Abstract
Background:
Concern exists that prednisone-free maintenance immunosuppression in kidney transplant recipients will increase acute and/or chronic rejection.
Methods:
From October 1, 1999, through February 29, 2004, at our center, 477 kidney transplant recipients (341 living donor, 136 cadaver) discontinued prednisone on postoperative day 6, per our protocol. Immunosuppression consisted of polyclonal antibody (Thymoglobulin) for 5 days, prednisone intraoperatively and for 5 days, a calcineurin inhibitor, and either sirolimus or mycophenolate mofetil. We compared outcome with that of historical controls who did not discontinue prednisone.
Results:
The recipients on prednisone-free maintenance immunosuppression had excellent 4-year actuarial patient survival (92%), graft survival (90%), acute rejection-free graft survival (86%), and chronic rejection-free graft survival (95%). The mean serum creatinine level (± SD) at 1 year was 1.6 ± 0.6; at 4 years, 1.6 ± 0.6. We noted that 8% of recipients had cytomegalovirus (CMV) disease; 4.5%, fractures; 2.8%, cataracts; 1%, posttransplant diabetes; 0.2%, avascular necrosis; 0.2%, posttransplant lymphoproliferative disease; and 0%, polyomavirus. In all, 85% of kidney recipients with functioning grafts remain prednisone-free as of April 1, 2004.
As compared with historical controls, the recipients on prednisone-free maintenance immunosuppression had better patient (P = 0.02) and graft survival (P < 0.0001) and lower rates of acute (P = 0.0004) and chronic (P = 0.02) rejection. In addition, they had a significantly lower rate of CMV disease (P < 0.0001), cataracts (P < 0.0001), posttransplant diabetes (P < 0.0001), and avascular necrosis (P = 0.0003).
Conclusions:
Prednisone-related side effects can be minimized without maintenance immunosuppression; our prednisone-free recipients do not have increased acute or chronic rejection.
Concern exists that prednisone-free maintenance immunosuppression after kidney transplants will be associated with increased acute and chronic rejection. Our series shows low rates of acute rejection and excellent 4-year graft survival.
Successful clinical allotransplants only became possible with the development of immunosuppression. Initially, 6-mercaptopurine, or its derivative azathioprine (AZA), was used.1–3 Shortly thereafter, Starzl et al4 reported improved graft survival when AZA was combined with prednisone. Since that time, transplant clinical research has focused on improving short- and long-term graft survival while simultaneously minimizing immunosuppression-related complications.
Prednisone has a well-defined side effect profile, which includes hypertension, osteoporosis (and fractures), avascular necrosis, cataracts, mood alterations, posttransplant diabetes, easy bruisability, and skin changes. Some of these side effects are related to the cumulative steroid dose.5 Others develop rapidly posttransplant6 and can occur early, even with use of relatively low-dose steroids.7–9 Consequently, even early in the history of transplantation, attempts were made to minimize the daily prednisone dose.10 In general, however, kidney transplant recipients who began prednisone at the time of their transplant tended to have better long-term graft survival than those who did not take prednisone.
Throughout the 1980s and 1990s, many transplant centers studied whether selected, clinically well, immunologically low-risk kidney transplant recipients, without a previous acute rejection episode, could undergo prednisone withdrawal. Those studies showed that, even in carefully selected recipients, prednisone withdrawal was associated with an increased risk of acute rejection11,12 and of graft loss.12 Of particular concern was a Canadian multicenter, prospective, randomized study in which recipients on cyclosporine (CSA) and prednisone, without active rejection, were randomized at 90 days posttransplant to continue on CSA and either low-dose prednisone or placebo. The Canadian study found no difference in graft survival for the first 3 years posttransplant, but thereafter the steroid-free group had significantly worse graft survival (P = 0.03 versus the low-dose prednisone group).13
More recently, with the introduction of new, more potent immunosuppressive agents, interest in steroid-sparing protocols has resurged. Two prospective randomized studies of steroid withdrawal in recipients on CSA, mycophenolate mofetil (MMF), and prednisone showed an increased incidence of acute rejection episodes in the steroid withdrawal arm.14,15 In contrast, numerous other studies have now shown a low acute rejection rate when steroids are completely avoided or discontinued in the first week after kidney or simultaneous kidney-pancreas transplants.16–24
Outcome at 1 year posttransplant has been excellent with protocols incorporating prednisone avoidance or rapid discontinuation. Yet concern remains, fueled by the results of the Canadian study, that long-term outcome will be worse in prednisone-free recipients. We herein report 4-year outcome in a cohort of kidney transplant recipients who discontinued their prednisone in the first posttransplant week.
MATERIALS AND METHODS
From October 1, 1999, through February 29, 2004, at our center, 477 kidney transplant recipients discontinued their prednisone on postoperative day (POD) 6, per our protocol. Initially, this protocol applied only to recipients of first living donor (LD) kidney transplants; in October 2000, we expanded it to apply to all recipients of first and second LD and first and second cadaver (CAD) donor kidneys. Currently, we exclude from this protocol recipients taking prednisone at the time of their transplant and recipients requiring prednisone for an underlying disease.
Immunosuppression for all recipients on our prednisone discontinuation protocol consisted of Thymoglobulin (SangStat; 1.25 to 1.5 mg/kg/d) for 5 days, with the first dose given in the operating room, and prednisone for 6 days (methylprednisolone 500 mg, given in the operating room, followed by prednisone, 1 mg/kg on POD 1; 0.5 mg/kg on POD 2 and 3; and 0.25 mg/kg on POD 4 and 5). From October 1, 1999, through March 1, 2001, recipients also received CSA (adjusted to achieve blood levels of 150 to 200 ng/mL by HPLC for the first 3 months) and MMF (1 g BID). Since March 1, 2000, we have been conducting a randomized study of CSA-MMF versus tacrolimus (TAC)-sirolimus (SRL) as part of our prednisone discontinuation protocol. To date, we have found no difference between the CSA-MMF and TAC-SRL groups in the incidence of acute rejection or in graft survival, so for the purposes of this report, we have analyzed all of our prednisone discontinuation data in the aggregate. Some recipients on our prednisone discontinuation protocol chose not to participate in our randomized study of CSA-MMF versus TAC-SRL and so were treated with CSA-MMF; thus, the total number of recipients in our current report is 477.
For recipients with delayed graft function, we extended the course of Thymoglobulin (to a maximum 10 doses) and delayed introduction of the calcineurin inhibitor. Recipients with >25% increase in serum creatinine level underwent percutaneous allograft biopsy. Rejection episodes were treated with a rapid steroid taper; steroid-resistant rejection episodes, and histologically severe rejection episodes were treated with antibody therapy. After antirejection therapy, most recipients had 5 mg of prednisone daily added to their maintenance immunosuppression; some (n = 22) insisted on returning to prednisone-free immunosuppression.
All recipients were treated with prophylactic ganciclovir or valganciclovir for 3 months posttransplant. Pneumocystis prophylaxis was with Bactrim; in patients with sulfa allergies, dapsone or aerosolized pentamidine was used. Fungal prophylaxis was with oral clotrimazole or nystatin for 3 months posttransplant.
For recipients on our prednisone discontinuation protocol, we studied actuarial patient and graft survival rates, death-censored graft survival rates, and acute and chronic rejection-free graft survival rates; renal function (mean serum creatinine levels) at each year posttransplant; and the incidence and number of steroid- and immunosuppression-related side effects, including cataracts, fractures, avascular necrosis, skin cancer, posttransplant lymphoproliferative disease (PTLD), posttransplant diabetes mellitus (PTDM), and cytomegalovirus (CMV) infection. We also obtained pre- and posttransplant weight, serum cholesterol, and serum triglyceride levels.
We also compared outcome—including patient, graft, and acute rejection-free graft survival, and the incidence of side effects—in the recipients on our prednisone discontinuation protocol with outcome in a historical cohort (n = 388; January 1, 1996, through December 31, 2000) of first and second LD and CAD transplant recipients treated with polyclonal antibody, a calcineurin inhibitor, either MMF or AZA, and a prednisone taper (1 mg/kg/d tapered to 0.4 mg/kg/d by 1 month and to 0.15 mg/kg/d by 1 year).
We estimated actuarial patient, graft, and rejection-free graft survival rates by using Kaplan-Meier life table analyses. To compare differences between groups, we used log-rank and Wilcoxon tests. Similarly, we used log-rank tests to compare the rates of development of side effects in our prednisone discontinuation group versus historical controls.
RESULTS
Of the 477 kidney transplant recipients on our prednisone discontinuation protocol, 341 were LD and 136 were CAD recipients; 431 (90%) were first transplant recipients and 46 (10%) were retransplant recipients. Mean recipient age (± SD) was 47 ± 12 years (range, 19 to 76 years). Most (91%) of the recipients were white; 61% were male. The most common primary renal disease was diabetes (33%).
For the entire group of 477 recipients, the actuarial patient survival rate at 1 year was 97%; at 4 years, 92%. We found no significant difference in patient survival rates between LD and CAD recipients (Table 1). A total of 23 recipients have died (as of April 1, 2004), 4 after graft failure.
TABLE 1. Actuarial Results (Prednisone Discontinuation Protocol)
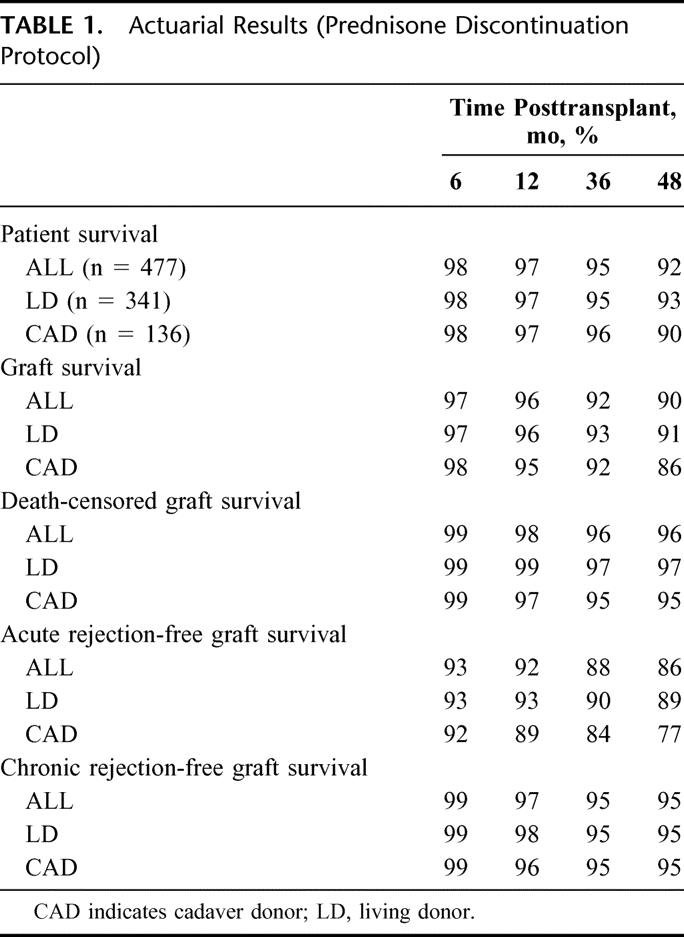
The actuarial graft survival rate at 1 year was 96%; at 4 years, 90% (Table 1). We found no significant difference in the actuarial graft survival rate between LD and CAD recipients. A total of 32 grafts failed (22 LD, 10 CAD): 19 due to death with function; 4, chronic rejection; 3, technical reasons; 2, primary nonfunction; 1, malignancy; 1, calcineurin toxicity; 1, overt noncompliance; and 1, other.
The death-censored graft survival rate at 1 year was 98%; at 4 years, 96% (Table 1). Again, we found no significant difference in the death-censored graft survival rate between LD and CAD recipients.
The acute rejection-free graft survival rate at 6 months was 93%; at 1 year, 92%; and at 4 years, 86% (Table 1). We noted a trend toward a higher acute rejection rate in CAD (versus LD) recipients (P = 0.06). A total of 50 recipients had a biopsy-proven acute rejection episode. Of those 50 first rejection episodes, 25 involved minimal to mild tubulointerstitial (ti) infiltrate; 10, moderate ti infiltrate; 2, severe ti infiltrate; 3, moderate to severe vascular infiltrate; and 7, C4d positivity without tubulitis. Median time to the first acute rejection episode was 1.4 months (range, 1 week to 3 years). Of those 15 first rejection episodes with moderate to severe changes, 11 occurred >5 months posttransplant. Of those 50 recipients with an acute rejection episode, 8 experienced a second episode during the study period. In addition, of those 50 recipients, 45 continue to have graft function; 5 grafts have failed: 3 because of chronic rejection; 1, death with function; and 1, malignancy.
The chronic rejection-free graft survival rate at 4 years was 95%; we found no difference between LD and CAD recipients (Table 1).
Mean serum creatinine level (± SD) at 1 year posttransplant was 1.6 ± 0.6 ng/dL; at 2 years, 1.6 ± 0.6 ng/dL; at 3 years, 1.7 ± 0.6 ng/dL; and at 4 years, 1.6 ± 0.6 ng/dL. We noted no significant difference in serum creatinine levels between LD and CAD recipients. However, recipients with ≥1 acute rejection episode had significantly higher serum creatinine levels at each interval studied (6, 12, 24, 36, and 48 months; P < 0.05).
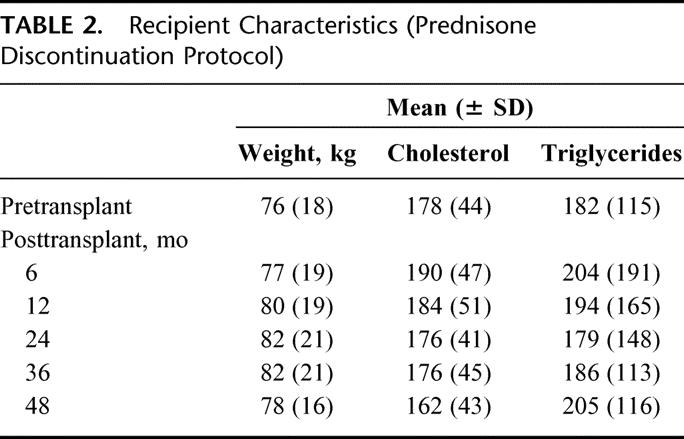
Weight, serum cholesterol, and triglyceride levels are shown in Table 2. Over 4 years, the average weight gain was 2 kg; we noted no significant change in either serum cholesterol or triglyceride levels. However, at each posttransplant interval studied (6, 12, 24, 36, and 48 months), >40% of recipients on our prednisone discontinuation protocol were treated with ≥1 lipid-lowering agents. In addition, at each interval studied, >80% were treated with ≥1 antihypertensive drug.
TABLE 2. Recipient Characteristics (Prednisone Discontinuation Protocol)
As of March 1, 2004, 39 (8%) recipients on our prednisone discontinuation protocol have had CMV disease; 21 (4.5%), fractures; 13 (2.8%), cataracts (1.6% of nondiabetics, 5% of patients with diabetes; P = 0.04); 5 (1%), PTDM; 1 (0.2%), avascular necrosis; and 1 (0.2), PTLD; none have had polyomavirus infection. We found no difference in the rate of these side effects between LD and CAD recipients.
Of the 477 recipients on our prednisone discontinuation protocol, 48 underwent a pancreas after kidney transplant, so were dropped from our analysis at the time of the pancreas transplant. Of the remaining recipients with functioning grafts, 85% were still prednisone-free as of April 1, 2004. The most common reason for resuming prednisone was acute rejection.
Outcome for the recipients on our prednisone discontinuation protocol versus the historical controls is summarized in Table 3. Recipients on our prednisone discontinuation protocol had significantly better actuarial patient survival (P = 0.02), graft survival (P < 0.001), death-censored graft survival (P < 0.001), acute rejection-free graft survival (P = 0.0004), and chronic rejection-free graft survival (P = 0.02).
TABLE 3. Actuarial Results (Prednisone Discontinuation Protocol versus Historical Controls)
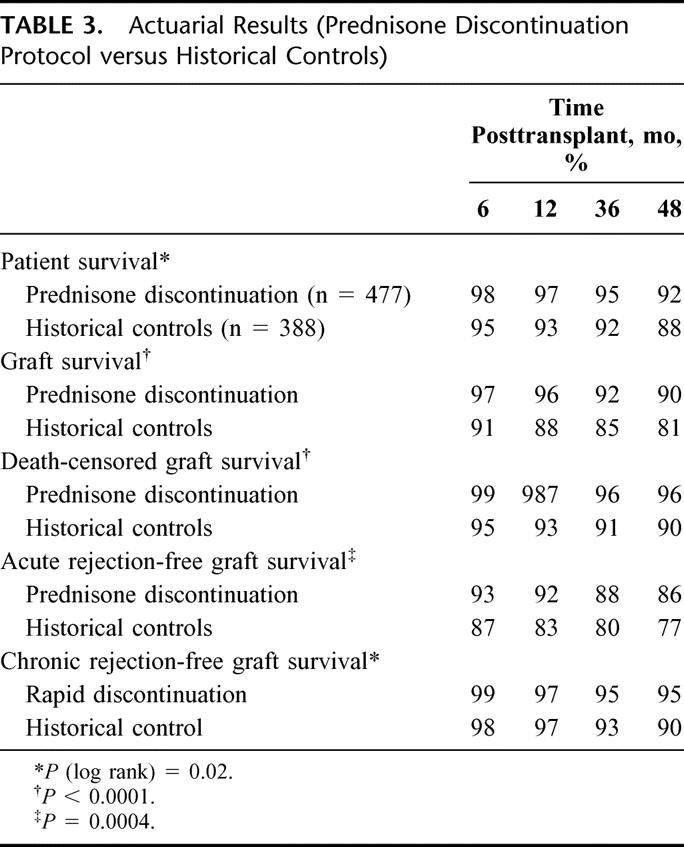
In addition, as compared with historical controls, the recipients on our prednisone discontinuation protocol had significantly lower rates (log rank) of CMV disease (P < 0.0001), PTDM (P < 0.0001), cataracts (P < 0.0001), and avascular necrosis (P = 0.003; Fig. 1). We found no difference between the 2 groups in the incidence of fractures (P = 0.07), wound complications, or polyomavirus infection.
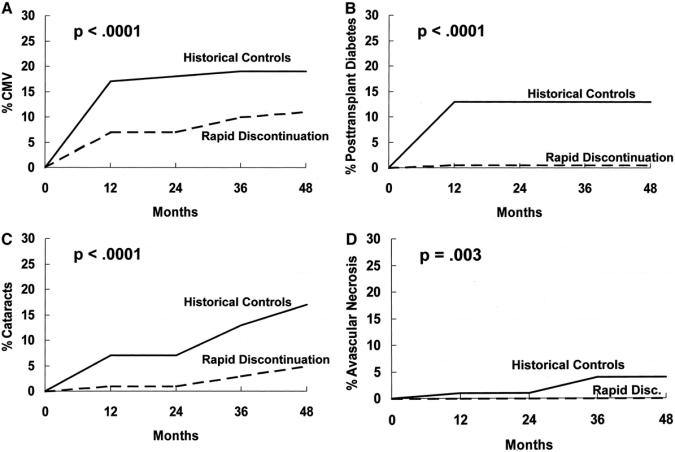
FIGURE 1. Significant side effect differences (prednisone discontinuation protocol versus historical controls). A, CMV; B, PTDM; C, cataracts; D, avascular necrosis.
DISCUSSION
The prednisone side effect profile provides a compelling reason to attempt steroid avoidance or discontinuation. In fact, when surveyed, kidney transplant recipients said they would prefer elimination of prednisone to elimination of other immunosuppressive agents.25 But, historically, randomized studies of low-dose prednisone or early prednisone discontinuation in recipients on CSA, AZA, and prednisone showed increased acute rejection rates in the low-dose or no prednisone groups.26,27 Moreover, even with newer immunosuppressive agents, prednisone withdrawal in selected recipients at 3 months posttransplant led to an increased incidence of acute rejection episodes.14,15 Thus, concern has persisted that, even with the newer immunosuppressive agents, prednisone avoidance or rapid discontinuation would lead to a similar increase in the incidence of acute rejection. Such an increase would be of significant concern: acute rejection episodes are the major risk factor for chronic graft dysfunction and for late graft loss.28 However, our current findings, and those of others, now show that the incidence of acute rejection can be low when prednisone either is completely avoided or is discontinued in the first posttransplant week.16–24
Of interest, 7 (14%) of our recipients with treated acute rejection episodes had C4d-positive episodes without cellular infiltrate.29–31 Studying biopsies for C4d positivity is relatively new at our center, so we have no comparative information for our historic controls. It may be that a significant percentage of recipients with renal dysfunction whose biopsy specimen did not reveal cellular infiltrate always would have had C4d positivity; alternatively, C4d positivity (thought to be evidence for antibody-mediated damage) may be higher in recipients on a rapid prednisone discontinuation protocol.
We also noted that most (11 of 15) rejection episodes involving moderate to severe histologic changes occurred late (>5 months) posttransplant. It is unclear whether this finding represents failure of our prednisone discontinuation protocol (the recipients had not been on prednisone since POD 5) or whether an element of noncompliance contributed to those late, severe, first rejection episodes.
Concern has also persisted that early prednisone discontinuation, even if not associated with acute rejection, would be associated with increased chronic graft dysfunction and graft loss after 3 years posttransplant.13,32 We previously noted excellent 3-year outcome.33 Perhaps the most important observation in our current study is that 4-year graft survival, death-censored graft survival, and renal function remained stable. Birkeland16 reported similar findings (n = 100) using a completely steroid-free protocol.
Why has rapid prednisone discontinuation succeeded when late prednisone withdrawal failed? Our maintenance immunosuppressive drugs for many of the recipients on our prednisone discontinuation protocol (ie, CSA and MMF) was identical to those used in 2 trials of late prednisone withdrawal, both of which showed increased acute rejection rates in the withdrawal groups.14,15 One major difference is the routine use, in our current study, of polyclonal antibody for induction therapy. Also important, perhaps, was that we gave the first antibody dose prevascularization. In fact, the European late prednisone withdrawal trial did not show an increased rejection rate in the subgroup receiving antibody induction therapy.14 An alternative explantation is that prednisone results in increased cytokine receptor expression on T cells, while simultaneously causing decreased cytokine release.34 Late withdrawal may result in cytokine release into an environment of up-regulated receptors.
All recipients on our prednisone discontinuation protocol received a calcineurin inhibitor (either CSA or TAC) as part of their immunosuppressive therapy. Long-term calcineurin inhibitor use has been associated with hypertension and nephrotoxicity.35 In addition, both CSA and TAC have drug-specific side effects. Thus, another immunosuppressive strategy might be to minimize or eliminate calcineurin inhibitors. Recently, some authors reported low acute rejection rates and excellent short-term graft survival using protocols that either avoided or discontinued calcineurin inhibitors.36–41 However, all of those protocols incorporated prednisone. If both calcineurin-sparing and steroid-sparing protocols are shown to have excellent long-term outcome, future patient care may involve individualized therapy to avoid specific side effects. Alternatively, protocols may be devised that minimize both prednisone and calcineurin inhibitors.42
Most reports on the side effects of calcineurin inhibitors in transplant recipients have involved recipients taking calcineurin inhibitors plus steroids. Our protocol provided an opportunity to study calcineurin inhibitor side effects in recipients not taking maintenance prednisone. Not surprisingly, we found a low incidence of the side effects usually associated with prednisone (eg, cataracts, avascular necrosis, diabetes). In fact, as compared with historical controls on prednisone maintenance immunosuppression, the recipients on our prednisone discontinuation protocol had a significantly lower rate of CMV disease, PTDM, cataracts, and avascular necrosis (Fig. 1). In addition, hyperlipidemia and polyomavirus infection did not occur as frequently as in protocols that combine calcineurin inhibitors and prednisone. However, our prednisone discontinuation group frequently had hypertension requiring blood pressure medication.
In conclusion, in spite of excellent short-term outcome, concern has persisted that immunosuppressive protocols incorporating prednisone avoidance or rapid discontinuation would lead to late graft dysfunction and increased graft loss. We now report a 90% 4-year graft survival rate and stable serum creatinine levels in the recipients on our prednisone discontinuation protocol.
ACKNOWLEDGMENTS
The authors thank Mary Knatterud for editorial assistance and Stephanie Daily for preparation of the manuscript. We also thank the transplant fellows and coordinators as well as all of the other physicians, nurses, and administrative staff who helped care for the patients and made this large-scale study possible.
Discussions
Dr. Marc I. Lorber (New Haven, Connecticut): Dr. Matas and his colleagues at the University of Minnesota should once again be congratulated for their forward-looking approach to the clinical care of transplant recipients. As graft survival has improved, and acute rejection rates have fallen, the major management challenges have changed, now relating largely to associated co-morbidities; some from underlying disease processes, and many resulting directly from immunosuppression.
Although problems associated with immunosuppression are multiple, the consequences of long-term steroids have long plagued transplant recipients. Patients regularly express their displeasure with steroid use, and the desire to eliminate corticosteroids from the regimen is far and away the most common medication-related request I hear from my patients.
Dr. Matas described the problems associated with steroids have been recognized for many years. However, despite considerable efforts, earlier attempts at elimination were discouraging, associated with increased acute rejection and graft loss. He has also reminded us that short-term success has been reported in the past, but long-term results have until recently been quite disappointing.
Although it is important to recognize the need for careful, ongoing attention, these 4-year outcome figures from the University of Minnesota are now beginning to address the relevant consideration. We should share cautious optimism in that regard.
Our group has also gained some experience with early steroid cessation. Initially, as part of a multicenter pilot, we have continued to use an approach focusing on steroid elimination after 5 days. Immunosuppression includes the monoclonal anti-IL-2 receptor antibody, basiliximab, for induction with maintenance using tacrolimus and sirolimus. Our patient numbers are smaller, and mean follow-up has only reached 2 years. However, the incidence of biopsy documented acute rejection has been low at 13% with no deaths or graft failures to date. Additionally, renal function has been good with a mean serum creatinine of 1.5 ± 0.4 mg/dL, and 87% of our recipients have remained off corticosteroids. Recognizing that our follow-up is still short and patient volume still small, we consider the low incidence of rejection and excellent outcomes encouraging.
Finally, beyond your encouraging findings, it is also important to emphasize that Dr. Matas and his group made seemingly subtle changes to existing immunosuppression with a dramatic change in outcome. The results emphasize the importance of critical observation, analysis and interpretation of available data when designing new studies. They also emphasize the importance of perseverance.
This report addresses an important, previously elusive clinical problem. Assuming the observations hold up over time, they represent an important advance in post-transplant management.
I have a few questions. You described a couple of potential explanations to explain your successful results. However, perhaps you might be willing to speculate in a little more detail about why you have achieved apparent success while others before you have reported disappointing outcomes with steroid withdrawal strategies?
You alluded to similar overall outcomes during your more recent experience, randomizing patients to receive either cyclosporine and mycophenolate mofetil or tacrolimus and sirolimus maintenance. Have you observed any differences? Do your results provide insights allowing you to favor one versus the other approach?
Recognizing the importance of long-term results, what data are needed before the transplant community should accept steroid free immunosuppression as the standard?
Again, thank you for the opportunity to review your data in advance, and congratulations on these excellent results!
Dr. Arthur J. Matas (Minneapolis, Minnesota): Thank you, Dr. Lorber. I think you raised some important questions.
Why rapid discontinuation of prednisone works when previous series of late steroid withdrawal failed is really unclear to me. We participated in the multicenter trial of late steroid withdrawal in recipients taking cyclosporine and CellCept; the trial showed an increased incidence of acute rejection in the steroid withdrawal group. And clearly a part of our current study is a cohort of patients who are getting cyclosporine and CellCept with early predisone discontinuation; yet with our current protocol we are not seeing an increased incidence of rejection.
I think the big difference is probably the use of antibody induction in the current patients, and particularly giving the antibody pre-vascularization. But that is a hypothesis. Another alternative is that with the improved CMV prevention that we currently have, we are capable of giving more immunosuppression. But it is unclear why early avoidance or rapid discontinuation works with the same background immunosuppression that failed in late withdrawal. Finally, it may be that early versus late alone is the difference.
A subset of recipients in the rapid discontinuation protocol are participating in a randomized study of cyclosporine and CellCept versus tacrolimus-sirolimus. After 2 years, we have seen no difference between the groups in acute rejection episodes, or in patient and graft survival. The only difference we have seen is a higher need for lipid-lowering medications in the sirolimus-treated recipients.
In terms of long-term results, I think the Canadian study showed a separation between those on versus off prednisone at around 2 to 3 years. Our data is out to 4 years. Dr. Kaufman at Northwestern has data, also out to 4 years. Neither of us sees any fall-off in graft survival similar to that seen in the previous Canadian study. And I think once we have 4- or 5-year data, it becomes important for the transplant community to start seriously thinking about elimination of steroids as a routine; again, as I said in my presentation, we need to balance steroid minimization with minimization of the nephrotoxicity from calcineurin inhibitors.
Dr. Dixon B. Kaufman (Chicago, Illinois): First, let me express my thanks to the officers and the members for the opportunity to comment on this important paper. I am here as a guest of Frank Stuart and speak, in part, to represent the views of our transplant program at Northwestern. We have become very strong advocates of steroid elimination because these agents simply no longer contribute to meaningful immunosuppression yet have a serious erosive effect on patient well-being. Dr. Matas, your experience and presentation demonstrated that beautifully.
We are proud of our use of steroid elimination protocols. Since 1998, virtually all kidney, pancreas, and islet transplant recipients, totaling over 700, have received this protocol successfully. My questions relate to our experience on this topic.
Minnesota has a very impressive multiorgan transplant center, and I would like to know how applicable your current protocols are to the other solid organ transplants done at the University of Minnesota.
Second, we started to coin a term “immunosuppressive tolerability” in distinction to the term “immune tolerance.” Your talk really relates to immunosuppressive tolerability. These agents are double-edged swords, and you are starting to pick and choose which ones carry the most benefit and eliminate those that have the highest risk. So your patients are on dual therapy. Where do you see this evolving? Can you get patients down to one immunosuppressive medicine? Which one and when posttransplant? Do you think you can improve on the concept of tolerability?
Dr. Arthur J. Matas (Minneapolis, Minnesota): First, I think your own data actually shows clearly that steroid-free protocols can be used in kidney-pancreas transplant recipients.
Our patients who are steroid-free after a kidney transplant, and who then come in for a pancreas after kidney transplant, remain steroid-free after their pancreas transplant. We also use a steroid-free protocol with kidney-pancreas transplants.
The Oschner clinic has shown that this protocol can be used in liver transplants. And in fact, in answer to your second question, our center is now using Campath for pancreas transplant recipients, with some exciting preliminary data.
I think the idea of “tolerability” is an important consideration. One concern, as Allen Kirk at the N.I.H. has previously mentioned, is that some patients who are off all immunosuppression posttransplant may be well for a little while. In fact, we have all seen patients who stopped their immunosuppression and occasionally do well for a little while, but then something happens, like a viral infection, that tips the balance.
It may be that what we should be aiming for is a very low-dose single-agent protocol. And I think the goal of combining these steroid-free and calcineurin-free protocols will be to aim for low-dose single, or perhaps low-dose dual, agents to minimize side effects (as we have shown here with the steroid-free protocol) yet simultaneously maximize long-term graft outcome.
Dr. Raymond Pollak (Peoria, Illinois): Very nice paper. I enjoyed it very much. Are your results applicable to other subpopulations of the patients who get kidney transplants, ethnic minorities and others? Second, with this large number of patients, have you done any in vitro work to demonstrate whether the tolerance you observed is long lasting, whether it is donor-specific or third-party-specific?
Dr. Arthur J. Matas (Minneapolis, Minnesota): We are using this protocol on all first and second transplant recipients. The only exception is recipients who are on predisone at the time of transplant. So the protocol is used for ethnic minorities as well. I think the Northwestern series, which has a larger percentage of minority recipients than we do, has shown clearly that steroid minimization can be done in ethnic minorities.
The Stanford group has shown clearly that a similar protocol is applicable to children. And our data has shown that this protocol can be used in high PRA and other high-risk recipients.
We are currently doing hyporesponsive and other substudies in this protocol, but there is no data yet to show whether these tests can predict who does, versus does not, have rejection.
Footnotes
Supported by grants from Fujisawa Healthcare, SangStat, and the NIH (DK13083).
Address correspondence to: Arthur J. Matas, MD, MMC, 328, 420 Delaware Street SE, Minneapolis, MN 55455. E-mail: matas001@umn.edu.
REFERENCES
- 1.Calne RY. The rejection of renal homografts: inhibition in dogs by 6-mercaptopurine. Lancet. 1960;1:417–418. [DOI] [PubMed] [Google Scholar]
- 2.Calne RY, Alexandre GPJ, Murray JE. The development of immunosuppressive therapy. Ann N Y Acad Sci. 1962;99:743–761. [DOI] [PubMed] [Google Scholar]
- 3.Kuss R, Legraine M, Mathe G, et al. Homologous human kidney transplantation. Postgrad Med J. 1962;38:528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Marchioro TC, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 5.Fryer JP, Granger DK, Leventhal JR, et al. Steroid-related complications in the cyclosporine era. Clin Transplant. 1994;8:224–229. [PubMed] [Google Scholar]
- 6.Cremer J, Struber M, Wagenbreth I, et al. Progression of steroid-associated osteoporosis after heart transplantation. Ann Thorac Surg. 1999;67:130–133. [DOI] [PubMed] [Google Scholar]
- 7.Laan RF, van Riel PL, van de Putte LB, et al. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis: a randomized, controlled study. Ann Intern Med. 1991;119:963–968. [DOI] [PubMed] [Google Scholar]
- 8.Saag KG, Koehnke R, Caldwell JR, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96:115–123. [DOI] [PubMed] [Google Scholar]
- 9.Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. [DOI] [PubMed] [Google Scholar]
- 10.McGeown MG, Douglas JF, Brown WA, et al. Advantages of low dose steroid from the day after renal transplantation. Transplantation. 1980;29:287–289. [DOI] [PubMed] [Google Scholar]
- 11.Hricik DE, O'Toole MA, Schulak JA, et al. Steroid-free immunosuppression in cyclosporine-treated renal transplant recipients: a meta-analysis. J Am Soc Nephrol. 1993;4:1300–1305. [DOI] [PubMed] [Google Scholar]
- 12.Kasiske BL, Chakkera HA, Louis TA, et al. A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol. 2000;11:1910–1917. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair NR. Low-dose steroid therapy in cyclosporine-treated renal transplant recipients with well-functioning grafts; the Canadian Multicenter Transplant Study Group. CMAJ. 1992;147:645–657. [PMC free article] [PubMed] [Google Scholar]
- 14.Vanrenterghem Y, Lebranchu Y, Hene R, et al. Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation. 2000;70:1352–1359. [DOI] [PubMed] [Google Scholar]
- 15.Steroid Withdrawal Study Group. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil: a prospective randomized study. Transplantation. 1999;68:1865–1874. [DOI] [PubMed] [Google Scholar]
- 16.Birkeland SA. Steroid-free immunosuppression in renal transplantation: a long-term follow-up of 100 consecutive patients. Transplantation. 2001;71:1089–1090. [DOI] [PubMed] [Google Scholar]
- 17.Matas AJ, Ramcharan T, Gillingham KJ. Rapid discontinuation of steroids in living donor kidney transplantation: a pilot study. Am J Transplant. 2001;1:278–283. [DOI] [PubMed] [Google Scholar]
- 18.Cantarovich D, Giral-Classe M, Hourmant M, et al. Prevention of acute rejection with antithymocyte globulin, avoiding corticosteroids, and delaying cyclosporin after renal transplantation. Nephrol Dial Transplant. 2000;15:1673–1676. [DOI] [PubMed] [Google Scholar]
- 19.Cole E, Landsberg D, Russell D, et al. A pilot study of steroid-free immunosuppression in the prevention of acute rejection in renal allograft recipients. Transplantation. 2001;72:845–850. [DOI] [PubMed] [Google Scholar]
- 20.Vincenti F, Monaco A, Grinyo J, et al. Rapid steroid withdrawal versus standard steroid therapy in patients treated with basiliximab, cyclosporine, and mycophenolate mofetil for the prevention of acute rejection in renal transplantation. Transplant Proc. 2001;33:1011–1012. [DOI] [PubMed] [Google Scholar]
- 21.Buell JF, Kulkarni S, Grewal HP, et al. Early corticosteroid cessation at one week following kidney transplant under tacrolimus and mycophenolate mofetil (MMF) immunosuppression: three-year follow-up. Transplant. 2000;69:5134. [Google Scholar]
- 22.Kaufman DB, Leventhal JR, Koffron AJ, et al. A prospective study of rapid corticosteroid elimination in simultaneous pancreas-kidney transplantation: comparison of two maintenance immunosuppression protocols: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Transplantation. 2002;73:169–177. [DOI] [PubMed] [Google Scholar]
- 23.Sarwal MM, Vidhun JR, Alexander SR, et al. Continued superior outcomes with modification and lengthened follow-up of a steroid-avoidance pilot with extended daclizumab induction in pediatric renal transplantation. Transplantation. 2003;76:1331–1339. [DOI] [PubMed] [Google Scholar]
- 24.Freise CE, Kang SM, Feng S, et al. Excellent short-term results with steroid-free maintenance immunosuppression in low-risk simultaneous pancreas-kidney transplantation. Arch Surg. 2003;138:1121–1125. [DOI] [PubMed] [Google Scholar]
- 25.Prasad GV, Nash MM, McFarlane PA, et al. Renal transplant recipient attitudes toward steroid use and steroid withdrawal. Clin Transplant. 2003;17:135–139. [DOI] [PubMed] [Google Scholar]
- 26.Schulak JA, Mayes JT, Moritz CE, et al. A prospective randomized trial of prednisone versus no prednisone maintenance therapy in cyclosporine-treated and azathioprine-treated renal transplant patients. Transplantation. 1990;49:327–332. [DOI] [PubMed] [Google Scholar]
- 27.Matas AJ, Payne WD, Gillingham KJ, et al. Optimizing the immediate post-transplant prednisone dose: a prospective randomized trial. Clin Transplant. 1992;6:73–76. [Google Scholar]
- 28.Matas AJ, Humar A, Payne WD, et al. Decreased acute rejection in kidney transplant recipients is associated with decreased chronic rejection. Ann Surg 1999;230:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lederer SR, Kluth-Pepper B, Schneeberger H, et al. Impact of humoral alloreactivity early after transplantation on the long-term survival of renal allografts. Kidney Int. 2001;59:334–341. [DOI] [PubMed] [Google Scholar]
- 30.Bohmig GA, Exner M, Habicht A, et al. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091–1099. [DOI] [PubMed] [Google Scholar]
- 31.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria: an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. [DOI] [PubMed] [Google Scholar]
- 32.Hricik DE. Steroid-free immunosuppression in kidney transplantation: an editorial review. Am J Transplant. 2002;2:19. [DOI] [PubMed] [Google Scholar]
- 33.Khwaja K, Asolati M, Harmon J, et al. Outcome at 3 years with a prednisone-free maintenance regimen: a single-center experience with 349 kidney transplant recipients. Am J Transplant. 2004;4:980. [DOI] [PubMed] [Google Scholar]
- 34.Almawi WY, Melemedjian OK, Rieder MJ. An alternate mechanism of glucocorticoid anti-proliferative effect: promotion of a Th2 cytokine-secreting profile. Clin Transplant. 1999;13:365–374. [DOI] [PubMed] [Google Scholar]
- 35.Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. [DOI] [PubMed] [Google Scholar]
- 36.Gonwa TA, Hrick DE, Brinker K, et al, for the Sirolimus Renal Function Study Group. Improved renal function in sirolimus-treated renal transplant patients after early cyclosporine elimination. Transplantation. 2002;74:1560–1567. [DOI] [PubMed] [Google Scholar]
- 37.Morales JM, Wramner L, Kreis H, et al, for the Sirolimus European Renal Transplant Study Group. Sirolimus does not exhibit nephrotoxicity compared to cyclosporine in renal transplant recipients. Am J Transplant. 2002;2:436–442. [DOI] [PubMed] [Google Scholar]
- 38.Johnson RWG, Kreis H, Oberbauer R, et al. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001;72:777–786. [DOI] [PubMed] [Google Scholar]
- 39.Flechner SM, Goldfarb D, Modlin C, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation. 2002;74:1070–1076. [DOI] [PubMed] [Google Scholar]
- 40.Kreis H, Cisterne JM, Land W, et al, for the Sirolimus European Renal Transplant Study Group. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation. 2000;69:1252–1260. [DOI] [PubMed] [Google Scholar]
- 41.Kreis H, Oberbauer R, Campistol JM, et al, for the Rapamune Maintenance Regimen Trial. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004;15:809–817. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro R, Jordan ML, Basu A, et al. Kidney transplantation under a tolerogenic regimen of recipient pretreatment and low-dose postoperative immunosuppression with subsequent weaning. Ann Surg. 2003;238:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]


