Abstract
Objective:
We sought to identify the rate of axillary recurrence after sentinel lymph node (SLN) biopsy for breast cancer.
Summary Background Data:
SLN biopsy is a new standard of care for axillary lymph node staging in breast cancer. Nevertheless, most validated series of SLN biopsy confirm that the SLN is falsely negative in 5–10% of node-positive cases, and few studies report the rate of axillary local recurrence (LR) for that subset of patients staged by SLN biopsy alone.
Methods:
Through December of 2002, 4008 consecutive SLN biopsy procedures were performed at Memorial Sloan-Kettering Cancer Center for unilateral invasive breast cancer. Patients were categorized in 4 groups: SLN-negative with axillary lymph node dissection (ALND; n = 326), SLN-negative without ALND (n = 2340), SLN-positive with ALND (n = 1132), and SLN-positive without ALND (n = 210). Clinical and pathologic characteristics and follow-up data for each of the 4 cohorts were evaluated with emphasis on patterns of axillary LR.
Results:
With a median follow-up of 31 months (range, 1–75), axillary LR occurred in 10/4008 (0.25%) patients overall. In 3 cases (0.07%) the axillary LR was the first site of treatment failure, in 4 (0.1%) it was coincident with breast LR, and in 3 (0.07%) it was coincident with distant metastases. Axillary LR was more frequent among the unconventionally treated SLN-positive/no ALND patients than in the other 3 conventionally treated cohorts (1.4% versus 0.18%, P = 0.013).
Conclusions:
Axillary LR after SLN biopsy, with or without ALND, is a rare event, and this low relapse rate supports wider use of SLN biopsy for breast cancer staging. There is a low-risk subset of SLN-positive patients in whom completion ALND may not be required.
Among 4008 consecutive patients having sentinel lymph node (SLN) biopsy for unilateral invasive breast cancer, we have observed axillary local recurrence in 10 individuals (0.25%) at a median of 31 months (10,354 woman-years) of follow-up. Axillary local recurrence following SLN biopsy is comparable to that of axillary dissection.
Since the initial reports of Krag et al1 and Giuliano et al2 describing sentinel lymph node (SLN) biopsy for breast cancer, more than 60 observational studies of SLN biopsy validated by a “backup” axillary lymph node dissection (ALND) demonstrate that SLN biopsy is feasible, accurate, and suitable for virtually all patients with operable, clinically node-negative disease.3 It works well in a wide range of practice settings4,5 and with a variety of techniques.6–8 Most important, SLN biopsy allows enhanced pathologic analysis (with serial sectioning and anticytokeratin staining) to be performed on a routine basis, increasing the accuracy of staging9 and reducing the proportion of false-negative results.10
Finally, the SLN biopsy procedure has proven to be safe; serious allergic reactions to isosulfan blue dye are infrequent (0.5% in 1 large series11), radiation exposure from 99mTc is trivial,12 and sensory morbidity is less than that of ALND.13 However, the long-term sequelae of SLN biopsy remain to be defined and especially the incidence of axillary local recurrence (LR). If the SLN is falsely negative in about 5% of node-positive patients,7 will SLN-negative patients (treated without ALND) be at an increased risk of subsequent axillary LR? Here, we aim to address this concern by examining our entire experience with SLN biopsy, with particular emphasis on the incidence and pattern of axillary LR.
METHODS
Between September 1996 and December 2003, 6278 patients underwent SLN biopsy at Memorial Sloan-Kettering Cancer Center (MSKCC), and were entered prospectively into the MSKCC Breast Cancer SLN Database. Review of clinicopathologic features and follow-up of all patients for this study was approved by the MSKCC Institutional Review Board.
Exclusion criteria included (1) failed SLN mapping, (2) benign disease (primarily cases of prophylactic mastectomy), (3) bilateral breast cancer, (4) intraductal carcinoma (DCIS), (5) inflammatory cancer, and (6) primary breast tumors of nonmammary origin. To allow at least 1 year of potential follow-up, patients treated after December 2002 also were excluded, leaving 4008 patients with unilateral invasive breast cancer for this analysis.
Our technique of SLN biopsy has previously been described in detail.14 Briefly, all patients had SLN mapping using a combination of radioisotope (unfiltered 99mTc sulfur colloid injected intradermally) and isosulfan blue dye (injected subdermally or peritumorally). All blue and/or focally hot and/or palpably suspicious nodes were considered to be SLN7 and were submitted for intraoperative frozen section (FS). Final pathologic examination of FS-negative SLN included serial sections and immunohistochemical (IHC) anticytokeratin staining, taking 1 hematoxylin-eosin and 1 IHC-stained section from each of 2 levels 50μ apart.
After a validation phase in which we performed a planned backup ALND on all patients having SLN biopsy, our mature treatment algorithm was to perform no further axillary surgery in SLN-negative patients, and ALND in all SLN-positive patients (whether positive on FS or on final pathology). For this analysis, patients were further categorized as SLN negative with ALND (n = 326, representing our validation phase), SLN negative without ALND (n = 2340, representing our current standard practice), SLN positive with ALND (n = 1132, also representing our current standard practice), and SLN positive without ALND (n = 210, representing a group of patients who either refused completion ALND or were felt to be at low risk of having residual axillary disease).
For uniformity of comparisons, “ALND” was defined as the removal of a total of at least 10 lymph nodes. Among the “ALND” group, 7.2% were considered by the operating surgeon not to have had ALND, and in the “no ALND” group, 4.9% were considered to have had ALND. After surgery, all decisions regarding systemic adjuvant treatment and radiotherapy were based on conventional criteria. Follow-up of all patients included a physical examination of the breast and axilla at 3–6 month intervals, and annual mammography (with or without screening ultrasound). Other imaging studies were done only on the basis of symptoms or physical findings. LR were categorized as (1) axillary LR as the first site of treatment failure, (2) axillary LR coincident with breast LR, and (3) axillary LR coincident with distant disease. Categorical variables were compared using the Fisher exact test and statistical analyses of continuous and categorical variables used SPSS (SPSS Inc., Chicago, IL) and StatXact 5 (Cytel Software Corp.).
RESULTS
Among all 6278 patients (and after excluding 81 patients with incomplete data) the SLN was identified by blue dye in 81.5% (5049/6197), by isotope in 93.5% (5796/6197) and by dye and/or isotope in 97.3% of cases. The SLN procedure failed in 2.7% of cases. Among the 4008 study patients with unilateral invasive breast cancers treated prior to December 2002, median follow-up was 31 months, or 10,354 woman-years.
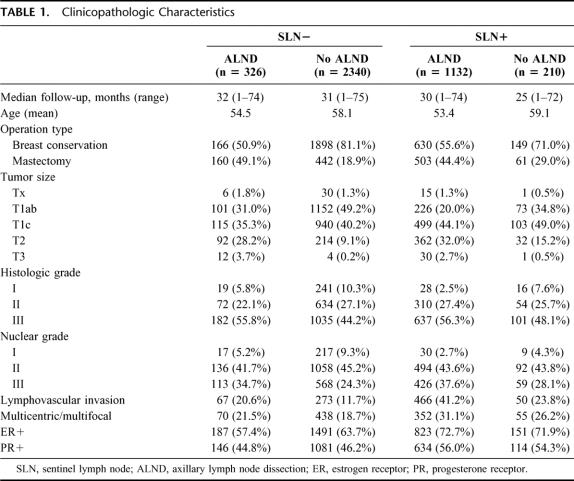
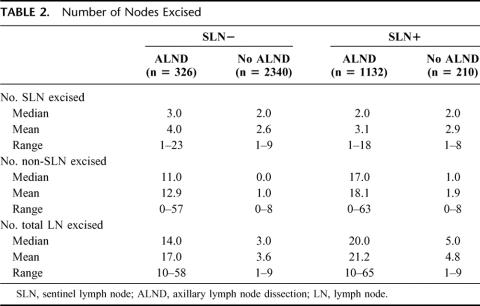
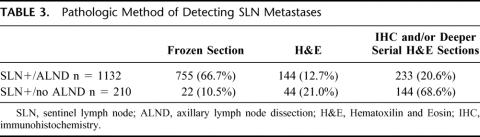
Clinicopathologic characteristics for each of the 4 patient cohorts are outlined in Table 1. Those patients who had SLN biopsy with ALND (compared with SLN biopsy without ALND) were characterized by higher rates of mastectomy, larger tumors, and more lymphvascular invasion. Numbers of SLN and of non-SLN removed were comparable in both of the no-ALND groups and in both of the ALND groups (Table 2). Of the SLN-positive patients, those who had ALND were more likely to have been diagnosed on FS, whereas those who did not have ALND were more likely to have been diagnosed by serial sections and/or IHC (Table 3).
TABLE 1. Clinicopathologic Characteristics
TABLE 2. Number of Nodes Excised
TABLE 3. Pathologic Method of Detecting SLN Metastases
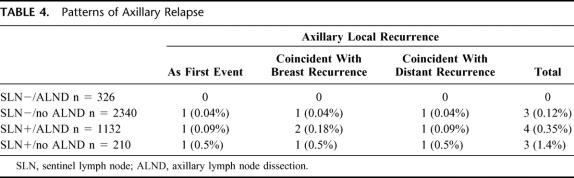
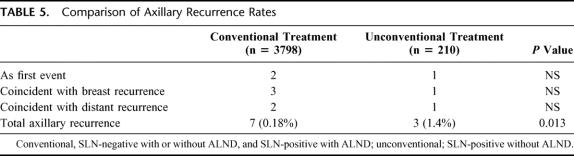
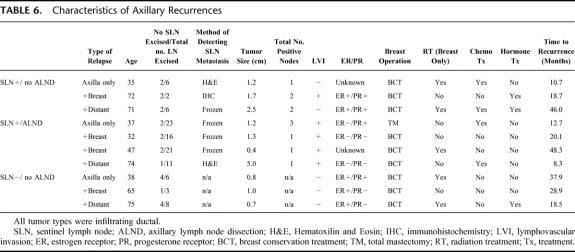
Across all 4008 patients (Table 4), there were a total of 10 axillary relapses (0.25%, Table 5), with only 3 (0.07%) as the sole initial site of treatment failure. Comparing the 3 cohorts treated “conventionally” (SLN negative/ALND, SLN negative/no ALND, and SLN positive/ALND) with those treated “unconventionally” (SLN positive/no-ALND), axillary LR was more frequent in the latter group (Table 5, 0.18% vs. 1.4%, P = 0.013). In summarizing the clinicopathologic characteristics of all 10 patients who had an axillary LR (Table 6), there was no pattern which could distinguish those who developed axillary LR from those who did not.
TABLE 4. Patterns of Axillary Relapse
TABLE 5. Comparison of Axillary Recurrence Rates
TABLE 6. Characteristics of Axillary Recurrences
Among 210 SLN positive/no ALND patients, 149 had breast conservation, and 53 of these had radiotherapy (RT) at MSKCC, allowing a detailed audit of their RT records and films. In this group, 43% (23/53) had RT to the breast only, and 57% (30/53) received additional tangent fields to the axilla. No patient in this subset developed axillary LR.
DISCUSSION
The historic rationale for ALND in breast cancer has been 3-fold: prognostication, the prevention of axillary LR, and the possibility of a small survival benefit from the removal of positive axillary nodes. During the past decade, SLN biopsy has emerged as a new standard of care in this setting and has allowed the elimination of ALND for a group of patients who are unlikely to benefit from it, those with negative axillary nodes. A substantial and largely observational literature has asked and answered many questions regarding the feasibility, accuracy, technique, case selection, learning curve, morbidity, and short-term safety of SLN biopsy. However, given the procedure's false-negative rate of approximately 5%, questions regarding long-term safety (and especially local control in the axilla) after SLNB remain unanswered. For SLN biopsy to replace ALND as a new standard of care, it is critical that axillary local control be at least comparable to that achieved after ALND.
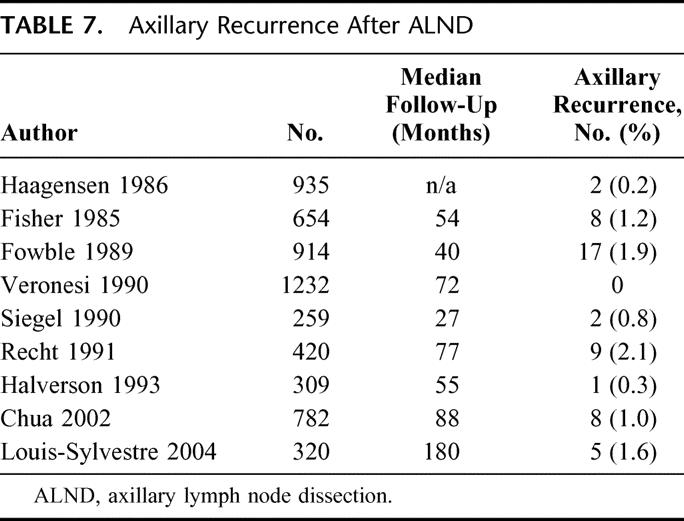
Recurrence in the axilla after ALND is quite infrequent, with 9 studies15–23 reporting axillary LR after ALND ranging from 0% to 2.1% at follow-up of 40–180 months (Table 7). The 10-year report from NSABP-B04 trial,16 which compared radical mastectomy (RM), total mastectomy (TM) plus radiation, and TM alone, in women with clinically node-negative breast cancers supports this finding; in the RM group, 40% of whom had positive axillary nodes, only 1% recurred in the axilla. In the TM group, 18.6% developed clinical axillary LR, requiring reoperative ALND at a median interval of 14.8 months (range, 3–134), with 75% of all axillary recurrences occurring within the initial 24 months of follow-up. The 25-year results of NSABP-B0424 are comparable, showing ALND to be very effective in preventing axillary LR but also showing no significant difference in distant disease-free survival among the 3 arms of the study. Of note, B-04 was underpowered to detect small survival differences; 2 randomized trials25,26 and a recent overview27 suggest that there is a small but significant impact of local control on survival.
TABLE 7. Axillary Recurrence After ALND
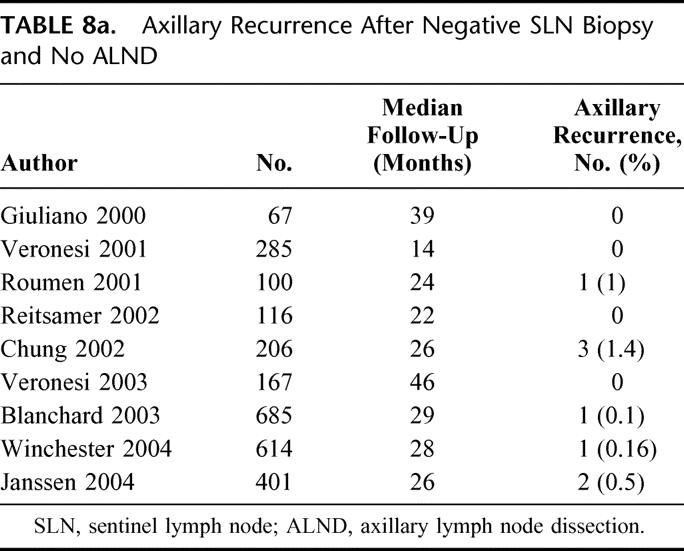
Given the low rate of axillary LR after ALND, it is understandable that LR after SLN biopsy would be of concern. Nine studies28–36 have now reported comparably good results in patients with a negative SLN biopsy and no ALND, with axillary LR ranging from 0% to 1.4% at 14–46 months of follow-up (Table 8a). Of particular note is Veronesi's randomized trial33 comparing SLN biopsy with ALND to SLN biopsy with ALND only if the SLN contained metastases. Although there was a false-negative rate of 9% in the SLN biopsy with ALND arm, there was no axillary LR in either arm of the trial at a median follow-up of 46 months. Our own results in SLN-negative patients with no ALND substantiate this finding, with an extremely low rate of axillary LR, 0.12% (3/2340), at a median follow-up of 31 months. This low recurrence rate is quite comparable to that following conventional ALND, and justifies the emerging consensus that ALND can be omitted safely when the SLN is negative.
TABLE 8a. Axillary Recurrence After Negative SLN Biopsy and No ALND
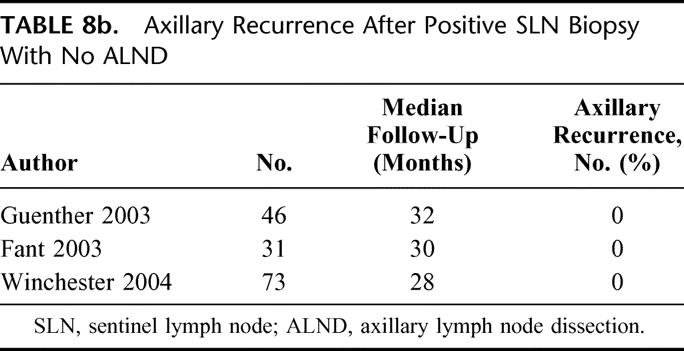
Across our entire experience with SLN biopsy, local control appears to be comparable to that after ALND, regardless of SLN status or performance of ALND. Axillary LR was infrequent both in SLN-positive patients who had completion ALND and those who did not, 0.35% and 1.4%, respectively. Although axillary LR was more frequent in the unconventionally treated SLN-positive/no ALND group than in the rest of our patients (Table 5), this small difference (while statistically significant) may lack clinical significance. Van Zee et al.37 have recently drawn on our experience to develop a multivariate nomogram for the prediction of non-SLN status in SLN-positive cases, and we have increasingly used this tool in counseling those patients whose SLN prove positive on final pathology. Not surprisingly, patients in the SLN-positive/no ALND cohort were older, had smaller tumors, less frequent lymphvascular invasion, and lower-volume SLN metastases. There are 3 reports35,38,39 in the literature regarding this subset of patients (Table 8b), with no axillary LR at 28–32 months of follow-up. These results together with our own suggest that there is a low-risk subset of SLN-positive patients in whom ALND might not be needed, but require longer follow-up to be substantiated. An ongoing prospective randomized trial, ACOSOG Z0011,40 aims to answer to this question.
TABLE 8b. Axillary Recurrence After Positive SLN Biopsy With No ALND
Some caveats apply to our findings. The most significant is the relatively short follow-up of 31 months. Although 75% of axillary LR after total mastectomy in the 25-year report of the NSABP B-04 trial24 occurred within 2 years, breast cancer has a long natural history and low-volume residual disease might take much longer to become clinically apparent. A second caveat is whether our observed low rate of axillary LR in the era of SLN biopsy is simply the result of contemporary adjuvant treatments; chemotherapy,41 tamoxifen,42 and RT43 all act to reduce LR independently of surgery. In our own internal audit, more than half of the SLN-positive/no ALND cohort patients who were treated at MSKCC received RT, which was modified to better treat the axilla. Finally, given the small number of events in our series, it is impossible to identify predictors of axillary LR at this time.
SLN biopsy is an increasingly well-accepted standard of care in breast cancer and appears equivalent if not superior to conventional ALND in accurately staging the axilla and preventing axillary LR. Across our entire experience of 4008 cases (representing 10,354 woman-years of follow-up), and even in our unconventionally-treated subset of 210 SLN-positive/no ALND patients, axillary LR appears to be infrequent, and comparable to that of ALND. Although longer follow-up is required to substantiate these results, the fear that axillary LR after SLN biopsy would exceed that of ALND appears to be unjustified.
Discussions
Dr. Kirby I. Bland (Birmingham, Alabama): President Jones, Secretary Pellegrini, Dr. Eberlein, Fellows and Guests of the Association: I wish to congratulate Dr. Cody and his colleagues at Memorial for bringing a most important study to our attention. This study precedes the long-awaited results of The American College of Surgeons Oncology Trial Z0011 evaluating the sentinel node positive patient having completion versus omission of the axillary dissection. The analysis draws on a large group of consecutive patients (n = 4008) representing a median follow-up of 31 months and over 10,000 woman years of evaluation.
Since sentinel node biopsy was introduced for melanoma by Don Morton in 1992, the application of the technique to breast cancer axillary metastasis by numerous institutions has allowed the procedure to become the new standard of care for axillary node staging. More than 60 observational studies using the biopsy technique have been validated with “back up” ALND demonstrating that SLNB is a highly accurate and reproducible technique with false-negative rates that approximate 5%. Thus, the question posed by the authors is most appropriate and one that should be answered in prospective randomized studies. However, this large prospective database at Memorial provides us excellent opportunity to provide contemporary endpoints prior to the prospective trial that we are trying to complete in the College trial which will address the incidence and patterns of axillary nodal recurrence as well as survival outcomes.
Questions for the authors: Among the 4,000+ patients evaluated in the analysis, there was a total of 10 axillary relapses representing a frequency of 0.25%. As presented, the greatest frequency of this recurrence rate was in the SLN-positive/no ALND group (n = 3; 1.5%). However, this frequency is 12.5 times that rate for the SLN-negative/no ALND group (0.12%; n = 0.013). It is thus this group that would potentially obtain the greatest benefit with subsequent ALND (Levels I and II) to abrogate the potential of local-regional recurrence and possibly enhance survival.
Thus my question, with the short-term follow-up in which failures were evaluated with a range of 8–46 months, do you have data relative to patterns of failure and survival rates? Your follow-up of 31 months, while showing this difference in local-regional recurrence, despite the small total numbers, could possibly be translated into a negative impact on survival.
In this era of contemporary adjuvant therapies with chemotherapy, antiestrogens (SERMs) and radiation therapy to reduce local recurrence, do you foresee additional analyses that will shed light on this critical question?
Third, do you think that the SLN+/no ALND cohort treated at Memorial should be treated with axillary radiation to better control subsequent disease? In the B-04 trial of the NSABP, a 19% failure rate within 24 months of total mastectomy without radiation treatment was observed; most of these patients were treated with Level I, II, and III dissections. Is it possible that the long natural history and low-volume nodal disease will confound this issue; what will be long-term follow-up outcomes on disease-free survival?
The authors indicate a greater than X4 recurrence in the axilla for the SLN+ patient who is not treated with ALND. While nodal recurrence was more frequent in this unconventionally treated group than for the rest of their patients, this small difference may lack clinical significance. As noted above, we do not know the ultimate impact of this variance on survival, which hopefully Z0011 of the College trials will answer. I would like to question the authors regarding objective pathological features that predict a greater frequency of recurrence rate in these subsets. Your group, similar to that of Van Zee et al, has drawn on multivariant nomograms for prediction of clinicopathologic features in SLN+ patients (eg, young age, larger tumors, extensive lymphovascular invasion, micro-metastases, etc). These nomograms can be utilized to counsel patients whose SLN proves positive or negative on final evaluation pathologically. Could you instruct us how these nomograms are routinely utilized at Memorial for the SLN-positive/no ALND cohort in whom 210 of these patients were managed with this “unconventional” treatment? It will be impossible to know the patterns of failure and impact on survival until the prospective randomized trial ACOSOG Z0011 is completed.
Again, I thank the authors for bringing this most important study to the attention of the Association which confirms that axillary recurrence following SLNB, with and without ALND, is uncommon. This study confirms that this low relapse rate corroborates wider use of the SLN biopsy for breast cancer staging. Further delineation of the low-risk subset of patients in whom sentinel lymph node positive nodes were not treated with conventional ANLD needs further clarification and this study will further our knowledge of this issue. I wish to thank the Association for the privilege of the floor and again thank the authors for the opportunity to review this manuscript in advance.
Dr. Hiram S. Cody, III (New York, New York): Thanks very much, Dr. Bland.
Regarding the first question—and this slide again summarizes it—there is approximately 10 times the rate of axillary recurrence in the unconventional group compared to the conventional treatment group, 1.4% versus 0.18%. This, in fact, is why we recommend to sentinel lymph node positive patients that they have a completion axillary dissection. I should add, though, that in breast cancer you have to have a high rate of local recurrence before you begin to see an adverse survival impact, and my educated guess is that a 1 to 2% increase in axillary recurrence would not change survival at all.
The use of adjuvant therapy will pose a major problem in interpreting the results of all our clinical trials from here on. Despite clear surgical endpoints, there will be heavy contamination by variations in systemic treatment, and this will confound the interpretation of current breast cancer trials as they begin to mature. In our unconventional group we were able to go back and audit the additional treatment they received based on not having an axillary dissection, and among patients treated at our institution about half had their radiotherapy parameters altered to encompass at least part of the undissected axilla.
Finally, regarding the nomogram, Kim Van Zee of our group has developed a multivariate nomogram, which allows us to predict for sentinel node positive patients the likelihood of residual axillary nodal disease. This has proven very useful, but different patients given the same results may still decide on different courses of action.
Dr. Merrick I. Ross (Houston, Texas): Dr. Cody, you continue to show us why your group continues to take a leadership role in this particular cancer-related issue.
Actually, Dr. Bland addressed most of my questions. But we didn't really talk Group 4, which is the 2000 group, the ones that had sentinel node negative and no axillary dissection. I think you showed very nicely that a negative sentinel node biopsy is a good way to accomplish durable local-regional control. And I think that is very convincing data. But there is a false negative rate of 8% based on the Veronesi study, which means that not all patients that have microscopic disease will become clinically relevant in the axilla. But we also know from the B-04 data that not all patients with microscopic disease actually failed in the axilla. And just because they don't fail in the axilla doesn't mean there isn't microscopic disease that may be clinically relevant.
The reason why I am bringing this up is because there are, as we go up and try to have really diagnosis with breast cancer, the vast majority of our patients will have small tumors, and, therefore, the role of adjuvant chemotherapy or systemic therapy is going to be decreased over time. That false negative rate of 5 to 8% is going to become more important as time goes on, because that is what is guiding our decision making in cases of small tumors. Patients with small tumors who have negative sentinel nodes will not be getting adjuvant systemic therapy, so that 5 to 8% becomes a little more relevant over time. I would like your comment about that.
Dr. Hiram S. Cody, III (New York, New York): I agree with you entirely, Dr. Ross, and this gets at several other interesting issues. First of all, as we currently treat breast cancer, almost everyone gets some form of systemic therapy. Sentinel node biopsy may point the way to giving fewer of those patients receiving systemic therapy, since those who are truly node negative after enhanced pathologic analysis have a better survival than historic norms would indicate. We will be very interested in looking at survival as we go forward, but for this study we kept the focus on axillary local recurrence, and will need much longer follow-up to address the issue of survival.
Dr. David W. Easter (San Diego, California): A really interesting and provocative study. Thanks for those results. I just want to push you a bit further on what we are all hinting at. Do you have any more data for us? Or even generalities about those 4 groups? Did you extend the radiation treatment field? And what percent in those 4 groups got systemic therapy? I think you almost said that you did extend radiation fields and that almost everybody got systemic therapy.
Dr. Hiram S. Cody, III (New York, New York): Thank you, Dr. Easter. Since we only did an audit of the radiotherapy parameters in the unconventional group, and have not extended that analysis to the rest of the patients, I just don't have that information for you. In general, radiotherapy in the remaining patients was not extended beyond the breast unless there was involvement of more than 3 axillary nodes.
Dr. Stanley P. L. Leong (San Francisco, California): I, too, would like to congratulate you, Dr. Cody, for an excellent paper from such an extensive database. There are 2 groups that I would like to concentrate on what you presented.
The first group is the sentinel lymph node negative group. Based on your data and other investigators’ data, would you like to make a statement that indeed it is standard of care performing sentinel lymph node dissection for breast cancer? If it is negative, no additional axillary lymph node dissection should be performed.
The second issue is the sentinel lymph node positive group and yet not having axillary lymph node dissection. This is a more provocative group. It appears that although statistically significant, the clinical significance, as you have mentioned, is not that impressive. So based on that database with over 200 patients, would you recommend that even in the positive sentinel lymph node group, perhaps with more restrictive criteria that certain patients may not need subsequent axillary lymph node dissection without waiting for the result of Z0011? Thank you.
Dr. Hiram S. Cody, III (New York, New York): Thank you, Dr. Leong. Regarding the first question, every time I have been tempted to say it is the standard of care someone from the audience predicts trouble with the lawyers. I am absolutely certain it is an equivalent standard of care, and that for us it is our standard of care. I hope that it will become the standard of care, even if it is not so at present.
Regarding your second question, we have tried to be supportive of the Z0011 trial, but it is very difficult to accrue to a study with randomization between surgery and no surgery. Having said that, this unconventional group is going to remain a dilemma for all of us. There will continue to be a small number of patients in whom for one reason or another a completion axillary dissection is not done, and I think the best response I can give is to say that we are very interested in following these patients long-term.
Dr. Monica Morrow (Chicago, Illinois): The group from Memorial, consisting of high volume specialty surgeons, has traditionally reported local recurrence rates that are a lot lower than what we see, for example, from the cooperative groups. Your local recurrence rate in the axilla after axillary dissection in this study was a factor of 10-fold lower for the node positive axilla than what was seen in the NSABP B-04 dissection arm.
You clearly proved that for your group local control after sentinel node biopsy is adequate, but do you think these results can be extrapolated to the average general surgeon who treats 10 breast cancers a year? Is the technique well described enough, well developed enough, and easy enough to adopt that everyone can presume their local control will be equivalent?
Dr. Hiram S. Cody, III (New York, New York): Thank you, Dr. Morrow. I think the best data on the generalizability of sentinel node biopsy comes from the Louisville Sentinel Node Trial, which encompasses the experience of about 220 surgeons and more than 2000 cases done in a wide variety of practice settings. Their results for all aspects of the sentinel lymph node biopsy procedure are remarkably consistent with our own. Their rate of local recurrence would be interesting as well, and I think would give us a good handle on whether what we are seeing in our own data is an unnaturally low rate of local recurrence. I do think sentinel lymph node biopsy can be generalized, and I think the learning curve may not be as long as we have all said. We started out recommending 50–60 validated cases, and 3 years ago Kelly McMasters presented to this Association a superb study which showed that 20 cases was the optimum number. Now we have data from the UK ALMANAC trial which suggests that by using a standardized technique from the outset the learning curve may be as few as 1–2 cases.
Dr. Steven M. Strasberg (St. Louis, Missouri): I rise on a point of terminology, not to having anything to do with breast cancer, but regarding the term “standard of care.” I think Dr. Cody's concern about the use of that term is appropriate.
“Standard of care” is a medico-legal term, which includes both the current norm of practice, and how that practice is delivered; that is, it is also concerned with whether care is delivered with skill, good judgment and diligence. It is preferable not to use the term “standard of care” to refer to simply norm of practice. Rather “standard practice” or “standard care,” which are medical and not medico-legal terms, should be used. They refer appropriately to only current norm of practice.
Dr. Hiram S. Cody, III (New York, New York): Thank you, Dr. Strasberg. I agree with you entirely. Let me just ask you in return, how should I best answer that question when I am asked it in the future?
Dr. Steven M. Strasberg (St. Louis, Missouri): I think one should say that “standard of care” is a medico-legal term and it is confusing to use it simply as a term to refer to what current accepted practice is. But it is being used in the literature that way, and this leads to confusion. In fact, there are now many manuscript titles in the recent literature that include the term “the new standard of care,” by which is meant “a new norm of practice.” But lawyers may latch onto that and conclude that that is the “standard of care” in the legal sense. It is doubly confusing because the medico-legal standard of care includes consideration of whether the care is delivered with diligence, while diligence, skill and judgment are not a part of what is meant by “standard of care” when used as a pure medical term.
Footnotes
Reprints: Hiram S. Cody, III, MD, The Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: codyh@mskcc.org.
REFERENCES
- 1.Krag DN, Weaver DL, Alex JC, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–340. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cody HS III. Sentinel lymph node biopsy for breast cancer: does anybody not need one? Ann Surg Oncol. 2003;10:1131–1132. [DOI] [PubMed] [Google Scholar]
- 4.McMasters KM, Tuttle TM, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560–2566. [DOI] [PubMed] [Google Scholar]
- 5.Tafra LC, Lannin DR, Swanson MS, et al. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001;233:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMasters KM, Wong SL, Martin RCG, et al. Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multi-institutional study. Ann Surg. 2001;233:676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin RCG, Derossis A, Fey J, et al. Intradermal isotope injection is superior to intramammary in sentinel node biopsy for breast cancer. Surgery. 2001;130:432–438. [DOI] [PubMed] [Google Scholar]
- 8.Kern KA. Concordance and validation study of sentinel lymph node biopsy for breast cancer using subareolar injection of blue dye and technetium 99m sulfur colloid. J Am Coll Surg. 2002;195:467–475. [DOI] [PubMed] [Google Scholar]
- 9.Giuliano AE, Dale PS, Turner RR, et al. Improved staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;3:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberman L. Pathologic analysis of sentinel lymph nodes in breast carcinoma. Cancer. 2000;88:971–977. [PubMed] [Google Scholar]
- 11.Montgomery LL, Thorne AC, Van Zee KJ, et al. Isosulfan blue dye reactions during sentinel lymph node mapping for breast cancer. Anesth Analg. 2002;95:385–388. [DOI] [PubMed] [Google Scholar]
- 12.Waddington WA, Keshtgar MR, Taylor I, et al. Radiation safety of the sentinel lymph node technique in breast cancer. Eur J Nucl Med. 2000;27:377–391. [DOI] [PubMed] [Google Scholar]
- 13.Temple LK, Baron R, Cody HS III, et al. Sensory morbidity after sentinel lymph node biopsy and axillary dissection: a prospective study of 233 women. Ann Surg Oncol. 2002;9:654–662. [DOI] [PubMed] [Google Scholar]
- 14.Cody HS, Borgen PI. State-of-the-art approaches to sentinel node biopsy for breast cancer: study design, patient selection, technique, and quality control at Memorial Sloan-Kettering Cancer Center. Surg Oncol. 1999;8:85–91. [DOI] [PubMed] [Google Scholar]
- 15.Haagensen CD. Diseases of the Breast. New York: Saunders; 1986:911–913. [Google Scholar]
- 16.Fisher B, Redmond C, Fisher E. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. New Engl J Surg. 1985;312:674–681. [DOI] [PubMed] [Google Scholar]
- 17.Fowble B, Solin LJ, Schultz DJ, et al. Frequency, sites of relapse, and outcome of regional node failures following conservative surgery and radiation for early breast cancer. Int J Radiat Oncol Biol Phys. 1989;17:703–710. [DOI] [PubMed] [Google Scholar]
- 18.Veronesi U, Salvadori B, Luini A, et al. Conservative treatment of early breast cancer: Long-term results of 1232 cases treated with quadrantectomy, axillary dissection, and radiotherapy. Ann Surg. 1990;211:250–259. [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel BM, Mayzel KA, Love SM. Level I and II axillary dissection in the treatment of early-stage breast cancer. An analysis of 259 consecutive patients. Arch Surg. 1990;125:1144–1147. [DOI] [PubMed] [Google Scholar]
- 20.Recht A, Pierce SM, Abner A, et al. Regional nodal failure after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1991;9:988–996. [DOI] [PubMed] [Google Scholar]
- 21.Halverson KJ, Taylor ME, Perez CA, et al. Regional nodal management and patterns of failure following conservative surgery and radiation therapy for stage I and II breast cancer. Int J Radiat Oncol Biol Phys. 1993;26:593–599. [DOI] [PubMed] [Google Scholar]
- 22.Chua B, Ung O, Boyages J. Competing considerations in regional nodal treatment for early breast cancer. Breast J. 2002;8:15–22. [DOI] [PubMed] [Google Scholar]
- 23.Louis-Sylvestre C, Clough K, Asselain B, et al. Axillary treatment in conservative management of operable breast cancer: dissection or radiotherapy? Results of a randomized study with 15 years of follow-up. J Clin Oncol. 2004;22:97–101. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. [DOI] [PubMed] [Google Scholar]
- 25.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer/. N Engl J Med. 1997;337:956–962. [DOI] [PubMed] [Google Scholar]
- 26.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. New Engl J Med. 1997;337:949–955. [DOI] [PubMed] [Google Scholar]
- 27.Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst. 2004;96:115–121. [DOI] [PubMed] [Google Scholar]
- 28.Giuliano AE, Haigh PI, Brennan MB, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol. 2000;18:2553–2559. [DOI] [PubMed] [Google Scholar]
- 29.Veronesi U, Galimberti V, Zurrida S, et al. Sentinel lymph node biopsy as an indicator for axillary dissection in early breast cancer. Eur J Cancer. 2001;37:454–458. [DOI] [PubMed] [Google Scholar]
- 30.Roumen RM, Kuijt GP, Liem IH, et al. Treatment of 100 patients with sentinel node-negative breast cancer without further axillary dissection. Br J Surg. 2001;88:1639–1643. [DOI] [PubMed] [Google Scholar]
- 31.Reitsamer R, Peintinger F, Prokop E, et al. Sentinel lymph node biopsy alone without axillary lymph node dissection - follow up of sentinel lymph node negative breast cancer patients. Eur J Surg Oncol. 2003;29:221–223. [DOI] [PubMed] [Google Scholar]
- 32.Chung MA, Steinhoff MM, Cady B. Clinical axillary recurrence in breast cancer patients after a negative sentinel node biopsy. Am J Surg. 2002;184:310–314. [DOI] [PubMed] [Google Scholar]
- 33.Veronesi U, Paganelli G, Viale G, et al. A Randomized Comparison of Sentinel-Node Biopsy with Routine Axillary Dissection in Breast Cancer. N Engl J Med. 2003;349:546–553. [DOI] [PubMed] [Google Scholar]
- 34.Blanchard DK, Donohue JH, Reynolds C, et al. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138:482–487. [DOI] [PubMed] [Google Scholar]
- 35.Winchester DJ, Sener SF, Brinkman EM, et al. Axillary recurrence following sentinel node biopsy. Ann Surg Oncol. 2004;11:S58. [DOI] [PubMed] [Google Scholar]
- 36.Janssen CMM, Smidt ML, Bruggink EDM, et al. Axillary recurrences after negative sentinel node biopsy for breast cancer: incidence and clinical significance. Ann Surg Oncol. 2004;11:S76. [DOI] [PubMed] [Google Scholar]
- 37.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–1151. [DOI] [PubMed] [Google Scholar]
- 38.Guenther JM, Hansen NM, DiFronzo LA, et al. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003;138:52–56. [DOI] [PubMed] [Google Scholar]
- 39.Fant JS, Grant MD, Knox SM, et al. Preliminary outcome analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Surg Oncol. 2003;10:126–130. [DOI] [PubMed] [Google Scholar]
- 40.Giuliano AE. Z0011: a randomized trial of axillary node dissection in women with clinical T1 or T2 N0 M0 breast cancer who have a positive sentinel node. Available at: http://www.acosog.org/studies/synopses/Z0011_Synopsis.pdf; accessed June 24, 2004.
- 41.Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomized trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 42.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomized trials/. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 43.Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and surgery in early breast cancer—an overview of the randomized trials. N Engl J Med. 1995;333:1444–1455. [DOI] [PubMed] [Google Scholar]