipients who exceed the criteria could live for at least 3 years. These recipients would be excluded from LDLTx if the Milan criteria were applied. Similar findings with DDLTx for HCC have led to proposals for expanding the criteria. The UCSF criteria39 expand the indications for transplant to a single tumor less than 6.5cm in diameter, or no more than 3 tumors, the largest of which is less than 4.5 cm, and a total diameter for all tumors of less than 8cm. Using these criteria, 1- and 5-year recurrence free survival were 90% and 75.2%, respectively. Herrero et al40 reported 87% and 70% 1- and 5-year recurrence-free survival in patients with single tumors less than 6 cm in diameter, or 2 or 3 lesions, none more than 5 cm. The Barcelona group35 is undertaking a trial of liver transplantation for patients with single tumors less than 7 cm, 3 nodules (none more than 5 cm) or 5 nodules (none more than 3 cm), aiming at 5-year survival of 50%. Finally, Iwatsuki et al41 have proposed a prognostic scoring system derived from lobar distribution, largest tumor size and vascular invasion. Tumor-free 5-year survival was 40% when the score was less than 15.
Pathologic risk factors for HCC recurrence in the present study were almost identical to those reported by others.42–45 Significant correlations of HCC recurrence with number, size, vascular invasion, distribution, and histologic grade were found. In particular, macroscopic vascular invasion was the most important risk factor for recurrence.42,43 Compared with no vascular invasion, HCC involvement of minor vessels (Vp1), the segmental branches (Vp2), or to more than the lobar branches (Vp3) was associated with 2-, 5-, and 8-fold increases in recurrence. In addition, close association of histologic differentiation with tumor characteristics and recurrence was found. This may suggest that the aggressiveness of HCC cells per se, particularly of poorly differentiated cancer cells, correlates with higher potential of recurrence, as has been described by others.43,44 Tamura et al44 reported that median recurrence-free survival of patients with poorly differentiated HCC was only 175 days, compared with 430 days in those with well to moderately differentiated HCC. Association of AFP level with HCC recurrence, as observed in this study, could be also explained by the relationship of AFP level with HCC differentiation.46
According to the recent analysis by Marsh and Dvorchick,47 with the 407 cases who underwent DDLTx at the University of Pittsburgh between 1981 and 2002, 5-year recurrence-free survival was 49.7% in patients who exceeded the Milan criteria, 45.3% outside the UCSF criteria, and 18.9% outside the Pittsburgh criteria. These figures suggest that no criteria for the selection of patients with advanced HCC can truly predict individual outcomes. Furthermore, even with current sophisticated imaging techniques, tumor stages are incorrectly diagnosed in 20–30% of patients.48 Pathologic staging can only be done in explanted livers. More importantly, vascular invasion has been identified as the most reliable predictor for HCC recurrence.41–43 If cancer cells that have infiltrated into the vasculature before transplantation or into the circulation by manipulation at operation are responsible for HCC recurrence, it may be worthwhile to determine the presence of such cells (ie, micrometastasis) before LTx. However, results with the analysis of peripheral blood and/or bone marrow have been contradictory to date.49,50 Genetic differences among HCC nodules were found to be a good predictor for recurrence,51 but the requirement of liver biopsy before transplantation may limit its universal application.
In conclusion, despite risks to donors, LDLTx can achieve acceptable survival in HCC patients, even when liver function is markedly impaired or HCC is uncontrollable by conventional antitumor treatments. Regardless of the method of pretransplant treatments, disease-free survival of LDLTx recipients who meet the Milan criteria is comparable to that after DDLTx, while recipients with advanced HCC can be rescued by LDLTx with 3-year survival of 60%. Further study is needed to establish precise prediction methods to avoid unnecessary risks to living liver donors.
ACKNOWLEDGMENTS
The authors express sincere gratitude to all of the 49 liver transplant programs, participating the HCC registry in Japan; Chiba University, Dokkyo University School of Medicine, Ehime University, Fukushima Medical University, Gunma University, Hirosaki University, Hiroshima University, Hyogo College of Medicine, Jichi Medical School, Kagoshima University, Kanagawa Children's Medical Center, Kanazawa Medical University, Kanazawa University, Kansai Medical College, Keio University, Kitasato University, Kobe University, Kumamoto University, Kyoto University, Kyushu University, Matsunami General Hospital, Mie University, Nagasaki University, Nagoya City University, Nagoya University, Nara Medical University, National Okayama Medical Center, Nihon University, Niigata University, Nippon Medical School, Okayama University, Osaka City University, Osaka Medical College, Osaka University, Sagamihara Kyodo Hospital, Shimane Medical University, Shinshu University, Showa University, Tohoku University, Tokyo Medical and Dental University, Tokyo Medical University, Tokyo Women's Medical University, Toyama Medical and Pharmacological College, University of Tokushima, University of Tokyo, University of Tsukuba, Yamaguchi University, Yokohama City University. We thank our colleagues in conducting the study, Mr. Minoru Ohta for statistic analyses, Miss. Kazuko Asari for typing the manuscript, and Ms. Nancy Ehrlich Lapid for editorial assistance.
Discussions
Dr. Ronald W. Busuttil (Los Angeles, California): Dr. Todo and his colleagues from Hokkaido have gathered a wealth of data from the Japan Transplant Registry on living donor liver transplants for patients with HCC. Their analysis of 316 cases of living donor transplant for HCC is truly the largest reported and provides us with valuable information regarding both selection criteria and outcome. The accumulation of such a large number of cases is owed to the lack of deceased donors in Japan, which was already mentioned to be only 24 such transplants in the last 6 years. From my perspective there are 4 lessons that can be learned from this series.
The first is that the preoperative variables that predict outcome for both hepatic resection and cadaveric liver transplant—namely tumor size, number of nodules, vascular invasion, and AFP, which is a surrogate marker for tumor differentiation—also apply to living donor transplantation for HCC.
The second is that the MELD score, the model for endstage liver disease, is an accurate predictor of early mortality after living donor transplant as it is for cadaveric liver transplant. In fact, in living donor transplantation the slope of the curve predicting mortality is raised for a given MELD score compared to cadaveric liver transplant.
The third is that the prognostic validity of the Milan criteria—1 tumor mass no greater than 5 centimeters, or 3 masses, none being greater than 3 centimeters—applies also to living donor transplant for HCC as it does for cadaveric transplant. Furthermore, beyond these criteria, the metrics portend a poor prognosis, with only 50% 3-year disease survival in living donor transplantation.
The final lesson is that this data, in my view, debunks the myth that living donor liver transplants are not suitable for HCC because the regeneration of a partial graft would accelerate tumor recurrence. In fact, the 3-year disease-free survival in this report of living donor transplants versus 5 other reports of cadaveric full size liver that have been reported in the literature is essentially identical at 75%.
Dr. Todo, I have 3 brief questions.
Since you use living donor transplant as rescue therapy in patients with HCC, what is your view and experience with resection as a bridge to transplant, since at this meeting last year a not very favorable report was made by Professor Bismuth's group?
Secondly, using living donors to transplant patients with tumors that exceed the Milan criteria is controversial, particularly in the United States when we now can transplant up to 25% of HCC patients with cadaveric transplants, and these are done for HCC because of the increased allocation system that UNOS provides. Do you use different criteria for these patients that exceed the Milan criteria, such as only donors with perfectly suitable anatomies or younger recipients?
Finally, what is your opinion about using living donor transplantation as the initial therapy for patients who were just put on the list to avoid the high risk of dropout and spread which you alluded to?
Dr. Satoru Todo (Sapporo, Japan): Thank you very much, Dr. Busuttil, for your generous comments and questions.
Let me answer to the last question first. We can offer live donor liver transplantation as the initial treatment for all HCC patients, as long as we are allowed to pay the penalty of the mortality of donors. The annual number of patient deaths by HCC is more than 20,000 in the United States and more than 30,000 in Japan. Currently, the mortality rate of the live donor is estimated at .5% to 1%. However, even if we could reduce the mortality to .1%, we will lose several 10 donors every year. My answer is YES, but we have to be very cautious in selecting the case for LDLTx.
With regard to the second question, as presented by Dr. Bismuth and his group last year, I believe the resection can be used as a bride to live donor liver transplantation. When we analyzed our cases with more than 500 hepatic resections that were performed over the last 10 years at my department, we found 4 different types of the patient outcome. Thirty percent of the patients developed hepatic and extrahepatic recurrence within 1 year and died very shortly. Thirty percent of the patients developed solely intrahepatic HCC recurrence approximately after 2 years. These patients were then treated by various conventional methods for several years, but finally died within 1 year after these treatments had become inapplicable. I believe they are the candidates who should have a chance to get the live donor liver transplantation before conventional treatments fail to prolong their survival. Ten percent of the patients developed liver failure within 2 or 3 years after resection without HCC recurrence, and would also be good candidates for live donor liver transplantation. The remaining 30% of patients died from various reasons. Thus, in our series, 40% of patients who underwent primary liver resection for HCC may be salvaged by LDLTx.
With regard to the Milan criteria, there is tendency nowadays that many of the transplant centers in the United States and Europe are trying to expand the criteria. According to the recent analysis by Dr. Marsh with more than 400 cases who underwent cadaveric transplantation at Pittsburgh from 1985 to 2000, a 5-year recurrence-free survival rate was estimated at nearly 45% in those who were outside of the Milan criteria. These and our results suggest that it is the time to reconsider the Milan criteria for the indication of patients with HCC, particularly in LDLTx.
Dr. Charles Miller (Cleveland, Ohio): Dr. Todo, I want to congratulate you and your many colleagues in Japan for this really excellent and informative series, your superb results, and, importantly, the worldliness of your community there to share in detail the cases amongst yourselves and now with us. I really appreciate being able to have read the manuscript in advance. I have a couple of comments and just a couple of questions basically, one about the patients within the Milan criteria and then one for the patients beyond.
It seems clear that prompt and expeditious transplantation of patients within the Milan criteria, HCC, have relegated these relatively small tumors really to the category of complication of liver disease, such as the size, encephalopathy, and bleeding varices. But you comment that you really wait and try all the other therapies before getting to transplant.
You also show that transplantation at the earlier phases of the MELD score is far better, these tumors don't recur, and they are unpredictable pre-transplant, even with conventional therapies. And you comment in the manuscript about the safety of left lobe transplantation. So I would like to take Dr. Busuttil's question one step further, because you said donor safety would predict its use as a preemptive therapy. Wouldn't left lobe transplantation and the safety with that allow this to be used as a preemptive therapy for Milan criteria patients?
For those patients with tumors exceeding the Milan criteria, Dr. Busuttil commented that the survival is the same for cadaveric transplantation. Well, that is true. We published a series in Annals of Surgery in 80 patients that we put on the list for exceeding Milan criteria, and we, too, got about 50% 5-year survival. The problem was, half of those patients died before they ever had an opportunity. So an intention-to-treat analysis shows that survival is probably no better than 20 to 25%. So do you think that living donor transplants is preferable to cadaveric transplantation for these patients?
And in terms of screening and new adjuvant therapies, could you educate us about what you are doing to best screen these patients and provide them with a multimodality pre- and post-transplant to try to get our survival from 50% up to 70%-75%?
Dr. Satoru Todo (Sapporo, Japan): Thank you very much for your comments and questions, Dr. Miller. With regard to the second question, the influence of tumor numbers or tumor size on the recurrence rate was not so strong than that of vascular invasion in our analysis. I believe that HCC recurrence after LDLTx is closely related to the aggressiveness of HCC itself, rather than numbers or the sizes themselves. Probably, HCC cells might have invaded into the vasculature before liver transplant is attempted, or migrated into the circulatin by manipulation of the liver at the time of recipient liver removal. If we could detect HCC cells in the circulation or the bone marrow before transplantation by using recent molecular technique, those patients could be eliminated from the waiting list. Similarly, as Dr. Busuttil reported 10 years ago and you reported several years ago in this meeting, adjuvant therapies, such as systemic chemotherapy and TAE, at preoperative period may be effective. However, it is not sure yet, if they can control HCC spreading and recurrence.
With regard to the left lobe, we should use the left lobe graft more often in HCC patients, because they have only slight portal hypertension. I had one such patient who developed multiple HCC lesions 1 year after left lobectomy. He had 1 donor; his wife who was very small in size and had anatomical problems. She could offer only the left lobe to her husband, with BV/SV ratio of only 23%. However, because the recipient didn't have any portal hypertension, the patient went well after LDLTx without any clinical complications associated with small-for-size syndrome. Thus, I believe that the use of the left lobe depends on the severity of the portal hypertension. The left lobe transplant is safer to the donor than using the right lobe, because the right lobe hepatotectomy has the morbidity rate of 20% compared to 10% after left lobe donations.
Dr. Christoph Broelsch (Essen, Germany): This is a very important paper that you presented to us today because it can show the way of the use of living related liver transplants not only in the Japanese background but also in the countries where we do have cadaveric organs. Because the standards for the use of these cadaveric organs, as has already been said, is limited to the state-of-the-art indication for transplantation, which is limited for tumors in the liver.
I have a question to your presentation. Several of your patients exceeded the so-called Milan criteria. How secure, how accurate was your preoperative staging for these particular patients? And did you deliberately exceed Milan criteria even when you saw in the staging that those were larger than 2 centimeters and more than three nodules?
My group and others have advocated the use of living related in expanded indications already for years and we have been very much criticized for that. I was very glad to see that your results indicate that there was 60% survival in an otherwise unresectable untreatable cancer. This is better than any other oncological treatment I know about. I congratulate you for your results.
Dr. Satoru Todo (Sapporo, Japan): Thank you for your question, Dr. Broelsch. Of course, all of the patients who have macrovascular invasions and evident extrahepatic spread are not candidates for LDLTx, but we have to be aware of the evidence that pre-transplant imaging studies have a limit in accuracy. We found 16% of patients in this study are underestimated and about 10% of patients are overestimated in preoperative imaging studies. Fortunately, however, none of our patients had lymph node metastases. With any suspicious patients, we always study the lymph node involvements before starting the dissectin of hepatic vasculatures.
Footnotes
Supported by the grant from the Ministry of Health, Labour and Welfare (# H-12-regenerative-017).
Reprints: Satoru Todo, MD, The First Department of Surgery, Hokkaido University Graduate School of Medicine, North 7, West 5, Kita-ku, Sapporo 060-8638 Japan. E-mail: stodo@med.hokudai.ac.jp.
REFERENCES
- 1.Starzl TE, Putnam CW. Candidacy. In: Starzl TE. Putnam CW, eds. Experience in Hepatic Transplantation. Philadelphia, PA: Saunders; 1969:3–8. [Google Scholar]
- 2.Penn I. Hepatic transplantation for primary and metastasis cancer of the liver. Surgery. 1991;110:726–735. [PubMed] [Google Scholar]
- 3.Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bismuth H, Chiche L, Adam R., et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinoma in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Bruix J, Fuster J, et al. Liver transplantation for treatment of small hepatocellular carcinoma: the TNM classification does not have prognostic power. Hepatology. 1998;27:15272–1577. [DOI] [PubMed] [Google Scholar]
- 7.Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311–322. [DOI] [PubMed] [Google Scholar]
- 8.De Carlis L, Giacomoni A, Lauterio, et al. Liver transplantation for hepatocellular carcinoma: Should the current indication criteria be changed? Transpl Int. 2003;16:115–122. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. [DOI] [PubMed] [Google Scholar]
- 10.Yao FY, Bas NM, Nikolai B, et al. Liver transplantation for hepatocellular carcinoma: Analysis of survival according to the intention-to-treat principle and drop-out from the waiting list. Liver Transpl. 2002;8:873–883. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Balan V, Hernandez JL, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. [DOI] [PubMed] [Google Scholar]
- 12.Bosch FX. Global epidemiology of hepatocellular carcinoma. In: Okuda K, Tabor E, eds. Liver Cancer. New York: Churchill Livingstone; 1997:13–28. [Google Scholar]
- 13.El-Serag H. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107. [DOI] [PubMed] [Google Scholar]
- 14.Strong RW, Lynch SV, Ong TH, et al. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322:1505–1507. [DOI] [PubMed] [Google Scholar]
- 15.Broelsch CE, Emond JC, Whitington PF, et al. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wachs ME, Bak TE, Karrer FM, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313–1316. [DOI] [PubMed] [Google Scholar]
- 17.Marcos A, Fisher RA, Ham JM, et al. Right lobe living donor liver transplantation. Transplantation. 1999;68:793–803. [DOI] [PubMed] [Google Scholar]
- 18.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplantation in adults and children: a single center experience. Ann Surg. 2001;234:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka K, Uemoto S, Tokunaga Y, et al. Surgical techniques and innovations in living-related liver transplantation. Ann Surg. 1993;217:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishizaki T, Ikegami T, Hirosige S, et al. Small graft for living donor liver transplantation. Ann Surg. 2001;233:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan ST, Lo CM, Lui CL, et al. Determinants of hospital mortality of adult recipients of right lobe live donor liver transplantation. Ann Surg. 2003;238:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiesner RH, McDiarmid SV, Kamath PS, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567–580. [DOI] [PubMed] [Google Scholar]
- 23.Shiffman ML, Brown RS, Olthoff KM, et al. Living donor liver transplantation: Summary of a conference at the National Institutes of Health. Liver Transpl. 2002;8:174–188. [DOI] [PubMed] [Google Scholar]
- 24.Trotter JF, Wachs M, Everson GT, et al. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. [DOI] [PubMed] [Google Scholar]
- 25.Strong RW. Whither living donor liver transplantation? Liver Trasnpl Surg. 1999;5:536–538. [DOI] [PubMed] [Google Scholar]
- 26.Umeshita K, Fujiwara K, Kiyosawa K, et al. Operative morbidity of living liver transplantation in Japan. Lancet. 2003;362:687–690. [DOI] [PubMed] [Google Scholar]
- 27.American Society of Transplant Surgeons’ Position Paper on Adult-to-Adult Living Donor Liver Transplantation. Liver Transpl. 2000;6:815–817. [DOI] [PubMed] [Google Scholar]
- 28.Kaihara S, Kiuchi T, Ueda M, et al. Living-donor liver transplantation for hepatocellular carcinoma. Transplantation. 2003;75:S37–S40. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi PH, Trotter JF, Forman L, et al. Impact of pretransplant diagnosis of hepatocellular carcinoma on cadaveric liver allocation in the era of MELD. Liver Transpl. 2004;10:42–48. [DOI] [PubMed] [Google Scholar]
- 30.Freeman RB, Harper A, Edwards E. The MELD system and hepatocellular carcinoma [Abstract]. Am J Transpl. 2003;3(suppl 5):284. [Google Scholar]
- 31.Fontana RJ, Hamidullah, Nghiem, H et al. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165–1174. [DOI] [PubMed] [Google Scholar]
- 32.Donckier V, Van Laethem JL, Van Gansveke D, et al. New considerations for an overall approach to treat hepatocellular carcinoma in cirrhotic patients. J Surg Oncol. 2003;84:36–44. [DOI] [PubMed] [Google Scholar]
- 33.Roayaies Frischer JS, Erme SH, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2000;235:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transplant lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruix J, Llovet J. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. [DOI] [PubMed] [Google Scholar]
- 36.Poon RTP, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection small hepatocellular carcinoma in patients with preserved liver function; Implication for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis; A reasonable strategy? Ann Surg. 2003;238:508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belghiti J, Cortes A, Abdalla EK, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. [DOI] [PubMed] [Google Scholar]
- 40.Herrero IJ, Sangro B, Quiroga J, et al. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Tranpl. 2001;7:631–636. [DOI] [PubMed] [Google Scholar]
- 41.Iwatsuki S, Dvorchil I, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma: A proposal of prognostic scoring system. J Am Coll Surg. 2000;191:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh JW, Dvorchik I, Bonham CA, et al. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538–543. [DOI] [PubMed] [Google Scholar]
- 43.Jonas SV, Bechsein WO, Steinmuller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation to hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. [DOI] [PubMed] [Google Scholar]
- 44.Tamura S, Kato T, Berho M, et al. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25–30. [PubMed] [Google Scholar]
- 45.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inamura H, Matsuyama Y, Miyagawa Y, et al. Prognostic significance of anatomical resection and des-γcarboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg. 1999;86:1032–1038. [DOI] [PubMed] [Google Scholar]
- 47.Marsch JW, Dvorchick I. Liver organ allocation for hepatocellular carcinoma: are we sure? Liver Transpl. 2003;41:693–696. [DOI] [PubMed] [Google Scholar]
- 48.Libbercht L, Bielen D, Verslype C, et al. Focal lesions in cirrhotic explant livers: Pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749–761. [DOI] [PubMed] [Google Scholar]
- 49.Sato Y, Ichida T, Suzuki S, et al. Living related donor liver transplantation for preoperative α-fetoprotein mRNA-positive patients of hepatocellular carcinoma: description of five cases. Transplant Proc. 2003;35:352–353. [DOI] [PubMed] [Google Scholar]
- 50.Lemonine A, Bricon TL, Salvucci M, et al. Prospective evaluation of circulating hepatocytes by alpha-fetoprotein mRAN in humans during liver surgery. Ann Surg. 1997;226:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finkelstein SD, Marsh W, Demetris AJ, et al. Microdissection-based allelotyping discriminates De novo tumor from intrahepatic spread in hepatocellular carcinoma. Hepatology. 2003;37:871–879. [DOI] [PubMed] [Google Scholar]