Abstract
Objective:
Venous thromboembolic events (VTE) are potentially preventable causes of morbidity and mortality after injury. We hypothesized that the current clinical incidence of VTE is relatively low and that VTE risk factors could be identified.
Methods:
We queried the ACS National Trauma Data Bank for episodes of deep venous thrombosis (DVT) and/or pulmonary embolism (PE). We examined demographic data, VTE risk factors, outcomes, and VTE prophylaxis measures in patients admitted to the 131 contributing trauma centers.
Results:
From a total of 450,375 patients, 1602 (0.36%) had a VTE (998 DVT, 522 PE, 82 both), for an incidence of 0.36%. Ninety percent of patients with VTE had 1 of the 9 risk factors commonly associated with VTE. Six risk factors found to be independently significant in multivariate logistic regression for VTE were age ≥40 years (odds ratio [OR] 2.01; 95% confidence interval [CI] 1.74 to 2.32), lower extremity fracture with AIS ≥3 (OR 1.92; 95% CI 1.64 to 2.26), head injury with AIS ≥3 (OR 1.24; 95% CI 1.05 to 1.46), ventilator days >3 (OR 8.08; 95% CI 6.86 to 9.52), venous injury (OR 3.56; 95% CI 2.22 to 5.72), and a major operative procedure (OR 1.53; 95% CI 1.30 to 1.80). Vena cava filters were placed in 3,883 patients, 86% as PE prophylaxis, including in 410 patients without an identifiable risk factor for VTE.
Conclusions:
Patients who need VTE prophylaxis after trauma can be identified based on risk factors. The use of prophylactic vena cava filters should be re-examined.
This large study from the American College of Surgeons National Trauma Data Bank defines the risk factors associated with venous thromboembolic events after trauma and presents a new algorithm for venous thromboembolic event prophylaxis based on the risk factor analysis.
The association between injury and venous thromboembolic events (VTEs) is well recognized. The reported incidence of VTE after trauma varies from 7% to 58% depending upon the demographics of the patients, the nature of the injuries, the method of detection (ie, surveillance imaging versus clinical detection), and the type of VTE prophylaxis (if any) used in the study population.1–5 Because the mortality of post-traumatic pulmonary embolism (PE) approaches 50% in some series, most trauma centers have developed protocols for VTE prophylaxis, although there are no large studies to document the efficacy of any method of prophylaxis in this heterogeneous population. What has emerged from the existing literature is a fairly consistent list of posttraumatic risk factors for VTE and these “high-risk” patients are usually targeted for prophylactic measures.2,6–11 It was our hypothesis that the number of patients sustaining clinically significant VTE after injury in recent years is actually relatively low. We additionally hypothesized that we could clearly identify the patients most likely to benefit from VTE prophylaxis. To test our hypotheses, we used the largest trauma database available, the American College of Surgeons (ACS) National Trauma Data Bank (NTDB). The NTDB was designed by a collaborative group of interested parties, including members of the ACS Committee on Trauma, emergency medical organizations, governmental agencies, trauma registry vendors and other interested parties. The NTDB now contains over 730,000 cases from 268 trauma centers in 36 states, United States territories, and the District of Columbia and thus provides a rich source of data for clinical benchmarking.
METHODS
The National Trauma Databank
The data for this study were obtained by querying the National Trauma Data Bank (NTDB). The NTDB is the most complete national trauma database currently available. Sponsored by the American College of Surgeons, it contains data regarding demographics, injury severity, and injury origin as well as descriptive accounts of the incident. Data submitted to NTDB are rigorously examined using both the National Trauma Registry of the American College of Surgeons and an additional logical checks system set into place by the NTDB administrators. Trauma centers of all levels of designation are encouraged to submit their data to NTDB. Investigators wishing to use the NTDB must submit an application to the American College of Surgeons, which includes the purpose of the study, the data elements requested, and how the study will be used. All data provided by the NTDB is de-identified. Additionally, this study fulfilled all of the requirements for research as outlined by the University of California at San Francisco Human Subjects Protection Program. The data used for this study included all of the patients contained in the NTDB from 1994 through the year 2001. During that time period, a total of 131 trauma centers were contributing data.
Data Analysis
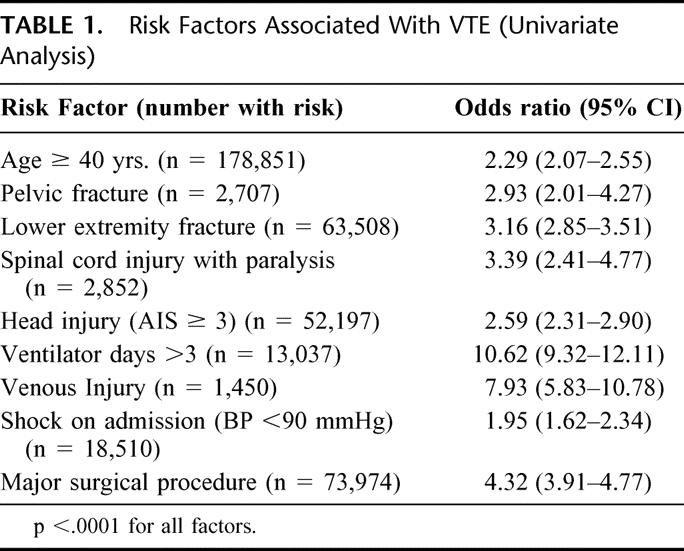
We first queried the database for the demographics of the entire population, including age, sex, mechanism of injury, and outcome. We searched for all episodes of deep venous thrombosis, pulmonary embolism or a combination of DVT/PE, which are listed under the complications section of the NTDB registry. We then compared the patients with and without DVT/PE for identifiable risk factors. This list of risk factors was developed by prospective studies conducted at our institution and those identified by a consensus panel of experts in the field of thromboembolism, lead by Dr. Lazar Greenfield and are summarized in Table 1. 2–6 For the purposes of this analysis, a major operative procedure was defined as one that lasted > 2 hours and was chosen from a list of procedures codes contained in the registry.
TABLE 1. Risk Factors Associated With VTE (Univariate Analysis)
Statistical Analysis
Individual risk factors for DVT/PE were identified using logistic regression. Risk factors found to be significantly associated with thromboembolism were then entered into a backwards, stepwise multivariate regression model.
Survey
A 9-element survey was sent to 131 trauma centers participating in the NTDB. The surveys were directed toward the trauma program manager or the trauma director. Participants were asked to identify risk factors for VTE from the list of risk factors outlined above. The preferred method of prophylaxis was indicated in a ranked fashion from a list that included pneumatic compression devices, foot pumps, low molecular weight heparin, subcutaneous regular weight heparin, and vena cava filters. Participants were then asked to repeat the ranking of prophylactic methods in the setting of a contraindication to heparin. The surveys assessed the use of CT and ultrasound for screening for VTE in high-risk patients. The indications for and the use of prophylactic vena cava filters were also assessed.
RESULTS
Demographics of the Population as a Whole
From a total of 131 participating trauma centers, 450,375 patients were available for analysis. The age range was from 1–90+, with a mean of 39.6 years. (Note: Because the data obtained from the NTDB must not be linked to an individual, all patients 90 years and older were made 90 years of age in the data set.) Male gender represented 65% of the population. Blunt mechanisms predominated (84.3%); 2.5% of the patients were burn victims, and 13.2% sustained penetrating trauma. 69% of the patients in the NTDB had an injury severity score (ISS) of ≤9, whereas 24% were moderately injured (ISS ≥10 but <25). The percentage of patients with severe trauma (ISS ≥ 25) was 7%. Of the contributing centers that were designated, most were level I or level II trauma centers. There were a number of missing data points from the 9 categories outlined above. Additionally, comorbid factors were missing from many records in the NTDB.
Patients With Thromboembolic Events
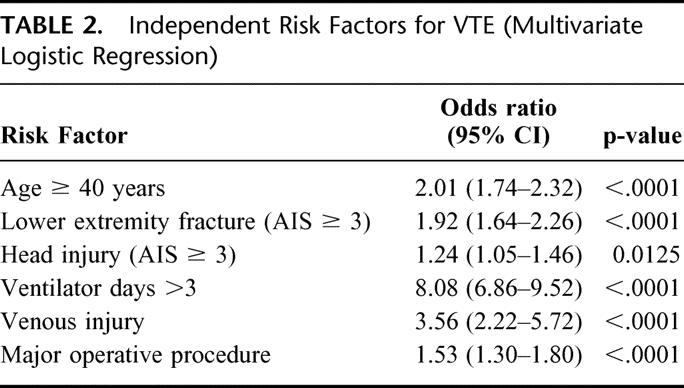
From the total of 450,375 patients, 998 had a DVT, 522 had a PE, and 82 sustained both DVT and PE, for an overall clinical incidence of 0.36% and a PE rate just greater than 0.13%. The mortality among the patients with PE was 18.7%. In patients with VTE, 69% were male and 88% had sustained blunt trauma, with a mean age of 49 years. Of the risk factors analyzed, all 9 were significantly associated with VTE on univariate analysis, with a P < 0.0001 for all factors. (Table 1) Risk factors identified as independently significant on logistic regression analysis included an age ≥ 40 years, the presence of lower extremity fracture (AIS ≥ 3), the presence of a major head injury (AIS ≥ 3), days requiring mechanical ventilation greater than 3, the presence of a venous injury, and the need for at least one major operative procedure (Table 2).
TABLE 2. Independent Risk Factors for VTE (Multivariate Logistic Regression)
Vena Cava Filters
During our analysis of procedure codes, we noted that 3883 patients had undergone “vena cava plication,” which is the code for a filter. Of these patients, 526 had DVT and or PE, whereas 3357 (86%) had neither, indicating that the filter was placed prophylactically. Further analysis of the group that received filters demonstrated that 410 patients had none of the 9 risk factors for VTE.
Survey Results
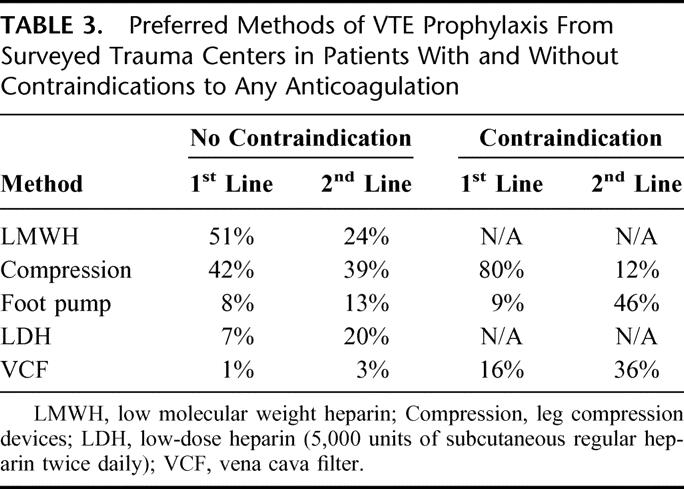
From a total of 131 surveys sent to participants in the NTDB, 56% were completed and returned. Most respondents were able to identify the risk factors for VTE. Nearly 50% of the surveyed trauma centers had an established protocol for DVT prophylaxis in trauma patients. In patients without a contraindication to heparin, the preferred method of prophylaxis was LMWH, followed by sequential compression devices (Table 3). For patients with a contraindication to heparin, most used mechanical compression devices. However, 16% of respondents replied that they would put in a prophylactic VCF in these cases. The use of prophylactic filters varied widely among surveyed trauma centers. Although filters were most often placed by radiologists, in 24% of the centers using prophylactic filters they were inserted by the trauma surgeons.
TABLE 3. Preferred Methods of VTE Prophylaxis From Surveyed Trauma Centers in Patients With and Without Contraindications to Any Anticoagulation
DISCUSSION
Historical Perspectives
That trauma patients are at risk for deep venous thrombosis and pulmonary embolism has been recognized for almost a century. In 1934 J.S. McCartney suggested that there was an association between trauma and death from pulmonary embolism, and that the association was particularly strong in patients with lower extremity fractures.12 This observation was followed by a number of autopsy studies that not only confirmed the relationship between injury and thromboembolic events (VTE) but also suggested that these events were rarely diagnosed premortem.13–15 These preliminary studies stimulated the sentinel work by Freeark and others (1967), who demonstrated venous thrombosis by venogram in 35% of patients with fractures.16 Thrombus formation was observed within 24 hours of injury and involved both the injured and the uninjured extremity. Importantly, the majority or these patients were asymptomatic.
The incidence of VTE after trauma is influenced by many factors, including the nature of the injuries, the method used to diagnose asymptomatic DVT and PE, and the type of prophylactic measures being used. Until now, no large-scale studies were available for analysis. The best estimate of incidence comes from the 1994 study by Geerts and others, who performed a single venogram during the hospital course of 349 injured patients who were NOT receiving any form of prophylaxis.4 Deep vein thrombosis was detected in 201 of the 349 patients (58%), although the incidence of proximal DVT was only 18%. However, 3 patients died of massive pulmonary embolism before a venogram could be performed, and at least 4 other patients were suspected or documented to have PE (2% incidence, 43% fatality rate). Using serial duplex examinations performed at least weekly on injured patients, we detected an overall rate of DVT in 6% of 251 patients, but 50% of these patients were receiving some type of prophylaxis.2 In our previous prospective investigations of prophylaxis in trauma patients, clinically significant PE occurred in 9 of 364 high-risk patients (2%) with an associated mortality rate of 22%.1,2 Until recently, screening for asymptomatic pulmonary embolism was impractical and, since most pulmonary emboli are clinically silent, the incidence of occult PE is surely much higher than appreciated. One recent study documented a 24% incidence of asymptomatic PE in a study of 90 moderately to severely injured trauma patients undergoing surveillance contrast-enhanced helical CT scanning.17 The overall rate of the clinically significant PE in the NTDB described herein was 0.13%. However, in the group of patients with at least one risk factor, the PE rate was 0.21% and the mortality associated with PE was 18.7%.
Risk Factors for VTE in Trauma Patients
In the recent practice management guideline review conducted by the Eastern Association for the Surgery of Trauma on the subject of risk factors for venous thromboembolism after injury, 4 studies received a Class I status (defined as prospective, randomized and controlled).9 These studies included our own 1994 and 1996 investigations, as well as 2 other studies examining the use of low molecular weight heparin in injured patients.2,6,10,11 In addition to these 4 studies, a consensus panel assembled by Greenfield compiled a list of risk factors for posttraumatic VTE, and later confirmed their utility in identifying high risk patients in a prospective study.7,8 By combining all of these studies, we developed the 9 risk factors used in the current investigation (see Table 1). These 9 risk factors identified 90% of the patients who developed a clinically significant VTE as reported in the NTDB.
Prophylactic Measures for VTE Prevention in Injured Patients
Definitive randomized controlled clinical studies on prophylactic measures in trauma patients with multiple injuries do not exist. Unlike other surgical patients with isolated disease, injured patients are a heterogeneous group who may have an isolated injury or any combination of injuries, making stratification extremely difficult. Additionally, many patients are excluded from one type of prophylactic measure or another by the very nature of their injuries. For example, bilateral full leg compression devices cannot be used with casts or external fixators, and some head-injured patients are not candidates for any type of anticoagulant. Despite these limitations, however, a few studies do exist to guide prophylactic measures. Three Class I studies have demonstrated that trauma patients receive no benefit from low-dose, unfractionated heparin administered subcutaneously.2,10,18 In contrast, Geerts and others demonstrated that patients receiving the low molecular weight heparin, enoxaparin, had a 6% rate of proximal DVT documented by venography, compared with a 15% rate in injured patients receiving low dose, unfractionated heparin as prophylaxis.10 Low molecular weight heparin has also been shown to be effective in patients with spinal cord injury.19,20 We have had similarly favorable experience with the use of the low molecular weight heparin enoxaparin and have not seen bleeding complications as long as coagulopathy is corrected and surgical bleeding is controlled before administration.6 It has also been our practice to withhold enoxaparin in patients with head injury and a documented intracranial hemorrhage until the hemorrhage has been documented to be stable on follow-up CT scan, and until the case has been discussed with the neurosurgical consultant.
The data on the efficacy of mechanical compression devices for VTE prophylaxis are less compelling, although they are attractive because of the low rate of associated complications. Only 1 study, which was a meta-analysis rather than a prospective randomized study, received a class I status by the EAST group.18 In that review, pneumatic compression appeared to offer little benefit over no specific prophylaxis in pooled randomized controlled studies of injured patients. In our previous study, only patients with neurosurgical injuries appeared to benefit from pneumatic compression, but others have found a 50% VTE rate in patients with head injuries wearing compression devices.2,21 Although calf-thigh compression may offer better compression than plantar venous compression alone, it appears that that most important factor is patient compliance with wearing these devices.22,23 Mechanical compression may be useful when combined with LMWH in very high-risk patients, but further research in the area of combined mechanical and chemical prophylaxis is clearly needed before recommendations can be made for injured patients.9,24
Vena Cava Filters (VCFs)
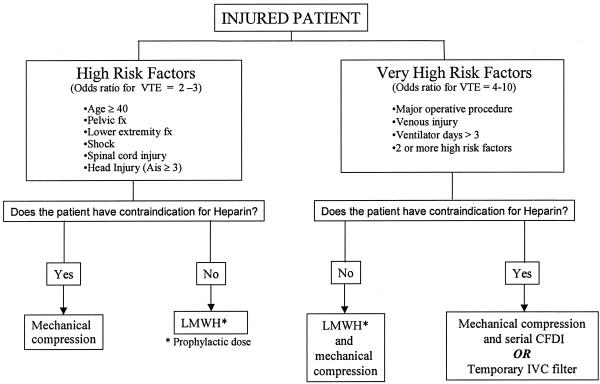
The effectiveness of a VCF in the prevention of pulmonary embolism in patients with proximal DVT has been well established. Traditionally, these filters have been placed in patients with acute proximal DVT or a recent PE who have either a contraindication to receiving anticoagulating doses of heparin, who have developed a bleeding complication while on heparin, or who have had a PE despite adequate anticoagulation. With concern about the apparent ineffectiveness of currently available VTE prophylactic methods in injured patients, some trauma surgeons have advocated for the placement of IVC filters in high-risk patients who have neither PE nor DVT.25–29 The recent development of ultrasound guided bedside techniques for IVC filter placement makes this procedure more cost-effective than the more traditional placement in radiology suites.30,31 However, several arguments can be made against the routine use of IVC filters as prophylactic devices in trauma patients. First, IVC filters do not prevent DVT, and, in fact encourage the development of DVT and may result in caval thrombosis and the long-term postphlebitic syndrome.27,32 Other complications with filters include migration, tilt, caval perforation and PE.33–35 Another argument against prophylactic filters is one of timing. At least 6% of PE episodes after trauma occur within 24 hours of the injury.36,37 Thus, to be most effective, filters would have to be placed right after admission. Temporary vena cava filters have recently been developed which appear to be safe and effective in critically ill patients, but experience in trauma patients is currently very limited.38–40 Concerns about embolization and the need for anticoagulation during retrieval of these temporary IVC filters also may offset their perceived benefit in trauma patients. Finally, one must examine the incidence of PE in large-scale studies before advocating for aggressive measures to prevent this potential complication. In a population based study from North Carolina reporting data from 1988 to 1993, the PE rate in injured patients was 0.30%, with a mortality rate among PE patients of 26%.41 In the current study, the overall PE rate was just over 0.13% but was 0.21% among the patients with risk factors. Of the 3883 patients who had IVC filters placed, only 526 had VTE (13.6%), 4.6% had PE, and 410 had no identifiable risk factor for VTE by the criteria established in this study. During the time period included in this study, the temporary filters were not in use. We currently advocate the use of IVC filters in a few selected patients who are considered to be at extremely high risk for VTE, who have the need for repeated operative procedures, and who have contraindications to anticoagulation, even at prophylactic doses (Fig. 1).
FIGURE 1. Proposed algorithm for VTE prophylaxis.
Serial Duplex Scanning
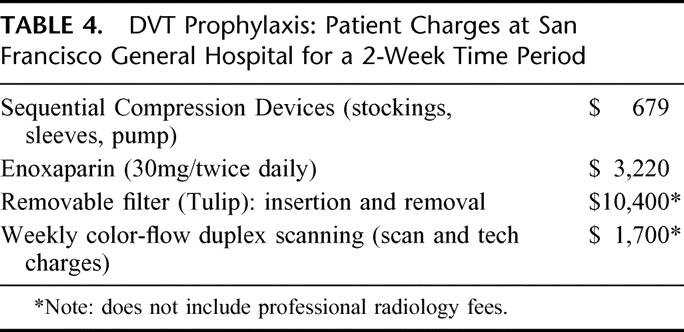
We have used serial duplex scanning as a method of detecting clinically occult DVT in our previous research studies.1,2,6 Duplex imaging, now more correctly termed color flow Doppler imaging (CFDI), has a reported range of sensitivities and specificities of 89–100% compared with contrast venography in detecting lower extremity DVT.42 As opposed to traditional lower extremity venography, color flow Doppler imaging can be performed serially and can query the veins of the upper extremity as well, which may be a source of PE.43–45 However, some authors have argued that the relatively low clinical yield associated with DVT detected only with surveillance and the relative high cost associated with serial scanning do not justify the routine use of CFDI46,47 In contrast, Brasel found that CFDI was more cost-effective in preventing PE than prophylactic IVC filters unless the patients were hospitalized for more than 2 weeks.31 The relative charges to the patients at San Francisco General Hospital for various prophylactic measures are summarized in Table 4. An additional limitation of CFDI is the difficulty in performing a complete examination in patients with lower extremity casts, external fixators etc. In our hands, we were able to work around these devices, and we continue to advocate for surveillance scanning in our high-risk patients who we feel are not receiving adequate prophylaxis.
TABLE 4. DVT Prophylaxis: Patient Charges at San Francisco General Hospital for a 2-Week Time Period
Study Limitations
There are several limitations in using a large database for research. First of all, the quality of the data cannot be assessed, and we noted a range of missing data points critical to this analysis in 5–8% of records. Second, we can make no conclusions about prophylactic measures aimed at reducing VTE. With a de-identified data set, we cannot link a thromboembolic event with a given institution and with their VTE protocols. Additionally the NTDB currently does not collect information on prophylactic measures. These data could easily be added to facilitate future investigations. Using the risk factors that we determined to be significant, we identified 90% of patients with a VTE, but missed 10%. It is probable, however, that these 10% had a preexisting risk factor for VTE that could not be identified from the database, such as obesity, malignancy, or a history of a previous VTE. Reporting of comorbid factors in the NTDB was inconsistent. Additionally, we and others have found that the use of large-bore venous catheters in the femoral vein for resuscitation is associated with DVT.48 The number of patients who had these catheters cannot be determined from the NTDB. Another area where our analysis may have been in error is in patients with spinal cord injury and paralysis. These patients have long been recognized to be at extremely high risk for VTE, and PE is one of the most common causes of death following spinal cord injury.49,50 At the time of this analysis, the number of patients with spinal cord injury entered into the database was 2886 with a DVT rate of 0.5% and a PE rate of 0.7%. The fact that this risk factor was not significant in multivariate analysis is likely due to the relatively small number of patients with this injury.
It appears from our analysis that clinically significant thromboembolic complications are relatively uncommon in trauma centers during recent years. There are a number of potential explanations for this finding. First, it must be noted that most patients reported in the NTDB are not seriously injured. Only 7% had an ISS of 25 or greater. In previous studies, including our own, only patients with risk factors were included, making the incidence of VTE much higher. Additionally, the number of patients with DVT in the NTDB is far lower than would be expected given that 522 patients had PE. This suggests that silent DVT is not being detected. However, it is also possible that aggressive and routine application of prophylactic measures have been effective in reducing clinically significant VTE. As can be seen from the survey results, most physicians and nurses caring for injured patients are aware of the risk factors for VTE. Nearly half of the trauma centers surveyed have a VTE prevention protocol in place in their institution. Other factors that might contribute to the low rate of VTE include earlier fixation of fractures, more aggressive fluid resuscitation, earlier ambulation, and earlier discharge from the hospital.
One other important finding of our study is that the risk factors for VTE after trauma are not all equal. Additionally, simply summing up the number of risk factors in a given patient does not convey the true VTE risk. These weighted risk factors should be helpful to providers who are making decisions on the type of or combination of prophylactic measures selected for an individual patient. Based on the results of this study and considering the contribution to risk to the various factors, we propose a new algorithm for VTE prophylaxis in injured patients. (Fig. 1) Clearly, a prospective multicenter study examining the effectiveness of this algorithm in preventing thromboembolic complications in injured patients is warranted.
CONCLUSIONS
We have demonstrated that the incidence of clinically significant thromboembolic events in injured patients is relatively low, and that those at risk can be identified with a high degree of certainty. Patients included in future studies on VTE prophylaxis should be stratified by the risk factors identified in this analysis. Vena cava filters should be reserved for very high risk patients who cannot receive any other form of prophylaxis, whose risk for VTE is considered extremely high and prolonged, and who have at least one of the following independently significant risk factors for VTE: a lower extremity fracture with AIS ≥ 3, a head injury with AIS ≥ 3, a need for ventilation > 3 days, the need for major operative procedures, an age >40 years, or the presence of a venous injury.
ACKNOWLEDGMENTS
The authors would like to express their appreciation to Dr. Peter Bacchetti and Alan Bostrom from the Department of Biostatistics at the University of California, San Francisco, for their assistance in analysis.
Discussions
Dr. Steven R. Shackford (Burlington, Vermont): Dr. Knudson and her colleagues are to be congratulated for confirming previous work identifying cohorts of patients at increased risk for venous thromboembolism following trauma and for stratifying those risks based on an analysis of a large set of data provided by The American College of Surgeons. I have 3 questions.
Peggy, your finding of an overall incidence of VTE is quite low compared to the literature, including well-done studies by yourself, Geerts and others. Unfortunately, your reporting of odds ratios does not give us a raw incidence rate for each risk factor. To determine this, Dr. Alan Cook, one of our surgical residents, and I undertook an analysis of the same NTDB data set. We found the risk-specific incidence to be between 10- and 200-fold less than has been reported from centers, such as yours, that are aggressive about VTE surveillance and prophylaxis. Furthermore, your finding of 522 patients with pulmonary embolism implies that there are many more than 998 patients with DVT if one conservatively assumes that 18 cases of proximal DVT will result in one PE. Doing the math suggests conservatively 9,400 DVTs are needed to get the 522 PEs that you reported, about 9 times higher. Do you think that your finding represents truly a low incidence, or simply a lack of aggressive surveillance in participating hospitals? What were the results regarding the process for surveillance from the centers that responded to your survey?
Finally, your prophylaxis algorithm suggests that very high-risk patients undergo serial color-flow Doppler imaging. Applying that recommendation to the data set would conservatively suggest that 10% of patients are at very high risk and have a relative contraindication to heparin. For a given trauma center admitting 100 patients a month, this means that 10 patients will undergo serial imaging. Conservatively assuming that each patient will require a minimum of 2 exams, this algorithm effectively consumes 10% of a full-time equivalent of a vascular technology per 100 patients per month. Is that an effective deployment of resources?
Also, what do we do with those patients who can't be scanned because of plaster immobilizers, wounds, and external fixators, which can include up to 35% of patients who are at high risk?
I would like to thank Dr. Knudson for the opportunity to discuss this important manuscript and for sending it to me many months in advance of the meeting.
Dr. M. Margaret Knudson (San Francisco, California): Thank you, Dr. Shackford, for agreeing to review this paper, for serving as a mentor to me in my career, and, most importantly, for providing me with your discussion ahead of time so I could be prepared.
Your first question relates to the extremely low rate of clinically occult VTE in this population. Is the rate really low or are we just missing them? I actually think both are true. Certainly the incidence of VTE is diminished in trauma centers, perhaps because of aggressive prophylaxis, earlier discharge from the hospital, and earlier fixation of fractures. However, as you point out, we must have missed a large number of DVT because of the associated PE rate that we have described in this population.
If you look at the data from Geert's study, the average Injury Severity Score of the patients was 26. In this study from the National Trauma Data Bank, the Injury Severity Scores were much lower, and in fact only 7% of the population had an ISS greater than 25. Thus, we are describing different populations.
That is also true of our previous work. Although we described a higher rate of DVT, we actually selected the patients to be in our study who would be able to be in the hospital for at least a week so that we could perform serial duplex scanning. So again we are describing different populations.
One other thing about the trauma centers that were surveyed. Several of them said they were doing serial duplex exams but they were only doing them in patients who were at high risk. So they did not survey their entire population who had risk factors, but only those that they considered to be high risk patients. So true surveillance is not going on nationally.
Secondly, I did not provide for you in the manuscript the raw data entered regarding risk factors. Of the 9 risk factors identified, 42% of the population had none of them; 38% had 1; 13% had 2; and 6% had more than 3 risk factors. Age >40 years was the most common risk factor. There were 200,000 patients in the database who were greater than 40 years old; 75,000 had major operations and 53,000 had head injuries. There were only 1,500 patients with a venous injury.
Your third question relates to the recommendation for serial color-flow imaging in very high risk patients who cannot receive heparin as we recommended in our algorithm. Certainly it is true that plaster and external fixators make this examination more difficult. We have been able to work around that fairly successfully. Perhaps in the future with multi-slice CT scanners we will be able to survey the venous system more effectively.
Finally, we did an analysis of the cost effectiveness of various prophylactic devices based on a 2-week stay at the San Francisco General Hospital as of last week. These are charges to the patient for each type of prophylaxis. And as you can see, getting a serial color-flow duplex exam is still a bargain compared to the placement and removal of a filter.
Dr. Lazar J. Greenfield (Ann Arbor, Michigan): This is a challenging problem. But the challenge has not been to identify the risk factors in the population that we know has DVT or PE, the challenge is to apply these markers prospectively. And I think that the database should give us a tremendous advantage in this regard.
My first question is, on the population of patients who did receive prophylactic filters, what was their incidence of DVT? One of the problems we have is that we have no Level 1 data on which patients ought to get filters. And filters have never been a prophylaxis for DVT. To direct our attention to DVT should have the higher priority.
The concern that I have with your advocacy of a temporary filter is the very large assumption that you make that you specifically know the period of risk for these patients. Because, after all, when you remove the filter, you are telling the patient that the risk no longer exists. And if you are wrong in that assumption and the patient subsequently develops PE or needs a filter, then the risk from PE is not only there but the cost goes up by another significant increment.
There are a lot of other assumptions related to temporary filters, but time doesn't permit going into that.
The final question is, what time interval do you use when you remove the filter and do you have any data on outcomes following the removal?
Dr. M. Margaret Knudson (San Francisco, California): I don't want to appear to be an advocate for the placement of a large number of temporary vena cava filters, I think our recommendation is going to apply to a very small percentage of the entire trauma population.
You asked about the DVT rate in the filter group. There were 256 patients that had either a DVT or a PE with a PE rate in the filter group of 4.5%. Their risk factors look similar to the risk factors that were described for the entire VTE population.
Regarding the risk period, and I am aware of your research where you found DVT that was evident after the patients have been sent home, some of those patients are now being sent home on an anticoagulant. I am very concerned about the fact that at 2 weeks when the filters are going to be removed, they actually have clot on them. So the patients have to be able to receive heparin at the time of removal or the filters have to be left in place for a longer period of time. We have put in only three removable filters and have removed 3 of them without complication. But I am sure as more and more people use them, there will be PEs and DVTs that are associated with the removal of the filter.
Dr. Richard J. Mullins (Portland, Oregon): Dr. Knudson, thanks again for an informative study that helps us make decisions in the care of trauma patients. I have 2 questions.
I know you take care of children. Is there a youngest age at which you would no longer apply your protocol; that is, is there a threshold age below which you are not going to put vena cava filters in?
The second question is related to the data. You said 18% of the patients died in this database. Does that mean that they had sudden death and had an autopsy and so the National Trauma Data Bank has autopsy diagnoses in it? Are you concerned that some of the trauma patients who died did not have an autopsy and thus you do not know if you are underreporting the pulmonary embolism death rate?
Dr. M. Margaret Knudson (San Francisco, California): First of all, regarding DVT and PE risk in children after trauma, they appear to have a very low rate of DVT and PE. The only children who have developed it have been children who are 13 and over who have spinal cord injuries or pelvic fractures. In those patients we might consider the use of sequential pneumatic compression not a filter. To my knowledge, low molecular weight heparin has not been used in children for that purpose and so we don't give it to the patients younger than 18.
Regarding the cause of death, we don't know that they died of pulmonary embolism. We don't know how much autopsy data is actually in the National Trauma Data Bank. Some states, like California, require an autopsy after trauma. But many states have difficulty in getting them. So I am assuming that they died from their pulmonary embolism and that is how they were recognized in that 18%, but it is possible that PE wasn't the cause of death.
Dr. Anna M. Ledgerwood (Detroit, Michigan): I just want to follow up on that point and emphasize that we really don't know how this diagnosis of PE is made in this database. And I think this is a real weakness to what is a valiant attempt to try to define risk factors for us.
Dr. M. Margaret Knudson (San Francisco, California): Thank you, Dr. Ledgerwood. I am hoping this will stimulate a prospective study where we can improve the data quality a little bit.
Dr. A. Brent Eastman (San Diego, California): Dr. Knudson, I congratulate you on using the National Trauma Data Bank. This is a very rich database, and you have provided an example of how it can be used in clinical research.
I have 1 question. In your algorithm on the high-risk patients, you suggest that there is an additive effect of low molecular weight, heparin, and compression. Does this database in any way support that assumption, and if so, why wouldn't you use it on both sides of the algorithm?
Dr. M. Margaret Knudson (San Francisco, California): There is very little data on using the mechanical compression with low molecular weight heparin except in patients with spinal cord injuries. My personal belief is that the effect of mechanical compression is variable and it really depends on how much the patient is willing to use the devices.
In our own experience, they were effective in patients with head injuries, probably because they were paralyzed and intubated and were very compliant. If you walk around the ward you find most of the patients that have mechanical compression devices are not in them. So there is room to improve on that side of the equation. We have been very happy with low molecular weight heparin for patients who are at moderate VTE risk.
Footnotes
Supported by the Centers for Disease Control and Prevention, Grant R49-CCR90369.
Reprints: M. Margaret Knudson, MD, Department of Surgery, Ward 3A, San Francisco General Hospital, 1001 Potrero Avenue, San Francisco, CA 94110. E-mail: pknudson@sfghsurg.ucsf.edu.
REFERENCES
- 1.Knudson MM, Collins JA, Goodman SB, et al. Thromboembolism following multiple trauma. J Trauma. 1992;92:2–11. [DOI] [PubMed] [Google Scholar]
- 2.Knudson MM, Lewis FR, Clinton A, et al. Prevention of venous thromboembolism in trauma patients. J Trauma. 1994;97:480–487. [DOI] [PubMed] [Google Scholar]
- 3.Rogers FB. Venous thromboembolism in trauma patients: a review. Surgery. 2001;130:1–12. [DOI] [PubMed] [Google Scholar]
- 4.Geerts WH, Code KI, Jay RM, et al. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601–1606. [DOI] [PubMed] [Google Scholar]
- 5.Shackford SR, Davis JW, Hollingsworth-Frielund P, et al. Venous thromboembolism in patients with major trauma. Am J Surg. 1990;159:365–369. [DOI] [PubMed] [Google Scholar]
- 6.Knudson MM, Morabito D, Paiment GD, et al. Use of low molecular weight heparin in preventing thromboembolism in trauma patients. J Trauma. 1996;41:446–459. [DOI] [PubMed] [Google Scholar]
- 7.Greenfield LJ, Proctor MC, Rodriguez JL, et al. Posttrauma thromboembolism prophylaxis. J Trauma. 1997;42:100–103. [DOI] [PubMed] [Google Scholar]
- 8.Gearhart MM, Luchette FA, Proctor MC, et al. The risk assessment profile score identifies trauma patients at risk for deep vein thrombosis. Surgery. 2000;128:631–640. [DOI] [PubMed] [Google Scholar]
- 9.Rogers FB, Cippole MD, Velmajos G, et al. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma 2002;53142–53164. [DOI] [PubMed] [Google Scholar]
- 10.Geerts WH, Jay RM, Code KI, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335:701–707. [DOI] [PubMed] [Google Scholar]
- 11.Spannagel U, Kujath P. Low molecular weight heparin for the prevention of thromboembolism in outpatients immobilized by plaster cast. Semin Thromb Hemost. 1993;19S1:31–41. [PubMed] [Google Scholar]
- 12.McCartney JS. Pulmonary embolism following trauma. Am J Pathol. 1934;10:709–710. [Google Scholar]
- 13.Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism: a clinico-pathological study in injured and burned patients. Br J Surg. 1960;48:475–488. [DOI] [PubMed] [Google Scholar]
- 14.Fitts WT, Lehr HB, Bitner RL, et al. An analysis of 950 fatal injuries. Surgery. 1964;56:663–668. [PubMed] [Google Scholar]
- 15.Coon WW. Risk factors in pulmonary embolism. Surg Gynecol Obstet. 1976;143:385–390. [PubMed] [Google Scholar]
- 16.Freeark RJ, Boswick J, Rostam F. Posttraumatic venous thrombosis. Arch Surg. 1967;95:567–573. [DOI] [PubMed] [Google Scholar]
- 17.Schultz DJ, Brasel KJ, Washington L, et al. Incidence of asymptomatic pulmonary embolus in moderately to severely injured trauma patients. J Trauma. 2004;56:727–731; discussion 731–733. [DOI] [PubMed]
- 18.Velmajos GC, Kern J, Chan LS, et al. Prevention of venous thromboembolism after injury: an evidence-based report: analysis of risk factors and evaluation of the role of vena cava filters. J Trauma. 2000;49:132–144. [DOI] [PubMed] [Google Scholar]
- 19.Green D, Lee MY, Lim AH, et al. Prevention of thromboembolism after spinal cord injury using low molecular weight heparin. Ann Int Med. 1990;113:571–574. [DOI] [PubMed] [Google Scholar]
- 20.Spinal Cord Injury Thromboprophylaxis Investigators: Prevention of venous thromboembolism, in the rehabilitation phase after spinal cord injury: prophylaxis with low-dose heparin or enoxaparin. J Trauma 2003;54:1111–1115. [DOI] [PubMed] [Google Scholar]
- 21.Gersin K, Grinlinger GA, Lee V, et al. The efficacy of sequential compression devices in multiple trauma patients with severe head injury. J Trauma. 1994;37:205–208. [DOI] [PubMed] [Google Scholar]
- 22.Elliott CG, Dudney TM, Egger M, et al. Calf-thigh sequential pneumatic compression compared with plantar venous pneumatic compression to prevent deep-vein thrombosis after non-lower extremity trauma. J Trauma. 1999;47:25–32. [DOI] [PubMed] [Google Scholar]
- 23.Morris RJ, Woodcock JP. Evidence-based compression-prevention of stasis and deep vein thrombosis. Ann Surg. 2004;239:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agnelli G, Piovella F, Buoncristiani B, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med. 1998;339:80–85. [DOI] [PubMed] [Google Scholar]
- 25.Greenfield LJ, Michna BA. Twelve-year clinical experience with the Greenfield vena caval filter. Surgery. 1988;104:706–712. [PubMed] [Google Scholar]
- 26.Rogers FB, Shackford ST, Ricci MA, et al. Routine prophylactic vena cava filter insertion in severely injured trauma patients decreases the incidence of pulmonary embolism. J Am Coll Surg. 1995;180:641–647. [PubMed] [Google Scholar]
- 27.Rodriguez JL, Lopez JM, Proctor MC, et al. Early placement of prophylactic vena caval filters in injured patients at high risk for pulmonary embolism. J Trauma. 1996;40:797–804. [DOI] [PubMed] [Google Scholar]
- 28.Sue LP, Davis JW, Parks SN. Iliofemoral venous injuries: an indication for prophylactic caval filter placement. J Trauma. 1995;39:693–695. [DOI] [PubMed] [Google Scholar]
- 29.Winchell RJ, Hoyt DB, Walsh JC, et al. Risk factors associated with pulmonary embolism despite routine prophylaxis: implications for improved protection. J Trauma. 1994;37:600–606. [DOI] [PubMed] [Google Scholar]
- 30.Nunn CR, Neuzil D, Naslund T, et al. Cost-effective method for bedside insertion of vena caval filters in trauma patients. J Trauma. 1997;43:752–758. [DOI] [PubMed] [Google Scholar]
- 31.Brasel KJ, Borgstron CD, Weigelt JA. Cost-effective prevention of pulmonary embolus in high-risk trauma patients. J Trauma. 1997;42:456–462. [DOI] [PubMed] [Google Scholar]
- 32.Patton JH, Fabian TC, Croce MA, et al. Prophylactic Greenfield filters: acute complications and long-term follow-up. J Trauma. 1998;41:231–237. [DOI] [PubMed] [Google Scholar]
- 33.Rogers FB, Strindberg G, Shackford SR, et al. Five-year follow-up of prophylactic vena cava filters in high-risk trauma patients. Arch Surg. 1998;133:406–411. [DOI] [PubMed] [Google Scholar]
- 34.Bochicchio GV, Scalea TM. Acute caval perforation by an inferior vena cava filter in a multitrauma patient: hemostatic control with a new surgical hemostat. J Trauma. 2001;51:991–993. [DOI] [PubMed] [Google Scholar]
- 35.Streib EW, Wagner JW. Complications of vascular access procedures in patients with vena cava filters. J Trauma. 2000;49:553–538. [DOI] [PubMed] [Google Scholar]
- 36.Owings JT, Kraut E, Battistella F, et al. Timing of the occurrence of pulmonary embolism in trauma patients. Arch Surg. 1997;132:862–867. [DOI] [PubMed] [Google Scholar]
- 37.O'Malley KF, Ross SE. Pulmonary embolism in major trauma patients. J Trauma. 1990;30:748–750. [DOI] [PubMed] [Google Scholar]
- 38.Offner PJ, Hawkes A, Madayag R, et al. The role of temporary inferior vena cava filters in critically ill surgical patients. Arch Surg. 2003;138:591–595. [DOI] [PubMed] [Google Scholar]
- 39.Millward SF, Bhargava A, Aquino J, et al. Gunther tulip filter: preliminary clinical experience with retrieval. J Vasc Interv Radiol. 2000;11:75–82. [DOI] [PubMed] [Google Scholar]
- 40.Hughes GC, Smith TP, Eachempati SR, et al. The use of a temporary vena caval interruption device in high-risk trauma patients unable to receive standard venous thromboembolism prophylaxis. J Trauma. 1999;46:246–249. [DOI] [PubMed] [Google Scholar]
- 41.Tuttle-Newhall JE, Rutledge R, Hulman CS, et al. Statewide, population-based, time-series analysis of the frequency and outcome of pulmonary embolus in 318,554 trauma patients. J Trauma. 1977;42:90–98. [DOI] [PubMed] [Google Scholar]
- 42.Hammers JW, Cohn SM, Brown JM, et al. Doppler color flow imaging surveillance of deep vein thrombosis in high-risk trauma patients. J Ultrasound Med. 1996;15:19–24. [PubMed] [Google Scholar]
- 43.Napolitano LM, Garlapatti VS, Heard SO, et al. Asymptomatic deep venous thrombosis in the trauma patient: is an aggressive screening protocol justified? J Trauma. 1995;39:651–659. [DOI] [PubMed] [Google Scholar]
- 44.Burns GC, Cohn SM, Frumento RJ, et al. Prospective ultrasound evaluation of venous thrombosis in high-risk trauma patients. J Trauma. 1993;35:405–408. [DOI] [PubMed] [Google Scholar]
- 45.Wibbenmeyer LA, Hoballah JJ, Amelon MJ, et al. The prevalence of venous thromboembolism of the lower extremity among thermally injured patients determined by duplex sonography. J Trauma. 2003;55:1162–1167. [DOI] [PubMed] [Google Scholar]
- 46.Spain DA, Richardson JD, Polk JC, et al. Venous thromboembolism in the high-risk trauma patient: do risks justify aggressive screening and prophylaxis? J Trauma. 1997;42:463–469. [DOI] [PubMed] [Google Scholar]
- 47.Piotrowski J, Alexander J, Brandt CR, et al. Is deep vein thrombosis surveillance warranted in high-risk trauma patients? Am J Surg. 1996;172:210–213. [DOI] [PubMed] [Google Scholar]
- 48.Meredith JW, Young JS, O'Neill EA, et al. Femoral catheters and deep venous thrombosis: a prospective evaluation with venous duplex sonography. J Trauma. 1993;36:187–191. [DOI] [PubMed] [Google Scholar]
- 49.Myllynen P, Kammonen M, Rokkanen P, et al. Deep venous thrombosis and pulmonary embolism in patients with acute spinal cord injury: a comparison with nonparalyzed patients immobilized due to spinal fractures. J Trauma. 1985;25:541–543. [DOI] [PubMed] [Google Scholar]
- 50.Clagett GP, Anderson FA, Geerts W, et al. Prevention of venous thromboembolism. Chest. 1998;114:531S–560S. [DOI] [PubMed] [Google Scholar]