Abstract
Objective:
We sought to evaluate the potential benefits of minimally invasive approaches for treatment of isolated aortic and mitral valve disease.
Methods:
From 7/96 to 04/03, we performed 1000 minimally invasive valve operations: 526 aortic (AV) procedures (64 years; mean, 25–95) and 474 mitral (MV) procedures (58 years; mean, 17–90).
Results:
In the AV group, an upper ministernotomy was used in 492/526 patients (93%) and a right parasternal approach in 34 (7%). Sixty-three patients had reoperative aortic valve replacements. In the MV group lower sternotomy was used in 260/474 (55%), right parasternal in 200/474 (42%), and a right thoracotomy in 14 patients. MV repair was performed in 416 and MV replacement in 58 patients. Operative mortality was 12/526 (2%) in the AV and 1/474 (0.2%) in the MV group. Freedom from reoperation at 6 years was 99% and 95% in the AV and MV group, respectively. Late mortality was 5% in the AV and 3% in the MV group, respectively.
Conclusions:
Minimally invasive valve surgery can be performed at very low levels of morbidity and mortality, with results equal to or better than conventional techniques. All forms of valve repair and replacement operations can be performed. Long-term survival and freedom from reoperation are excellent.
Minimally invasive valve surgery decreases surgical trauma, recovery time, and resource use. We present our experience with a series of 1000 consecutive cases with low perioperative mortality and good midterm survival.
Minimally invasive methods have been successfully implemented and represent valuable alternatives or even standards for some procedures in general surgery, orthopedics, gynecologic, and thoracic surgery. Reduction in surgical trauma results in increased patient comfort as well as shorter hospital stay, thus saving valuable health care resources. Recognizing the benefits of such approaches in other surgical fields, minimally invasive surgery for valvular operations was introduced in 1996.1 Our institution was among the first centers to implement this concept and the initially reported results were encouraging, leading to the wider acceptance of these approaches.2 We now present our experience with the first 1000 minimally invasive valvular operations performed during a 7-year period.
MATERIALS AND METHODS
From July 1996 to April 2003 we performed 1853 isolated aortic and mitral procedures (aortic n = 1042, mitral n = 811). One thousand procedures were performed using the minimally invasive surgical approach (aortic n = 526, mitral n = 474). Patient data were prospectively entered in the electronic database to document indications, in-hospital morbidity and mortality, need for blood products use, total length of stay, disposition upon discharge, and postoperative morbidity and mortality. After obtaining the institutional review board approval, patient's data were analyzed retrospectively. Patients with concomitant coronary artery disease were excluded from the analysis.
Statistical Analysis
Data were expressed as median or percentage. Wilcoxon signed-rank test was used for continuous variables, and a two-tailed Fisher exact test was used for categorical variables. Long-term survival rates were calculated using the Kaplan-Meier method, and statistical significance was calculated by the log-rank test. A P value < 0.05 was considered to be statistically significant. The statistical software package used was Stata 7.0 for Windows (College Station, TX).
Operative Techniques: Aortic Valve Surgery
Two different minimally invasive approaches were used for aortic valve surgery: right parasternal (n = 34) and mini-upper sternotomy (n = 492). Right parasternal approach was performed through a 6- to 10-cm incision overlaying the second and third costal cartilage. After the complete resection of costal cartilages pericardium is opened and the aorta identified. Simultaneously, a 5-cm oblique inguinal incision was used to expose and cannulate femoral vessels. Upon the initiation of cardiopulmonary bypass, the aorta is cross-clamped trough the parasternal incision, under direct vision. Oblique aortotomy is made and extended into the midportion of the noncoronary cusp.
Incision for the partial upper sternotomy is 6–8 cm long, beginning halfway between sternal notch and angle of Loui. The partial upper sternotomy is than performed from the sternal notch extending toward the right fourth intercostal space, forming a reversed L shape sternotomy (Fig. 1). Cardiopulmonary bypass is established by cannulation of the distal ascending aorta and right atrium. Venous cannulation can be performed by using a small (21-F) venous cannula, which can be placed percutaneously in the femoral vein and advanced into the right atrium.
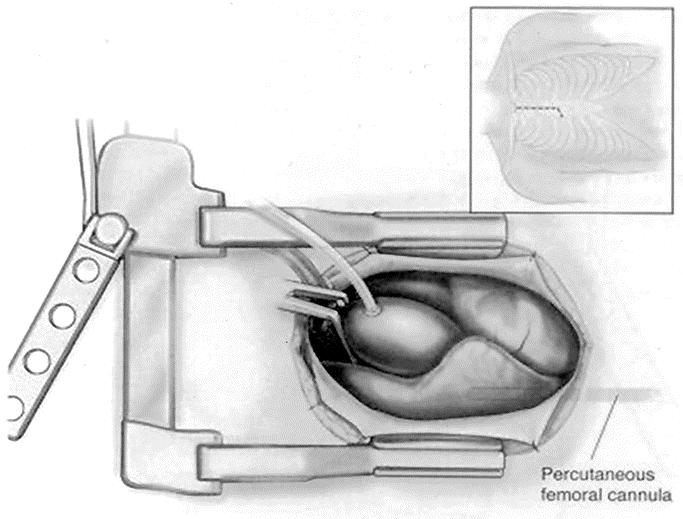
FIGURE 1. Partial upper sternotomy approach. Operative field and sternal incision (frame).
In our cohort of patients undergoing minimally invasive aortic valve surgery, there was a subgroup of patients undergoing reoperative procedures. All patients were placed on cardiopulmonary bypass (CPB) using peripheral arterial (percutaneous femoral or axillary artery) and venous cannulation sites. The anterior sternal table was divided with an oscillating saw and the posterior was divided using Mayo scissors under direct vision. Mediastinal dissection was limited to the aorta to enable clamping and aortotomy, and right atrium (RA). In patients with previous coronary artery bypass grafting and patent left internal mammary artery graft (LIMA), we have used moderate-to-deep hypothermia (20–22°C) with antegrade cardioplegia delivery, but without dissection of the LIMA pedicle. Retrograde cardioplegia was used in some patients, delivered by a transjugular coronary sinus catheter. Appropriate venting of the left ventricle was assured by using a small flexible transaortic left ventricular vent.3
Operative Techniques: Mitral Valve Surgery
Right parasternal approach was used in 200 patients undergoing mitral valve surgery. After a longitudinal pericardial incision above the phrenic nerve course, pericardium is suspended to the skin to elevate the right side of the heart. Superior vena cava is cannulated directly with a right-angled cannula. Mitral valve can be approached either directly or trans-septally via the RA. In the latter case, retrograde cannula is inserted into the coronary sinus under direct vision and secured by a purse string.
A small number of patients underwent mitral valve surgery via a limited anterolateral thoracotomy trough the right fourth intercostal space. Exposure technique resembles the one used for parasternal approach.
Most of the patients in our cohort underwent mitral valve surgery via the lower partial mini-sternotomy. Lower partial sternotomy (“mini-sternotomy”) is performed through a small 6- to 8-cm incision overlying distal aspect of the sternum. The sternotomy is extended from the sternoxiphoid junction upwards to the second intercostals space and extended into the right intercostal space (Fig. 2). Care is taken to prevent injury to right internal mammary artery. The ascending aorta is cannulated through the incision using a small cannula. In cases where small incision does not allow adequate aortic exposure, peripheral arterial cannulation via femoral was used as an alternative. Venous cannulation is usually accomplished by direct cannulation of the superior vena cava through the sternotomy. Inferior vena cava is cannulated with a small percutaneous transfemoral cannula advanced over the guide-wire and under guidance of the transesophageal echocardiography.4
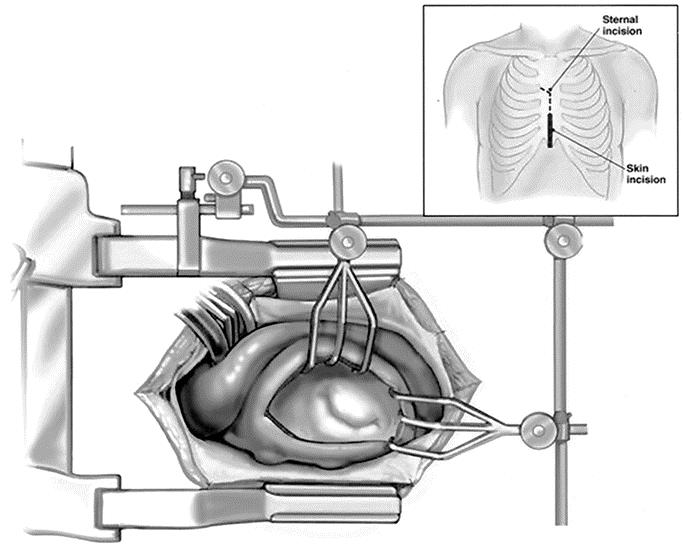
FIGURE 2. Lower partial sternotomy. Operative field and sternal and skin incision (frame).
RESULTS
The study population included a total of 1853 patients, 1042 patients undergoing aortic valve surgery (615 male, 427 female), and 811 patients undergoing mitral valve surgery (412 male, 399 female). Median follow-up was 39 months (range, 1–84).
Aortic Valve Surgery
Of 1042 patients undergoing aortic valve surgery, minimally invasive approach was used in 526. Three hundred seventy-six patients received a bioprosthetic valve (pericardial, n = 269; homograft, n = 69; porcine, n = 38) whereas 150 patients received a bileaflet mechanical prosthesis. Table 1. represents demographic data for both minimally invasive and conventional aortic valve procedures. Median cardiopulmonary bypass time (110 vs. 124 minutes) and aortic cross clamp time (77 vs. 86 minutes) were shorter in the minimally invasive group. Patients undergoing minimally invasive aortic surgery had shorter length of stay and were more frequently discharged to home without the need for additional stationary rehabilitation services. Table 2 outlines other intraoperative data and postoperative complications. There were 12/526 (2%) perioperative deaths in minimally invasive group and 14/516 (2.7%) in the conventional group (P = 0.80). In the minimally invasive group, 4 deaths were attributed to cardiac causes (low cardiac output syndrome, n = 4) whereas 8 deaths were attributed to noncardiac causes (stroke, n = 3; renal failure, n = 2; multisystem organ failure, n = 2; and respiratory failure, n = 1). Of 14 deaths in the conventional group 3 were from cardiac causes (arrhythmia, n = 2; biventricular failure, n = 1) and 11 were from noncardiac causes (stroke, n = 3; bowel ischemia, n = 2; multisystem organ failure, n = 1; renal failure, n = 1; coagulopathy, n = 1; acute pulmonary embolism, n = 1; ischemic brain injury after repair of iatrogenic pulmonary artery lesion, n = 1).
TABLE 1. Demographic Data for Patients Undergoing Aortic Valve Surgery
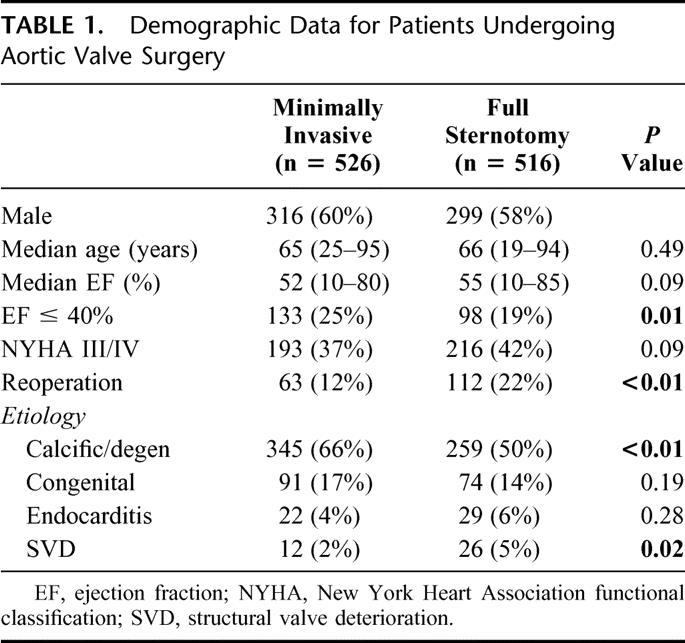
TABLE 2. Operative Data and Postoperative Complications for Patients Undergoing Aortic Valve Surgery
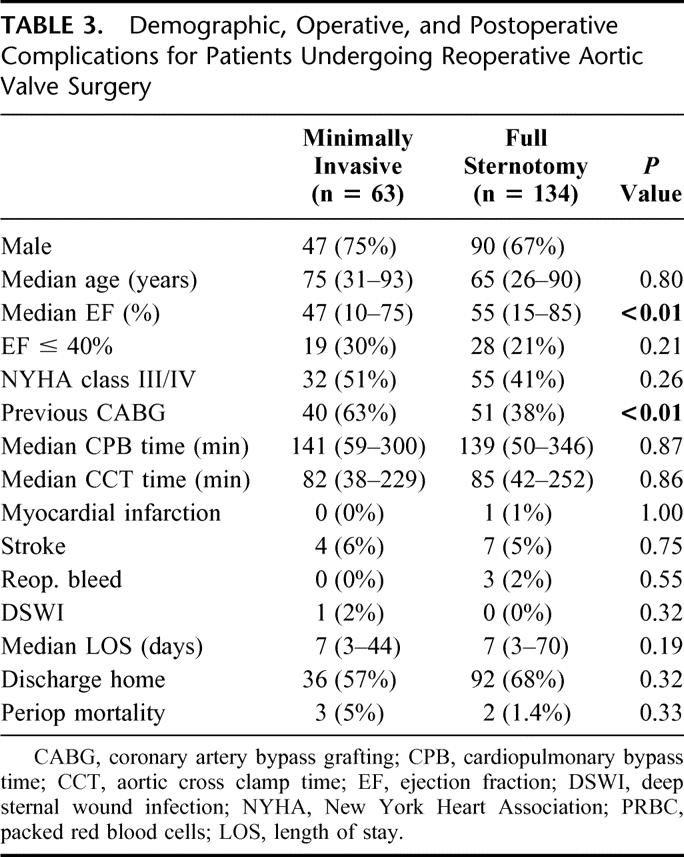
Median follow-up was 39 months (range, 1–84) for a total of 3448 patient years. There were 24/526 (5%) deaths in the follow-up period in the minimally invasive group, for the actuarial survival of 98%, 94%, and 82% at 1, 3, and 5 years respectively, and 56/516 deaths (10.8%) in the conventional group, for the actuarial survival of 94%, 90%, and 86% at 1, 3, and 5 years respectively (P = 0.006; Fig. 3). Sixty-three patients underwent reoperative aortic valve surgery via a minimal incision and 134 via sternotomy. Clinical and demographic data for this subgroup are presented in Table 3.
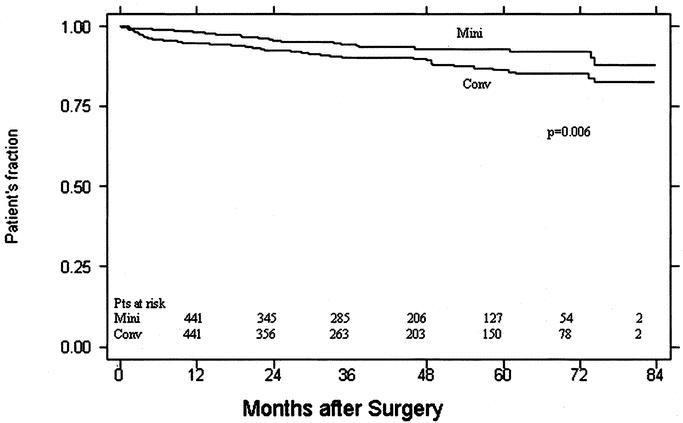
FIGURE 3. Kaplan-Meier survival curves for patients undergoing aortic valve surgery (minimally invasive versus conventional approach).
TABLE 3. Demographic, Operative, and Postoperative Complications for Patients Undergoing Reoperative Aortic Valve Surgery
Mitral Valve Surgery
Minimally invasive approach was used in 474/811 (58%) of patients undergoing mitral valve surgery. The mitral valve was repaired in 416 and replaced in 58 patients. Table 4 outlines demographic data for patients undergoing mitral valve surgery. Other intraoperative data and postoperative complications are presented in Table 5. One patient (0.2%) died in the perioperative period in the minimally invasive group as the result of cardiac failure. There was 1/337 (0.3%) deaths in the conventional group caused by multiple organ failure. Patients undergoing minimally invasive mitral valve surgery had shorter length of stay (5 versus 7 days) and were discharged home more frequently. Median follow-up was 42 months (range, 1–84 months) for a total of 1727 patient years. Sixteen patients (3%) who underwent minimally invasive mitral valve surgery died during follow-up for an actuarial survival of 98%, 97%, and 95% at 1, 3, and 5 years respectively. Among patients undergoing conventional sternotomy 35/337 (10.3%) died during follow-up for an actuarial survival of 97%, 91%, and 86% at 1, 3, and 5 years respectively(P = 0.03; Fig. 4).
TABLE 4. Demographic Data for Patients Undergoing Mitral Valve Surgery
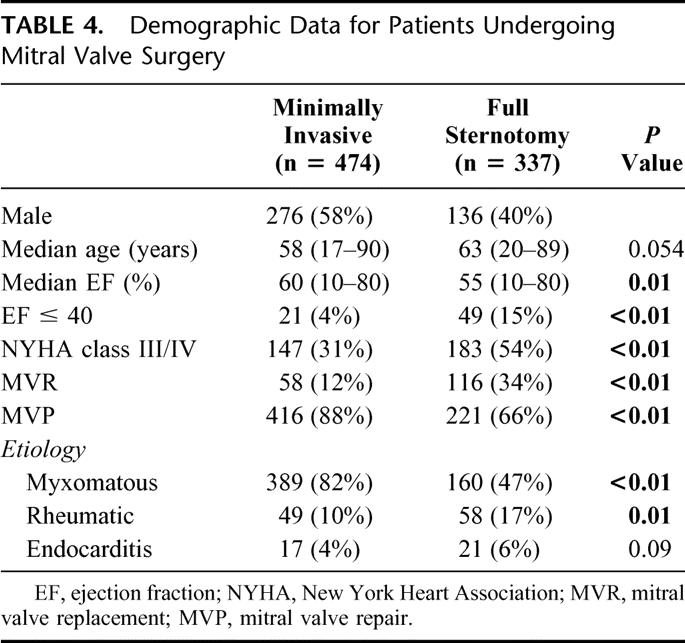
TABLE 5. Operative Data and Postoperative Outcome for Patients Undergoing Mitral Valve Surgery
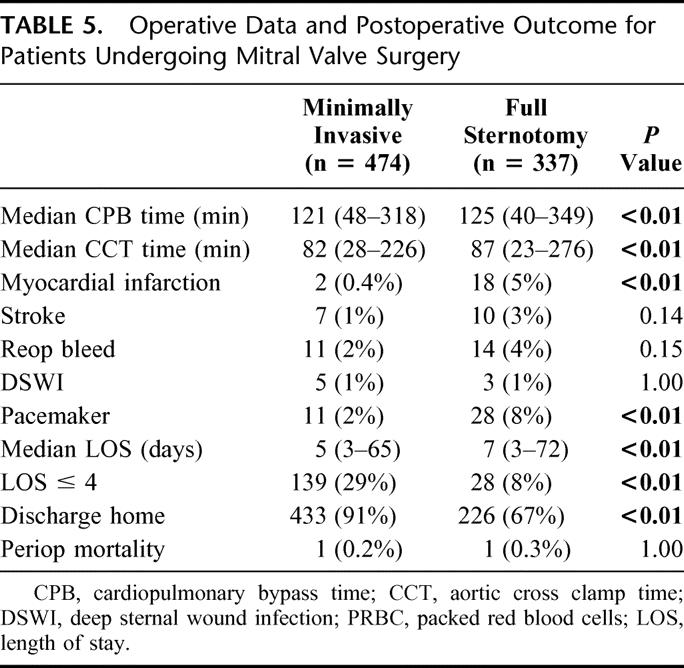
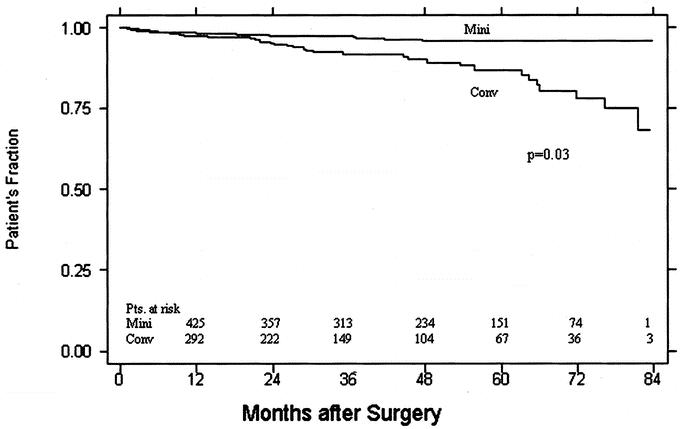
FIGURE 4. Kaplan-Meier survival curves for patients undergoing mitral valve surgery (minimally invasive versus conventional approach).
DISCUSSION
This study represents one of the largest single-center studies comparing the outcomes of minimally invasive and conventional valvular surgery. The main goal of this approach was reducing surgical trauma, increasing patient's satisfaction, and better use of healthcare resources. Parallel development of novel technologies (ie, robotics, video-assisted thoracoscopic surgery) enabled the implementation of such approaches into everyday practice.5
One of principal concerns involving minimally approach was a reasonable concern about the “trade-off” of surgical incision and limited exposure versus safety of the established approach through the full sternotomy.6 In our first report on minimally invasive valve surgery we also reported longer CPB and cross-clamp time.2 With more cases performed, centers that routinely use this approach still report on CPB times equal or slightly longer than conventional.7,8 In our present series CPB time is significantly shorter than in conventional group in both aortic and mitral groups although that difference is clinically less relevant. With more experience gained and shorter CPB time, these operations might benefit the patients more than standard procedures because of fewer pulmonary and cerebral complications. Stroke rates in our cohort of patients undergoing minimally invasive valve surgery were 2% in the aortic (significantly lower than conventional) and 1.4% in the mitral group, correspond to those previously reported.7,9,10
Another reason that might be responsible for the reluctance to use minimally invasive techniques is that the surgical field is limited to the valve and ascending aorta only. Major concerns include volume overload of the heart and de-airing. In our experience, routine use of transesophageal echo, use of CO2 in the operative field can eliminate these concerns.11 Percutaneous placement of venous cannula improves visualization in the operative field by eliminating the need for cannulation of the right atrium. Vacuum-assisted venous drainage represents a major improvement of extracorporeal bypass technology, which allows adequate venous drainage through small-diameter cannulas.
An important adjunctive aspect of minimally invasive surgery is the decrease of resource utilization.2,7 Significantly decreased hospital stay is reported in all reports on minimally invasive valve surgery. More patients discharged to home as opposed to skilled nursing facilities reflect improved functional status after surgery.
With increasing number of patients referred for reoperative valve surgery, minimally invasive approaches have to be considered for those patents as well. Our experience of aortic valve reoperations via the partial upper sternotomy, especially in patients with patent LIMA-left anterior descending graft, are very favorable, providing similar results as the standard approach.12 Similar results were reported by Svensson et al.13 We have previously reported the safety of minimally invasive mitral valve repair that led us to a conclusion that mitral valve repair should be offered to patients with myxomatous valve disease in the earlier phase of their disease.14
The retrospective nature is a limitation of this study. However, there have been very few large prospective randomized trials on this subject. Another limitation is lack of long-term functional status follow-up. Although less early postoperative pain and discomfort was reported,2,15 we feel that midterm postoperative functional status is not likely to be related to incision type.
Our early and midterm results for minimally invasive valve operations suggest the appropriateness of that approach for isolated primary mitral and primary and reoperative aortic valve operations. Minimally invasive approach does not compromise surgical exposure and enables the performance of all types of complex valve procedures without additional risk.
Discussions
Dr. Irving L. Kron (Charlottesville, Virginia): I very much appreciate the opportunity to discuss these excellent results from the Brigham. The authors discuss an evolution of their approach. The bottom line is the incisions are smaller and the mortality and reoperation rates are low. Most importantly, the operation can be done expeditiously in contrast to other reports on minimally invasive cardiac surgery. Shorter pump and ischemic times are inherently an advantage since there is nothing physiologic about the pump.
I have 3 questions for the authors. Why have they given up the parasternal approach? Were there issues with pain or exposure? Secondly, in the absence of coronary disease when do the authors advocate a standard surgical approach for valvular heart disease? Finally, are they convinced it is the approach that has caused the reduction in length of stay, or are they real good at selecting their patients?
Dr. Joseph M. Van De Water (Macon, Georgia): I didn't hear you say what the difference in pump time was, the difference in OR time, or the difference in cost.
Dr. Frederick L. Grover (Denver, Colorado): I wonder if you have any comments about time to return to work, activity, and pain scales.
Dr. Tomislav Mihaljevic (Boston, Massachusetts): Thank you very much for your questions. I will answer them in the order they were asked.
The first question relates to the parasternal approach and the utility of the parasternal approach. We used this approach in the early days of our minimally invasive valvular surgery and abandoned it for a main reason of a slower healing and a relatively high incidence of lung hernias in this particular group. We have switched to the partial upper sternotomy or partial lower sternotomy for most of our patients, as you saw in our presentation.
The question about when to use the standard versus minimally invasive approaches. I think in our group that particular issue gets addressed by an individual surgeon. For those of us who use the minimally invasive approaches on a majority of our patients, we think there are few absolute contraindications to use the minimally invasive approaches. The obesity limits the valvular exposure, and should be considered as a relative contraindication. There are no absolute contraindications for, in our opinion, use of the minimally invasive approach for aortic valve procedures.
Whether our results with minimally invasive approach are the results of a selection bias or whether the favorable outcomes are the result of a smaller incision is a good question. I think that, as you can see, our selection bias is mostly based on the surgeon's preference. There are surgeons in our group who prefer to use the conventional approaches. And I think that the results are comparable in both groups, and that both of these groups of patients had similar morbidity going into the surgery, and we firmly believe that the minimally invasive approaches result in faster recovery patients.
In the interest of time, we did not show you all of our data for both of these groups. There was no significant difference in the pump time or aortic cross clamp time between these 2 groups. Initial reports actually showed that the minimally invasive approaches did result in a slight increase in the pump time, but as the learning curve gets flatter those differences were actually eliminated and, as you will be able to see in our paper, our minimally invasive approach actually resulted in the shorter pump times and the shorter cross clamp times, in particular for the aortic valve surgery.
We have not performed any analysis in this particular population of patients in terms of their return to work, primarily because these are all elderly patients and most of them do not work anymore. But our experience is that those patients who are operated through the smaller incision with the less invasive approach, with the less trauma, do return to their regular daily activities faster than those operated with conventional approaches.
Footnotes
Reprints: Lawrence H. Cohn, MD, Division of Cardiac Surgery Brigham and Women's Hospital 75 Francis Street, Boston, MA 02115. E-mail: lcohn@partners.org.
REFERENCES
- 1.Navia JL, Cosgrove DM 3rd. Minimally invasive mitral valve operations. Ann Thorac Surg. 1996;62:1542–1544. [DOI] [PubMed] [Google Scholar]
- 2.Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg.1997;226:421–426; discussion 427–428. [DOI] [PMC free article] [PubMed]
- 3.Byrne JG, Karavas AN, Filsoufi F, et al. Aortic valve surgery after previous coronary artery bypass grafting with functioning internal mammary artery grafts. Ann Thorac Surg. 2002;73:779–784. [DOI] [PubMed] [Google Scholar]
- 4.Cohn L. Operative incisions for minimally invasive cardiac surgery. Op Tech Thoracic Cardiovasc Surg. 2000;5:146–155. [Google Scholar]
- 5.Chitwood WR. Minimally invasive valve surgery: trends for the future. Eur J Cardiothorac Surg. 1999;16(Suppl 1):S64–S65. [PubMed] [Google Scholar]
- 6.von Segesser LK, Westaby S, Pomar J, et al. Less invasive aortic valve surgery: rationale and technique. Eur J Cardiothorac Surg. 1999;15:781–785. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove DM 3rd, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg. 1998;65:1535–1538; discussion 1538–1539. [DOI] [PubMed]
- 8.Bonacchi M, Prifti E, Giunti G, et al. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg 2002;73:460–465; discussion 465–466. [DOI] [PubMed]
- 9.Sharony R, Grossi EA, Saunders PC, et al. Minimally invasive aortic valve surgery in the elderly: a case-control study. Circulation. 2003;108(Suppl 1):II43–II47. [DOI] [PubMed] [Google Scholar]
- 10.Grossi EA, Galloway AC, LaPietra A, et al. Minimally invasive mitral valve surgery: a 6-year experience with 714 patients. Ann Thorac Surg. 2002;74:660–663; discussion 663–664. [DOI] [PubMed]
- 11.Svenarud P, Persson M, van der Linden J. Effect of CO2 insufflation on the number and behavior of air microemboli in open-heart surgery: a randomized clinical trial. Circulation. 2004;109:1127–1132. [DOI] [PubMed] [Google Scholar]
- 12.Byrne JG, Aranki SF, Couper GS, et al. Reoperative aortic valve replacement: partial upper hemisternotomy versus conventional full sternotomy. J Thorac Cardiovasc Surg. 1999;118:991–997. [DOI] [PubMed] [Google Scholar]
- 13.Svensson LG, Nadolny EM, Kimmel WA. Minimal access aortic surgery including re-operations. Eur J Cardiothorac Surg. 2001;19:30–33. [DOI] [PubMed] [Google Scholar]
- 14.Greelish JP, Cohn LH, Leacche M, et al. Minimally invasive mitral valve repair suggests earlier operations for mitral valve disease. J Thorac Cardiovasc Surg. 2003;126:365–371; discussion 371–373. [DOI] [PubMed]
- 15.Walther T, Falk V, Metz S, et al. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Ann Thorac Surg. 1999;67:1643–1647. [DOI] [PubMed] [Google Scholar]


