Abstract
Background:
Multisystem injury and major surgical stress result in a hypermetabolic state with accelerated breakdown of protein stores. Loss of lean muscle mass impairs wound healing, increases infection rates, and weakens respiratory musculature. Oxandrolone is an anabolic steroid that attenuates loss of lean body mass and improves wound healing in burn patients. We hypothesized that oxandrolone would improve outcome for ventilator-dependent surgical patients.
Methods:
We conducted a randomized, double-blind, placebo-controlled trial of oxandrolone therapy for surgical/trauma patients requiring >7 days of ventilation. The primary end point was time on the ventilator.
Results:
Forty-one patients were enrolled between January 1, 2001, and June 15, 2003, 18 received oxandrolone (10 mg po BID) and 23 received a placebo. Groups were comparable for age, gender, injury severity score, and APACHE II score. The majority were trauma patients (83%), and 90% received enteral feeding. The oxandrolone group received higher caloric and protein intake before enrollment, but these differences were not significant. Contrary to our hypothesis, patients receiving oxandrolone spent significantly longer time on the ventilator than the placebo group (mean 21.7 days vs. 16.4 days, P = 0.03). There was no difference in infectious complications, acute respiratory distress syndrome, or multiple organ failure scores. Patients receiving oxandrolone had a longer intensive care unit stay but no difference in total hospital stay.
Conclusion:
Ventilator-dependent surgical patients receiving oxandrolone had a more prolonged course of mechanical ventilation, suggesting that oxandrolone may be detrimental in this circumstance. Oxandrolone may enhance collagen deposition and fibrosis in the later stages of acute respiratory distress syndrome and thus prolong recovery.
This was a randomized controlled trial of oxandrolone therapy for ventilator-dependent surgical/trauma patients. Oxandrolone therapy resulted in a prolonged duration of mechanical ventilation in these patients, which may suggest a negative impact of this therapy for patients with late-stage fibroproliferative acute respiratory distress syndrome.
Multisystem traumatic injury or severe surgical stress result in a hypermetabolic state, leading to a stress-induced catabolism and the accelerated breakdown of protein stores. If this process continues unchecked, the result is loss of lean body mass, leading to muscle weakness and depression of the immune response, making the patient more susceptible to infectious complications. Weakness of the respiratory musculature can inhibit ventilator weaning, and lack of protein leads to significant impairment in wound healing. These complications are observed with a loss of only 10–15% of lean body mass. A loss of lean body mass greater than 40% is usually fatal as the result of infectious complications.1 Recognition of these concerns has lead to a more aggressive pursuit of early nutritional support for critically ill patients, including replacement of protein losses. Despite this approach, however, several studies have shown that aggressive nutritional support alone does not prevent substantial body protein loss during the catabolic state of severe illness.2 As a result, attention has turned to the development of adjuvant nutritional therapies which, when administered in conjunction with aggressive protein and calorie support, may help reverse the catabolic state. These include the use of recombinant human growth hormone and anabolic steroids. Although initial studies using recombinant human growth hormone in trauma and postsurgical patients demonstrated improvements in nitrogen balance and lean body mass,3,4 a recent multicenter trial demonstrated a significant increase in mortality for critically ill patients treated with growth hormone.5 In addition, growth hormone is expensive, requires parenteral administration, and is complicated by significant hyperglycemia. As a result, this therapy has fallen out of favor and focus has shifted to the role of anabolic steroid supplementation in critically ill or malnourished patients.
Oxandrolone is an oral anabolic steroid with enhanced anabolic activity and minimal androgenic activity when compared with testosterone. In chronically malnourished patients including renal dialysis patients, chronic obstructive pulmonary disease patients, and HIV patients, anabolic steroids in combination with an enhanced calorie and protein diet have been shown to significantly improve lean body mass and muscle strength.6–9 In burn patients, Demling et al10,11 have demonstrated improvements in lean body mass and strength training during the rehabilitation phase and improved nutritional parameters and wound healing for patients beginning oxandrolone therapy 7 days after injury. Based on these studies, oxandrolone has achieved approval from the Food and Drug Administration as an adjunctive therapy to promote weight gain after extensive surgery, chronic infections, and severe trauma.
Despite this approved indication for severe trauma, very few data exist regarding oxandrolone therapy for trauma patients. Only 1 previous study has evaluated the effects of oxandrolone in the acute phase after traumatic injury, and it failed to show any significant difference between the oxandrolone and placebo groups.12 Patients in this trial were started on oxandrolone when enteral feeding was started, within 1 to 5 days of injury, and it was administered for a maximum of 28 days. We hypothesized that oxandrolone would be most effective in a population of critically ill surgical patients who are receiving full nutritional support, but are failing to thrive in the ICU as manifest by the need for prolonged ventilatory support. We hypothesized that off-setting the protein losses and enhancing lean body mass in this group would lead to a decrease in infectious complications and a shorter duration of mechanical ventilation. Thus we selected postsurgical/trauma patients who were ventilator dependent based on the need for at least 7 days of mechanical ventilation prior to enrollment in this trial.
METHODS
We conducted a prospective, randomized, double-blind placebo controlled trial of oxandrolone therapy for ventilator-dependent surgical patients. All patients admitted to our surgical/trauma intensive care unit (ICU) requiring mechanical ventilation for greater than 7 days were screened for enrollment between 1/1/01 and 6/15/03. Patients were excluded based on defined exclusion criteria, including renal failure (serum creatinine >2.0), hepatic insufficiency (total bilirubin > 2.0 or elevated AST/ALT), metastatic malignancy, chronic ventilator dependence or quadriplegia with ventilator dependence, age <18 years, pregnancy, planned extubation within 24 hours, inability to receive oral medications, already receiving oxandrolone or testosterone, or enrollment in other studies that precluded coenrollment. The legal next of kin for patients who did not meet exclusion criteria were approached for informed consent for study enrollment. The study was approved by the University of Washington IRB for Human Subjects. Once consent was obtained, patients were randomized by random number generation to receive oxandrolone 10 mg po BID or placebo BID for the duration of their ICU stay. Oxandrolone and placebo were donated by BTG Pharmaceuticals (Iselin, NJ). The dose selected is the standard dose used after burn injury and is reported to have an oral bioavailability greater than 97%. All care providers and investigators were blinded as to the randomization status.
The primary outcome was time on the ventilator, as a marker of restoration of lean muscle mass and improved respiratory function. Secondary outcomes included nutritional parameters, lean muscle mass as measured by bioelectrical impedance, infectious complications, development of acute respiratory distress syndrome (ARDS) or multiple organ dysfunction syndrome (MODS), rate of reintubation, length of ICU and hospital stay and mortality. For trauma patients the injury severity score was used as a marker of injury severity. The APACHE II score was also calculated on admission and day of study enrollment for all patients. During the study period, ventilator weaning was managed by a standard protocol implemented by the respiratory therapists. All patients requiring less than 50% inspired oxygen and positive end expiratory pressure <10 cm H2O were given spontaneous breathing trials daily and extubated if they did not develop tachypnea (respiratory rate <30), hypoxia, or retain carbon dioxide.
Nutritional monitoring included: (1) weekly assessment of albumin, prealbumin, C-reactive protein (CRP), liver function tests, prothrombin time, and serum creatinine; (2) 24-hour total urinary nitrogen: d1, d3,d5, d7, d14, d21, weekly until discharge from ICU; (3) daily monitoring of protein and calorie intake; and (4) daily weight, with baseline dry weight obtained from family or drivers license. Patients were fed via the enteral route whenever possible. Metabolic needs were estimated by the Harris-Benedict equation ×1.5 and daily Fick equations if pulmonary artery catheter monitoring was in place.13 When these estimates did not correlate, indirect calorimetry was performed using a Med Graphics CPX Express device. Indirect calorimetry was also conducted on patients who did not have pulmonary artery catheters. Lean body mass was estimated using bioelectric impedance on d1, 7, 14, 21, and weekly until discharge. The machine to measure bioelectrical impedance was provided by BTG pharmaceuticals. Bioelectrical impedance was measured as previously described.14
Infectious complications were defined as outlined in Table 1. ARDS was determined based on the American European consensus conference definition.15 These criteria include: (1) hypoxia with a PaO2/FiO2 ratio <200, (2) bilateral infiltrates on chest x-ray, and (3) no clinical evidence of increased left atrial pressure or a pulmonary artery wedge pressure of <18 mm Hg. The development of MODS was determined based on the worst daily MODS score achieved after enrollment.16 The MODS score was recorded on enrollment as a baseline assessment.
TABLE 1. Definitions for Infectious Complications
Statistical analysis was conducted on an intention to treat basis. Data were collected on a standardized data abstraction form and entered into a computerized database. The statistical software program, Stata (Stata Corporation, College Station, TX) was used for analysis. Differences between the treatment groups were determined by the χ2 test for dichotomous variables and the Student t test for continuous variables. Multivariate analysis was conducted using linear regression to assess the primary outcome.
RESULTS
During the 2.5-year study period there were 412 surgical patients requiring mechanical ventilation ≥7 days. Of these, 210 met exclusion criteria, 161 either refused participation or could not be consented, and 41 were enrolled (Fig. 1). Of the 41 enrolled, 18 were randomized to oxandrolone therapy, and 23 to placebo. The randomization scheme was originally designed based upon a planned enrollment of 144 patients and thus the initial randomization numbers were uneven. This original estimate was based upon a power calculation using time on the ventilator as the primary outcome variable. Using a 2-sided test, with a power of 0.8 and significance of P < 0.05, we estimated needing 72 patients per group to demonstrate a 10% change in number of ventilator days. We elected to discontinue enrollment after 41 patients due to an interim analysis suggesting a significant difference between the groups in respect to the primary outcome and the futility of continuing with the current rate of patient accrual.
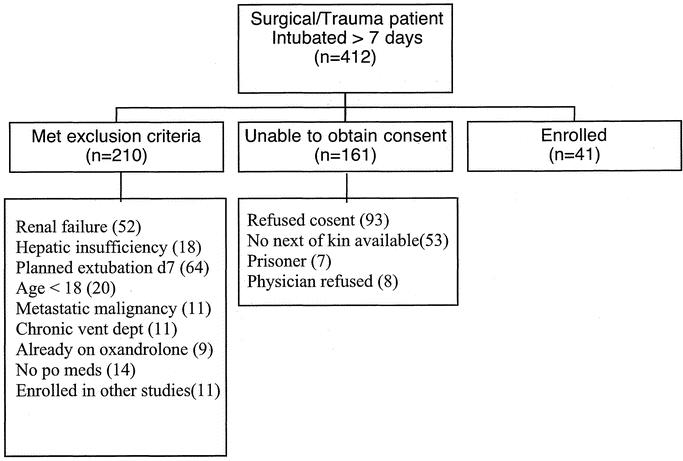
FIGURE 1. Enrollment screening: all patients admitted to the surgical/trauma ICU who required mechanical ventilation for ≥7 days were screened for study enrollment. Distribution of patients is illustrated here.
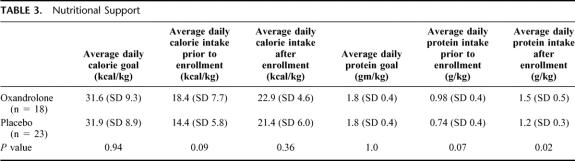
The groups were comparable for baseline demographics and injury severity or APACHE score (Table 2). The majority of the patients were victims of multisystem trauma with a mean injury severity score of 31 in both groups. There was no significant difference in chest injury severity as manifested by the abbreviated injury score for the chest. Patients were enrolled on average on day 9 of mechanical ventilation. Respiratory mechanics at the time of enrollment were comparable between the groups. The mean negative inspiratory force for patients in the oxandrolone group was −35 ± 13 versus −38 ± 15 for the placebo group (P = 0.6) and the mean rapid shallow breathing index was 108 ± 49 for the oxandrolone group versus 125 ± 45 for the placebo group (P = 0.4). The nutritional parameters are demonstrated in Table 3. The majority of patients in both groups were receiving enteral nutrition (90%) and had received approximately half of their total calorie and protein goals during the first week after injury. There was higher protein and calorie intake in the oxandrolone group prior to enrollment, but this difference was not statistically significant. After enrollment, both groups continued to improve their calorie and protein intake. A higher mean daily protein intake persisted in the oxandrolone group but there was no difference in total calorie intake.
TABLE 2. Demographics and Severity of Injury/Illness
TABLE 3. Nutritional Support
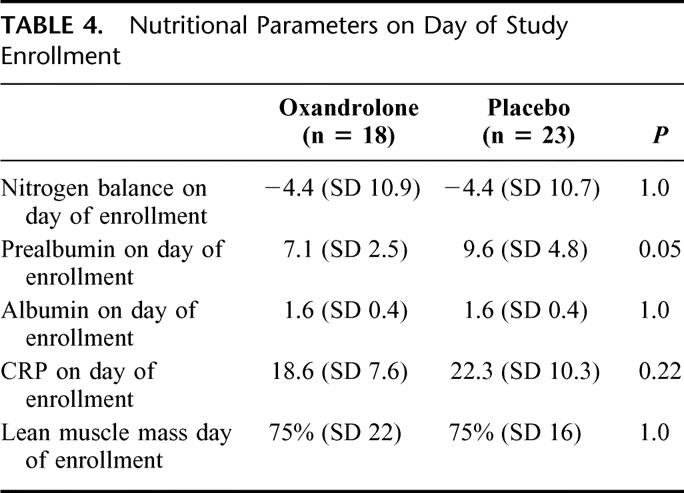
Despite the higher protein intake in the oxandrolone group, there was no difference in nitrogen balance, albumin, CRP, or lean muscle mass on the day of enrollment (Table 4) Prealbumin levels prior to enrollment were higher in the placebo group. In general, despite the fact that these patients were enrolled on average 9 days after injury they continued to have an elevated interstitial volume as evidenced by a mean body weight of 15 to 19 kg over their dry weight. This factor likely reduced the accuracy of the bioelectrical impedance calculations for lean muscle mass. Bioelectrical impedance measures the relationship between impedance through a biologic system and the fluid volume of that system so excessive interstitial volume may invalidate the results.17
TABLE 4. Nutritional Parameters on Day of Study Enrollment
In evaluating the change in nutritional parameters over the first 7 days after enrollment, the results were mixed. The placebo group had an overall better improvement in nitrogen balance with an average change of +6.6 g/d (SD 11), range (−8.2 to 26) versus, −0.7 g/d (SD 12) range (−16 to 20) for the oxandrolone group (P = 0.17). However, the prealbumin improved in the oxandrolone group an average of 6.9 mg/dL (SD 10) range (−9 to 35) versus 1.5 mg/dL (SD 6.6) range (−14 to 13) for the placebo group (P = 0.06). There was no significant difference in lean muscle mass between the groups over time. CRP levels were also not significantly different between the groups, suggesting similar levels of acute phase response in each group.
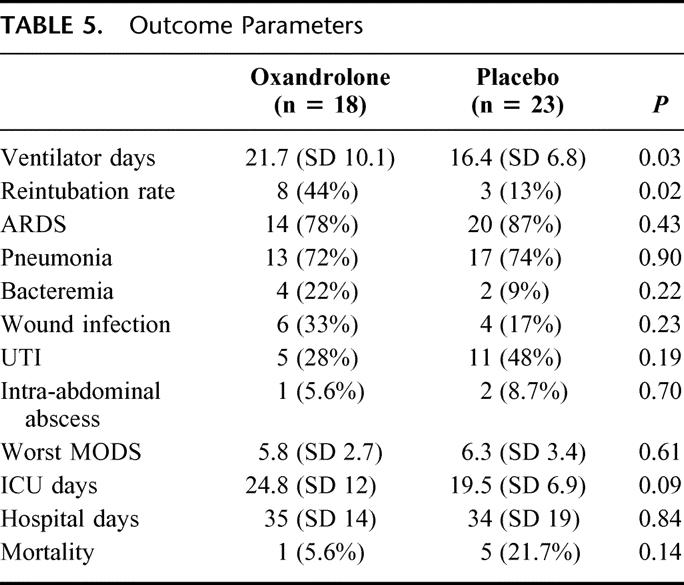
The primary and secondary clinical outcome parameters are illustrated in Table 5. Patients in the oxandrolone group had a significantly longer time on the ventilator at a mean of 21.7 versus 16.4 days (P = 0.03). Linear regression demonstrated an increase of 5.3 ventilator days for the oxandrolone group (95% CI 0.7 to 11, P = 0.053) This difference persisted even when the analysis was repeated excluding patients who died (5.6 days, 95% CI: 0.6–12, P = 0.07). Because there was a trend toward a higher percentage of males in the oxandrolone group and males may be more sensitive to anabolic steroids, we added gender to the linear regression model and this did not significantly alter the results (5.2 days, 95% CI −0.34–10.7, P = 0.065). The oxandrolone group also had a higher rate of reintubation (44% vs. 13%, P = 0.02). There was no significant difference in infectious complications, or the rate of ARDS or MODS. There was a trend toward a longer ICU stay for the oxandrolone group but no difference in total hospital stay. There were more deaths in the placebo group; however, none could be directly attributed to the nutritional therapy.
TABLE 5. Outcome Parameters
DISCUSSION
Anabolic agents, such as oxandrolone, have gained popularity for critically ill patients based upon experience in the burn population which demonstrates attenuation of the loss of lean muscle mass during rehabilitation and improvement in nutritional parameters and donor-site wound healing during the acute hospital phase.10,11,18 The role for oxandrolone therapy in other states of critical illness remains to be defined. The previous study by Gervasio et al12 demonstrated no benefit from oxandrolone therapy administered during the acute phase following traumatic injury. Our study focused on a more select group of critically injured patients who remained ventilator dependent after 7 days. We also fail to demonstrate any benefit from oxandrolone therapy for these patients. In fact, contrary to our hypothesis, patients receiving oxandrolone required a significantly longer duration of mechanical ventilation than those in the placebo group.
Despite the fact that patients in the oxandrolone group had a higher average daily protein intake during the study period, there was no significant improvement in nitrogen balance after initiation of oxandrolone therapy. There was an improvement in prealbumin levels for the oxandrolone group, which argues that the agent was having a metabolic impact on the patient, but this did not translate into any apparent clinical benefit. We attempted to measure lean muscle mass by bioelectrical impedance and found no significant difference between the groups. We believe that the absolute values for lean muscle mass may be inaccurate due to elevated interstitial fluid volumes that persisted in these patients.17 Another important observation from this study was the fact that these patients only received approximately 50% of their calorie and protein goals during the first 7 days of their hospital stay. This was primarily due to frequent interruptions in enteral feeding for operative and diagnostic interventions, as well as an inability to tolerate early enteral feeding in the postoperative period. Both groups demonstrated improved caloric intake in the second week of hospitalization (average 69% of goal), but still fell short of their goal. These findings are consistent with previous reports demonstrating difficulty in achieving full nutritional support in this population.19 The fact that patients receiving oxandrolone did not meet their nutritional goals could limit our ability to show a beneficial effect of this agent on the nutritional parameters measured, however, this does not account for the prolonged duration of mechanical ventilation seen in this group.
The primary limitation of this study is the relatively small number of patients enrolled. The number of patients required was lower than our original power calculations, but the fact that we demonstrate a statistically significant difference between the groups suggests adequate power for the trial. Study enrollment was stopped early based on an interim analysis demonstrating a significant difference in outcome based on the primary end point. We recognize that stopping boundaries usually require a lower P value to eliminate the chance of a Type I error, however, our data-monitoring group recommended ceasing enrollment based on the results of the interim analysis coupled with the difficulties with patient accrual.
We demonstrated a significantly longer duration of mechanical ventilation for patients treated with oxandrolone, which was contrary to our initial hypothesis. The reintubation rate was also higher in the oxandrolone group, but as all patients were managed with a standardized weaning and extubation protocol, we do not believe that this is a manifestation of a treatment difference between the groups but rather failure of patients in the oxandrolone group to sustain themselves without ventilator support. We hypothesize that in the setting of prolonged respiratory failure, oxandrolone may be contributing to excess scarring or collagen deposition in the lung, which could prolong the resolution of ARDS. The late stage of ARDS is referred to as the fibroproliferative phase and has been the subject of clinical trials suggesting that corticosteroids may be beneficial in reducing an excessive fibrotic response.20,21 Previous studies have demonstrated that oxandrolone enhances wound healing in patients with chronic wounds and for those recovering from burn excision and grafting.18,22,23 This improvement in wound healing was initially attributed to weight gain and improved tissue utilization of protein in patients receiving oxandrolone. A recent study, however, investigated the mechanisms responsible for improved wound healing in a rat model and demonstrated that administration of oxandrolone led to improved tensile strength of the wounds and enhanced collagen deposition.24 This report notes that fibroblasts have a high concentration of androgen receptors and thus may be activated by anabolic agents such as oxandrolone to enhance wound healing. Although this effect may be beneficial to the patient with cutaneous wounds, the same enhanced fibrosis could prolong the recovery of patients with late stage ARDS. We hypothesize that this effect may be independent of the nutritional effects of oxandrolone on lean body mass.
Another recent study examined gene expression patterns in the skeletal muscle of children receiving oxandrolone therapy after burn injury.25 Among their findings was the observation that the Growth Arrest and DNA damage inducible genes (GADD) were significantly suppressed in patients receiving oxandrolone. Depression of GADD-45 expression was also confirmed by Western blot. GADD proteins have been shown to inhibit proliferation and stimulate DNA repair and/or apoptosis and are induced in the setting of hyperoxic lung injury.26 Suppression of GADD-45 in pulmonary fibroblasts could be one mechanism of enhanced fibrosis induced by oxandrolone therapy.
In conclusion, this study demonstrates no benefit from oxandrolone therapy during the acute recovery of ventilator dependent surgical patients. Although there was no apparent direct toxicity related to oxandrolone, patients in this group had a prolonged period of mechanical ventilation compared with well-matched placebo controls. Further study of the effect of anabolic steroids on pulmonary fibrosis is warranted before the use of these agents is generalized to the critically ill postsurgical and trauma population.
ACKNOWLEDGMENTS
The authors wish to thank BTG Pharmaceuticals for donating the oxandrolone, placebo, and bioelectrical impedance machine.
Discussions
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): Dr. Bulger and her colleagues have injected a note of skepticism in the generally optimistic literature about hormonal support of stressed surgical patients. Before we abandon these agents entirely, I think we need a little more information. So I have some questions that will help me evaluate this information.
Since readiness for cessation of ventilatory support can be a variable and highly individualistic determination, we need to know how many surgeons cared for the study patients and whether one or more surgeons who applied more stringent criteria were over-represented as the attending surgeons of the oxandrolone treated patients.
The oxandrolone group received more calories during the study period, which raises the possibility that greater carbon dioxide production complicated ventilatory weaning and prolonged the need for ventilatory support, and we will welcome your comments on that.
Our early studies of human growth hormone in burn patients showed that the beneficial effect on nitrogen metabolism was observed only when calorie supply matched measured metabolic rate. Since that balance was never achieved in your patients, in the second week, both groups averaged only 69% of goal, is it fair to say that oxandrolone failed, or should the trial be repeated with adequate nutritional support?
In that same vein, were there some oxandrolone patients in whom calorie goal was achieved? If there were, was the duration of ventilatory support reduced in that subset of patients?
Lastly, you speculate that collagen production by fibroblasts stimulated by oxandrolone caused early pulmonary fibrosis which could have delayed weaning and prolonged the need for mechanical ventilation. To support that possibility, do you have confirmatory measurements of pulmonary compliance or resistance or measurements of serum levels of hydroxyproline as an index of collagen metabolism?
I compliment the authors on bringing this information to the Association and I thank the Association for the privilege of the floor.
Dr. Eileen M. Bulger (Seattle, Washington): Thank you, Dr. Pruitt, for those insightful questions.
Your first question regarded the mechanisms by which patients were weaned from the ventilator and the decision to extubate and whether this varied between the groups. Our patients are on a closed ICU unit and although cared for by a number of different surgeons rotating through that unit, they are all on a standardized weaning protocol, which is actually managed not by the physicians but by the respiratory therapists. We have found that this is a much more efficient way of weaning patients from a ventilator than allowing physicians to make the decisions. Thus patients were not treated differently between the groups. They were weaned on the standard protocol. When they met specific criteria, they underwent spontaneous breathing trials. When they passed those trials, then they were ready for extubation.
Your second question regarded whether or not patients in the oxandrolone group who got more calorie intake may have had increased CO-2 production. Patients in the subsequent period after beginning oxandrolone actually received fairly equal amounts of calorie intake, and, as you mentioned, only met 69% of their goals. So we do not feel that they were overfed. Generally, increased CO-2 production results from overfeeding, and we don't think this is a significant factor in this population. Thus we do not feel that this affected their ability to wean from the ventilator.
Your third question was regarding the calorie support. We were actually discouraged to see that patients received only about 50% of their goals despite our aggressive attempts at enteral feeding. This is reality. There are several studies in the literature not only in surgical patients, but in medical patients where they have tried to aggressively institute enteral feeding and only achieved 50% of the calorie goals. So I think when we are evaluating oxandrolone therapy we have to look at it in the setting of what we can realistically achieve in our patients. Thus repeating the trial with additional calorie would be difficult, because it would be hard to achieve adequate nutritional support without adding parenteral nutrition, which has potential negative effects as well.
Your fourth question was, did some subset of the population receive adequate calorie support and have a shorter duration of mechanical ventilation? I cannot answer that question because we did not divide the patients into any subsets.
Your fifth question was regarding confirmatory tests to evaluate pulmonary fibrosis. We think this is an excellent suggestion. We did not anticipate this negative result, contrary to hypothesis. As we did not anticipate that patients were going to spend a longer time on the ventilator, we did not plan any testing of markers of pulmonary fibrosis. When we went back and tried to figure out why this might be, we proposed this potential theory, which remains obviously to be proven. I think the next step, and what we plan to do, is to take this back to the laboratory to look at the effect of oxandrolone and other anabolic agents on fibroblast activity and the effect in lung injury models in animals before any other studies in humans.
Dr. William G. Cioffi (Providence, Rhode Island): I also congratulate Dr. Bulger and her group for carrying out this randomized blinded prospective trial of anabolic steroid efficacy in this group of extremely ill surgical patients. But, like Dr. Pruitt, I agree that before concluding that anabolic steroids are deleterious, we need more data. These groups are extremely small, making it possible that despite randomization, differences still exist given that only 20% of eligible patients enroll.
Although age, gender, ISS and APACHE scores are similar, what about pulmonary function at the time of enrollment? Do you have any gas exchange data or pulmonary function tests such as compliance? This is important since the length of ventilatory support was your primary endpoint.
Most of the patients were trauma patients, and although they had similar ISS scores you haven't given us any regional injury scores such as a chest injury score. If these were different, it would have significant impact.
Dr. Pruitt commented on the nutritional aspects and the inability to achieve appropriate nutritional support in these patients. But what about bioavailability of this orally administered agent to a group of very sick, critically ill patients? Is it possible that they were under-dosed?
You mentioned that the complication rates were grossly similar. But 2 of them were very different. Excluding pneumonia, bacteremia and wound infection rates were different, 60% versus 27%. And more importantly, as you noted, the extubation failure rate was extremely different, 44% versus 13%. That is important because your primary endpoint was ventilator dependence, and failed extubation has a significant impact on that. You noted that the extubation criteria were similar, but what data do you have such as rapid shallow breathing or other data to substantiate that indeed the patients were extubated at the same time?
Finally, you hypothesized that the difference observed may be secondary to increased pulmonary collagen deposition during the reparative phase of ARDS. Although you have no data to substantiate this, do you really think that is possible given the lack of difference in other nutritional parameters indicating that protein kinetics were not altered in your patients, so why would it be pulmonary specific?
I commend you for carrying out this transitional study and I appreciate the opportunity to comment.
Dr. Eileen M. Bulger (Seattle, Washington): Thank you, Dr. Cioffi, for those many insightful questions as well.
Your first question was on the pulmonary function at the time of enrollment. We did not do detailed pulmonary function testing so I don't have detailed data for you. We did collect the data that the respiratory therapists routinely collect and I can tell you that there was no difference in the negative inspiratory force between the groups. But other than that, I don't have any detailed pulmonary function data.
You asked about the chest ISS score. This was not different between the groups. The mean AIS chest score for both groups was 3. So I do not think there was a significant difference in chest injury between the groups.
You asked about the bioavailability of the oral agent. The company reports this agent to be 97% bioavailable. We were concerned about this as well, and this is another reason for not enrolling patients until they have been in the hospital for seven days. We thought that at seven days they would be more likely to tolerate enteral feeds and be more likely to have adequate absorption of the drug.
Your fourth question regarded the subtle differences in some of the infectious complications. Although these did not reach statistical significance, we agree there was a trend towards more bacteremia and wound infections in the oxandrolone group. However, at the same time there was a trend towards more urinary tract infections and intra-abdominal abscesses in the placebo group. We did repeat the regression analysis including all infectious complications and this did not change our primary outcome as well.
You asked about reintubation between the 2 groups. We actually took a high rate of reintubation to be an encouraging factor in that people were aggressively trying to get these patients off the ventilator and they were not able to thrive. Again, they were managed under the standard protocol, which includes primarily spontaneous breathing trials. We have largely abandoned the rapid shallow breathing index and thus didn't have that information available.
Your final question was regarding protein kinetics and whether we thought that oxandrolone would work if it wasn't altering the nutritional parameters. I think our concern here is that oxandrolone may be having effects on fibroblast activation that are not dependent on nutritional support. There may be an androgenic side effect of the medication or something that is yet to be understood. Again, fibroblasts have a high concentration of androgen receptors and we not sure how they respond to this agent. I think that this is a theory that warrants further investigation.
Dr. David B. Hoyt (San Diego, California): Dr. Bulger, very nice study, very nice presentation. I have 2 questions.
One, it is very disappointing when a trial like this doesn't work after it is based on reasonable animal data. That would be my first question. Is the animal data there to support this kind of trial design? Do we in fact need to have better animal models, maybe even multicenter animal models, before we go into clinical testing in this kind of study?
The second is, in animal models the use of androgens has been shown, at least if used early, to decrease the inflammatory response and increase perfusion. Dr. Chaudvey and the group in Alabama have done studies like that. I wonder if in retrospect this kind of trial design shouldn't be with earlier treatment?
Dr. Eileen M. Bulger (Seattle, Washington): I think we do not have animal data. This is a medication that was studied and showed benefit in burns, and those data were translated into all post-injury patients, including an FDA indication for the use of the drug in this setting.
With only one study in trauma patients, it didn't show a benefit. So I think that we need to take this back to the laboratory, we need to look at the effect of oxandrolone, how much androgenic activity it has. It was thought to have fairly minimal androgenic activity compared to testosterone, so it was felt that it would not have the anti-inflammatory effects that testosterone has. But I think we really don't know and it hasn't been studied in the laboratory.
Dr. Joseph M. Van De Water (Macon, Georgia): While you said that there was no difference in inspiratory pressures in both groups, what did happen to lean body mass when you measured it?
Dr. Eileen M. Bulger (Seattle, Washington): We thought we were going to be very high tech and measure lean body mass with bioelectrical impedance. However, we found what others have found, that it is very difficult to do that. In a critically ill ICU population, you have a lot of extra interstitial fluid on board. In fact, the numbers were equal between the groups but they were very high, probably because of the amount of extra fluid on board. Even 7 days after hospitalization, patients were still an average of 15 kilograms above their dry weight. So we found that essentially measurement of lean body mass by bioelectrical impedance is very inaccurate in an ICU population.
Dr. Paul R. Schloerb (Kansas City, Kansas): It was shown years ago by Don Whedon at N.I.H., and our studies confirmed at Francis Moore's laboratory, that if you and I ate the same food this week as we did last week and went to bed in an ICU we would be a negative nitrogen balance. So I wonder if we are not observing here what could be called obligatory negative nitrogen balance, a form of total body disuse atrophy.
Dr. Eileen M. Bulger (Seattle, Washington): I absolutely agree with you. In fact, in the burn studies of oxandrolone during acute hospitalization it attenuated nitrogen loss. But they all stayed in negative nitrogen balance. It is very hard to get these patients out of negative nitrogen balance with almost any therapy.
Footnotes
Supported by Pilot & Feasibility Award, Clinical Nutrition Research Unit, University of Washington, NIH 5P30 DK035816. Oxandrolone and placebo donated by BTG Pharmaceuticals, Iselin, New Jersey.
Reprints: Eileen M. Bulger, MD, Assistant Professor of Surgery, Box 359796, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104. E-mail: ebulger@u.washington.edu.
REFERENCES
- 1.Demling RH, DeSanti L. Use of anticatabolic agents for burns. Curr Opin Crit Care. 1996;2:482–491. [Google Scholar]
- 2.Wilmore DW. Catabolic illness. Strategies for enhancing recovery. N Engl J Med. 1991;325:695–702. [DOI] [PubMed] [Google Scholar]
- 3.Byrne TA, Morrissey TB, Gatzen C, et al. Anabolic therapy with growth hormone accelerates protein gain in surgical patients requiring nutritional rehabilitation. Ann Surg. 1993;218:400–416; discussion 416–418. [DOI] [PMC free article] [PubMed]
- 4.Petersen SR, Holaday NJ, Jeevanandam M. Enhancement of protein synthesis efficiency in parenterally fed trauma victims by adjuvant recombinant human growth hormone. J Trauma. 1994;36:726–733. [DOI] [PubMed] [Google Scholar]
- 5.Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. [DOI] [PubMed] [Google Scholar]
- 6.Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA. 1999;281:1275–1281. [DOI] [PubMed] [Google Scholar]
- 7.Schols AM, Soeters PB, Mostert R, et al. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med. 152:1268–1274, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira IM, Verreschi IT, Nery LE, et al. The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Chest. 1998;114:19–28. [DOI] [PubMed] [Google Scholar]
- 9.Grinspoon S, Corcoran C, Askari H, et al. Effects of androgen administration in men with the AIDS wasting syndrome. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;129:18–26. [DOI] [PubMed] [Google Scholar]
- 10.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma. 1997;43:47–51. [DOI] [PubMed] [Google Scholar]
- 11.Demling RH. Comparison of the anabolic effects and complications of human growth hormone and the testosterone analog, oxandrolone, after severe burn injury. Burns. 1999;25:215–221. [DOI] [PubMed] [Google Scholar]
- 12.Gervasio JM, Dickerson RN, Swearingen J, et al. Oxandrolone in trauma patients. Pharmacotherapy. 2000;20:1328–1334. [DOI] [PubMed] [Google Scholar]
- 13.Cobean RA, Gentilello LM, Parker A, et al. Nutritional assessment using a pulmonary artery catheter. J Trauma. 1992;33:452–456. [DOI] [PubMed] [Google Scholar]
- 14.Kushner RF. Bioelectrical impedance analysis: a review of principles and applications. J Am Coll Nutr. 1992;11:199–209. [PubMed] [Google Scholar]
- 15.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–232. [DOI] [PubMed] [Google Scholar]
- 16.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. [DOI] [PubMed] [Google Scholar]
- 17.Steingrub JS. Bioelectrical impedance monitoring: is this a bull market for electric utilities? Crit Care Med. 2000;28:273–275. [DOI] [PubMed] [Google Scholar]
- 18.Demling RH, Orgill DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care. 2000;15:12–17. [DOI] [PubMed] [Google Scholar]
- 19.McClave SA, Sexton LK, Spain DA, et al. Enteral tube feeding in the intensive care unit: factors impeding adequate delivery. Crit Care Med. 1999;27:1252–1256. [DOI] [PubMed] [Google Scholar]
- 20.Meduri GU, Chinn AJ, Leeper KV, et al. Corticosteroid rescue treatment of progressive fibroproliferation in late ARDS. Patterns of response and predictors of outcome. Chest. 1994;105:1516–1527. [DOI] [PubMed] [Google Scholar]
- 21.Meduri GU. Late adult respiratory distress syndrome. New Horiz. 1993;1:563–77. [PubMed] [Google Scholar]
- 22.Spungen AM, Koehler KM, Modeste-Duncan R, et al. 9 clinical cases of nonhealing pressure ulcers in patients with spinal cord injury treated with an anabolic agent: a therapeutic trial. Adv Skin Wound Care. 2001;14:139–144. [DOI] [PubMed] [Google Scholar]
- 23.Demling R, De Santi L. Closure of the “non-healing wound” corresponds with correction of weight loss using the anabolic agent oxandrolone. Ostomy Wound Manage 1998;44:58–62, 64, 66. [PubMed] [Google Scholar]
- 24.Demling RH. Oxandrolone, an anabolic steroid, enhances the healing of a cutaneous wound in the rat. Wound Repair Regen. 2000;8:97–102. [DOI] [PubMed] [Google Scholar]
- 25.Barrow RE, Dasu MR, Ferrando AA, et al. Gene expression patterns in skeletal muscle of thermally injured children treated with oxandrolone. Ann Surg. 2003;237:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Reilly MA, Staversky RJ, Watkins RH, et al. p53-independent induction of GADD45 and GADD153 in mouse lungs exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2000;278(3):L552–L529. [DOI] [PubMed] [Google Scholar]