Abstract
Objective:
Severe acute respiratory distress syndrome (ARDS) is associated with a high level of mortality. Extracorporeal life support (ECLS) during severe ARDS maintains oxygen and carbon dioxide gas exchange while providing an optimal environment for recovery of pulmonary function. Since 1989, we have used a protocol-driven algorithm for treatment of severe ARDS, which includes the use of ECLS when standard therapy fails. The objective of this study was to evaluate our experience with ECLS in adult patients with severe ARDS with respect to mortality and morbidity.
Methods:
We reviewed our complete experience with ELCS in adults from January 1, 1989, through December 31, 2003. Severe ARDS was defined as acute onset pulmonary failure, with bilateral infiltrates on chest x-ray, and PaO2/fraction of inspired oxygen (FiO2) ratio ≤100 or A-aDO2 >600 mm Hg despite maximal ventilator settings. The indication for ECLS was acute severe ARDS unresponsive to optimal conventional treatment. The technique of ECLS included veno-venous or veno-arterial vascular access, lung “rest” at low FiO2 and inspiratory pressure, minimal anticoagulation, and optimization of systemic oxygen delivery.
Results:
During the study period, ECLS was used for 405 adult patients age 17 or older. Of these 405 patients, 255 were placed on ECLS for severe ARDS refractory to all other treatment. Sixty-seven percent were weaned off ECLS, and 52% survived to hospital discharge. Multivariate logistic regression analysis identified the following pre-ELCS variables as significant independent predictors of survival: (1) age (P = 0.01); (2) gender (P = 0.048); (3) pH ≤7.10 (P = 0.01); (4) PaO2/FiO2 ratio (P = 0.03); and (5) days of mechanical ventilation (P < 0.001). None of the patients who survived required permanent mechanical ventilation or supplemental oxygen therapy.
Conclusion:
Extracorporeal life support for severe ARDS in adults is a successful therapeutic option in those patients who do not respond to conventional mechanical ventilator strategies.
At the University of Michigan, we have treated over 400 adult patients with extracorporeal life support for cardiac or pulmonary failure. Of these patients, 255 were placed on extracorporeal life support for severe acute hypoxic respiratory failure. One hundred thirty-two (52%) survived to hospital discharge with minimum long-term disability.
The acute respiratory distress syndrome (ARDS) refers to patients with an acute and progressive respiratory disease of a noncardiac nature, in association with diffuse bilateral pulmonary infiltrates demonstrated on chest radiograph, and with hypoxemia.1 In recent years, significant advances in our understanding of the pathophysiology of ARDS and lung injury have been made. This has led to prospective randomized clinical trials and attempts to improve therapy based on this scientific knowledge. Despite these recent advances, the mortality from ARDS in adults remains high at 40% to 60% in contemporary series.2–7 Approximately 10% of patients with ARDS are unsalvageable because of fatal illness (eg, head injury, massive burns, and metastatic cancer), so the mortality in ARDS patients selected for potential recovery is 30% to 50%. ARDS patients with a very high mortality risk can be identified early in the course of pulmonary failure. This group of “severe” ARDS patients is characterized by profound hypoxemia (PaO2/FiO2 ratio ≤100 and/or A-aDO2 >600 mm Hg) despite optimal conventional treatment. The use of extraordinary lifesaving treatment is indicated in these critically ill patients.
The technique of extracorporeal life support (ECLS, or ECMO, extracorporeal membrane oxygenation) for patients with severe ARDS involves placing them on a veno-venous (VV) or veno-arterial (VA) life support circuit with a membrane oxygenator to temporarily take over the gas exchange function of the lung. While on ECLS, the mechanical ventilator settings are adjusted to minimize ventilator-induced lung injury and to maximize the recruitment of functional residual lung capacity. Prolonged ECLS can sustain life even in the absence of native pulmonary function. ECLS is standard treatment of neonatal and pediatric respiratory failure unresponsive to other modes of treatment, and the survival rate in these patients is 70% to 90%.8–10 ECLS is also used for emergent cardiac support in pediatric and adult patients.11,12 In 1975, we participated in a prospective randomized trial of VA ECLS in adults for ARDS sponsored by the National Institutes of Health. This 9-center study demonstrated only 10% survival in both control and ECLS-treated patients.13 In retrospect, it was naive to conduct this study of an immature but complex technology in centers with no prior experience. Nonetheless, report of this study halted significant clinical research on ECLS for ARDS in adults for at least a decade.
More recently, investigators have reported improved survival with ECLS in severe ARDS patients failing all other means of respiratory support.14–17 In 1989, based on our successful experience with neonatal and pediatric respiratory failure, we returned to evaluation of ECLS for severe ARDS in adults. We previously reported our results with ECLS for respiratory failure in adults and documented a 52% survival in the first 100 patients.18 Other major centers also report 50% to 70% survival in high-mortality-risk adult ARDS patients.14–17
Our treatment protocol for adults involves an algorithm which aims to normalize body physiology, aggressively recruit functional residual lung capacity, and minimize ventilator-induced lung injury (Fig. 1). This algorithm used in 141 patients with respiratory failure referred for consideration of ECLS yielded a survival rate of 62% in patients with severe ARDS (median initial PaO2/FiO2 ratio of 66).19 The purpose of this study is to describe and update our experience with this algorithm and ECLS for severe ARDS in adult patients.
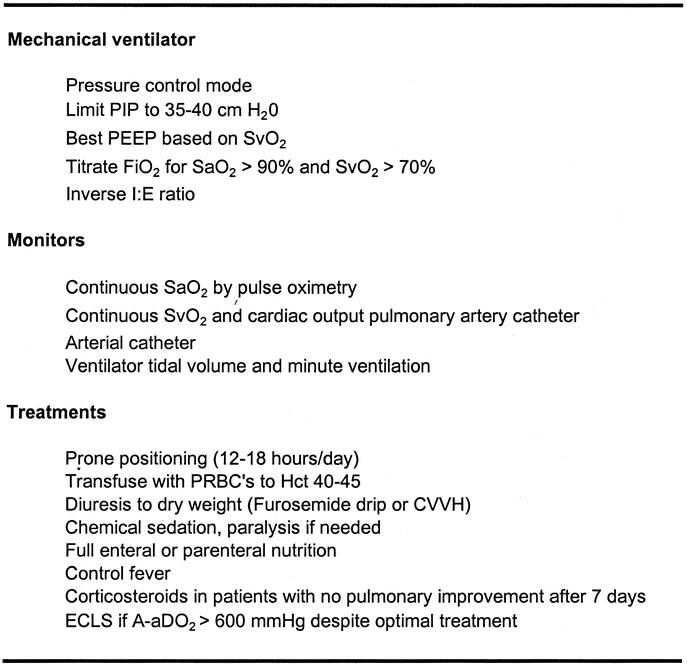
FIGURE 1. University of Michigan algorithm for treatment of severe ARDS. PIP, plateau inspiratory pressure; PEEP, positive end-expiratory pressure; FiO2, fraction of inspired oxygen; SaO2, arterial oxygen saturation; SvO2, mixed venous oxygen saturation; I:E, inspiratory to expiratory; Hct, hematocrit; PRBC's, packed red blood cells; CVVH, continuous veno-venous hemofiltration; ECLS, extracorporeal life support.
PATIENTS AND METHODS
From January 1989 to December 2003, 405 adult patients 17 years of age or older were placed on ECLS. Of these 405 patients, 255 had severe ARDS, 18 had pulmonary embolism, 7 had hypercarbic respiratory failure, and the remaining 125 patients were placed on ECLS for cardiac failure or cardiopulmonary resuscitation (CPR). The patients who formed the basis of this study were those placed on ELCS for severe ARDS.
Patients at outside hospitals who were potential candidates for ECLS were transferred using the facilities of Survival Flight (University of Michigan Health System ground and aero-medical patient transport service). Some patients were too clinically unstable for conventional transport. In these instances, the ECLS team and equipment were transported to the referring hospital and the patient was placed on ECLS. The patient was then transported back to the University of Michigan on active ECLS.
Selection Criteria and Pre-ECLS Management
After admission to the ECLS service, a standardized algorithm for management of severe respiratory failure was followed (Fig. 1). If patients fail to improve on this algorithm, ELCS is instituted. The indications for ECLS (and the definition of “failure to improve on algorithm treatment”) were based primarily on lung dysfunction measured as PaO2/FiO2 ratio <100 on FiO2 of 1.0, alveolar-arterial gradient (A-aDO2) >600 mm Hg, or transpulmonary shunt fraction >30% despite and after optimal treatment. These parameters describe a patient population with a >80% predicted mortality risk from ARDS.2,6,20 Early in our experience contraindications were age greater than 50 years, time on the mechanical ventilator more than 5 days, and severe systemic sepsis. As our experience grew, the age contraindication was advanced first to 60 and then to 70, while time on the mechanical ventilator was advanced to 7 then 10 days; severe sepsis is no longer a contraindication.
ECLS Methods
The technique of ECLS is well described.9,18 It includes major vascular cannulation in VV or VA mode. A drainage-infusion bridge (requiring regular unclamping and flushing) was used in all cases until mid-2003. Continuous on-line circuit monitors included venous drainage blood oxygen saturation, preoxygenator and postoxygenator pressure, and blood pump flow rate. VV access is the preferred mode of support for isolated respiratory failure. This was achieved by cannulation of the right atrium and inferior vena cava with the drainage and return catheters identified by optimal performance.21 VA access was used when systemic arterial perfusion support was necessary in addition to respiratory support. Venous access was gained by right atrium and/or inferior vena cava cannulation with arterial return into the common femoral or right carotid artery.
The algorithm for managing ECLS included maintaining blood flow to meet the goal of SaO2 >85% (VV) or >90% (VA). Standard heparin was given by continuous infusion at a rate titrated to maintain the desired whole blood activated clotting time 160 to 180 seconds (normal = 120 seconds). Patient management during ECLS included all of the measures listed in Figure 1, with the exception that the mechanical ventilator settings were reduced to “rest” or nondamaging settings (typically FiO2 0.3–0.5, rate 6–10, pressure control ventilation with PIP 30 cm H2O and positive end-expiratory pressure (PEEP) 10 cm H2O, inspiratory to expiratory [I:E] ratio >2:1).
Antibiotics were not given as a routine but were administered for proven microbial infection. Methylprednisolone (2 mg/kg/d)22 was given to patients who failed to improve their lung function after 7 days on ELCS. Renal failure was managed by continuous hemofiltration with or without countercurrent dialysis fluid. This was easily accomplished by adding a hemofilter to the ECLS circuit. Hemofiltration was usually performed in the continuous hemodiafiltration mode with a dialysis flow rate of 2 L/min. Fluids were managed to maintain a desired negative hourly balance until “dry weight” status was reached. Full nutritional support was given by the enteral or parenteral routes, depending on patient tolerance.
ECLS flow rate was adjusted to maintain gas exchange and hemodynamic stability at “rest” ventilator settings. Patients were weaned from ECLS when they were hemodynamically stable, sepsis had cleared, and they had adequate oxygenation by SaO2 and SvO2 at FiO2 0.5 or less. Some patients were weaned from ECLS at higher ventilator settings because of bleeding complications. When lung function improved, patients were then trialed off of ECLS at moderate ventilator settings (FiO2 0.5–0.6, PIP 30 cm H2O, PEEP 10 cm H2O, I:E 1:1) and decannulated if gas exchange was adequate. ECLS was discontinued before lung recovery in instances of irreversible brain damage, diagnosis incompatible with life, or irreversible pulmonary failure. Irreversible pulmonary failure was evident from elevations in pulmonary vascular resistance leading to pulmonary artery pressures greater than two thirds systemic blood pressure. This clinical finding was consistent with right heart failure, lung necrosis, and severe pulmonary fibrosis at autopsy.
After successful weaning from ECLS, patients remained on mechanical ventilation until standard extubation criteria were met. Patients were discharged from the intensive care unit (ICU) when they were stable off mechanical ventilation. Many of our patients were transferred to physical medicine and rehabilitation for 1 to 2 weeks of therapy and subsequently discharged from the hospital when capable of independent living. All patients are followed up at regular intervals by letter, telephone, and personal contact in the clinic.
Data Analysis
For purposes of this review the primary diagnoses leading to ARDS were divided into primary lung disease (ARDS, International Classification of Diseases, Ninth Revision [ICD-9] 518.81) and secondary lung disease (ARDS pulmonary insufficiency following trauma and/or surgery, ICD-9 518.5). Pulmonary embolism is discussed as a cause of respiratory failure in this series but is treated as a separate class of patients because this condition often requires both cardiac and pulmonary extracorporeal support. Lung recovery is defined as successful weaning and decannulation from extracorporeal support. Although there are many physiologic, radiologic, and chemical variables which describe the course of these patients, the results here are reported as primarily as survival or nonsurvival. Previously we found that younger age and fewer days on mechanical ventilation prior to ECLS were associated with higher survival.18 We evaluated the current database by multivariate logistic regression analysis to determine which additional factors were associated with survival.
Statistical Methods
The primary outcome measures were lung recovery (successful weaning and survival off ECLS), survival to hospital discharge, and complications. Data were analyzed using multivariate stepwise logistic regression. The dependent variable for logistic regression was mortality. First, clinical parameters associated with mortality were identified using univariate analysis (P value < 0.2). Continuous variables were analyzed using an unpaired 2-tailed Student t test. Discrete variables were compared using a 2-tailed Fisher exact test analysis. These potential confounders were entered in a multivariate stepwise (backward elimination) regression model with mortality as the dependent variable. Variables were removed and reentered into the model using a significance level for removal and reentry of 0.2 and 0.1, respectively. First-order interaction terms were investigated in the final model. All statistical analysis was performed using STATA Statistics/Data Analysis 8.0 software (Stata Corporation, College Station, TX). Results are presented as mean values ± SD unless otherwise noted. Statistical significance was defined as a P value ≤ 0.05. Approval for this study was obtained from the University of Michigan Health System institutional review board.
RESULTS
A total of 280 adult patients were identified who underwent treatment with ECLS for the diagnosis of respiratory failure, and 125 patients received ECLS for either support of failing cardiac function or ELCS CPR. Since 1989, the University of Michigan has averaged 18 to 20 cases per year of severe adult respiratory failure requiring ECLS therapy, with a maximum of 36 cases in 1 year. Successful weaning and survival off of ECLS was 68%, and survival to hospital discharge was 53% in patients treated with ELCS for respiratory failure (Table 1). The annual rate of weaning off ECLS and survival to hospital discharge has remained nearly constant since 1992, the point at which a total of 30 patients had been treated.
TABLE 1. Adult Respiratory ECLS Survival
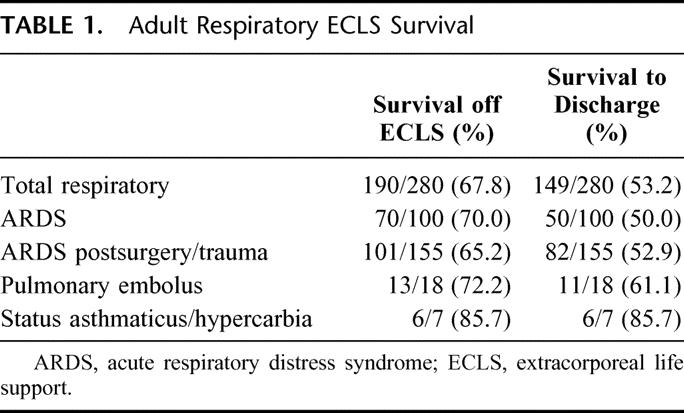
One hundred ninety-one of the 255 (75%) patients with severe ARDS were referred to the ECLS service from outside the University of Michigan. Ninety-nine of these patients required transport to the University of Michigan on ELCS, and those patients transported on ELCS had a 59% survival to hospital discharge. Two hundred six patients (81%) with severe ARDS required ECLS therapy within the first 6 hours of referral to the University of Michigan ECLS service. Of the adult patients treated with ELCS for severe ARDS, 67% were weaned from ECLS and 52% survived to hospital discharge.
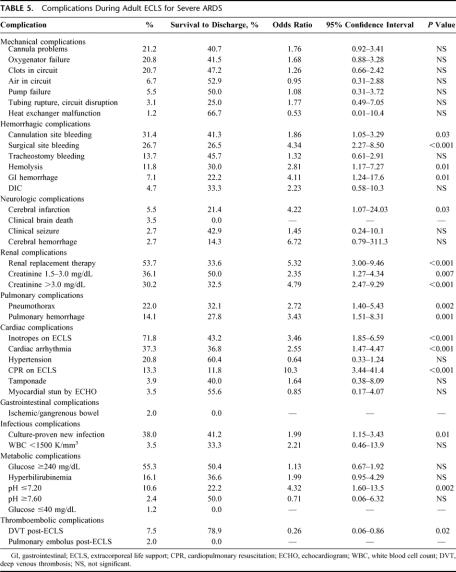
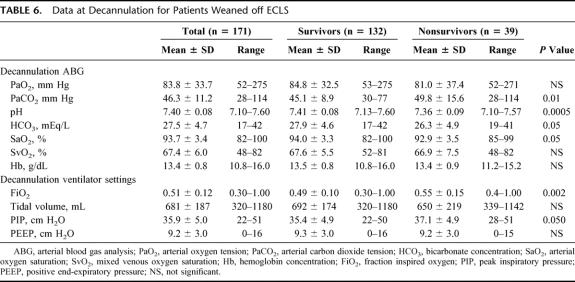
Characteristics of the adult patients treated for severe ARDS with ECLS are listed in Tables 2 through 7. Different modes of ELCS were employed, depending upon each patient's clinical condition and physiologic needs. The VA + V mode involves venous drainage of deoxygenated blood and venous and arterial reinfusion of oxygenated blood. This mode was primarily used in patients who had a common femoral artery infusion cannula and poor pulmonary function to improve the oxygen saturation of blood perfusing the brain and coronary arteries. The outcome for each different mode of ECLS used in these ARDS patients is described in Table 4. Mechanical and physiologic complications that occurred during ECLS are listed in Table 5. Cannulation site bleeding, surgical site bleeding, hemolysis, gastrointestinal hemorrhage, cerebral infarction, renal replacement therapy, creatinine >1.5 mg/dL, pneumothorax, pulmonary hemorrhage, inotropes on ECLS, cardiac arrhythmia, CPR on ECLS, culture proven new infection, pH ≤7.20, and deep venous thrombosis (DVT) post-ECLS were all associated with significantly decreased survival in univariate analysis. Physiologic and mechanical ventilator data at the time of decannulation for patients successfully weaned off of ECLS are listed in Table 6. These data are shown for patients who ultimately survived to hospital discharge (77%) and those who died in the hospital after discontinuation of ELCS (23%).
TABLE 2. Adult ECLS for Severe ARDS Survival by Diagnosis
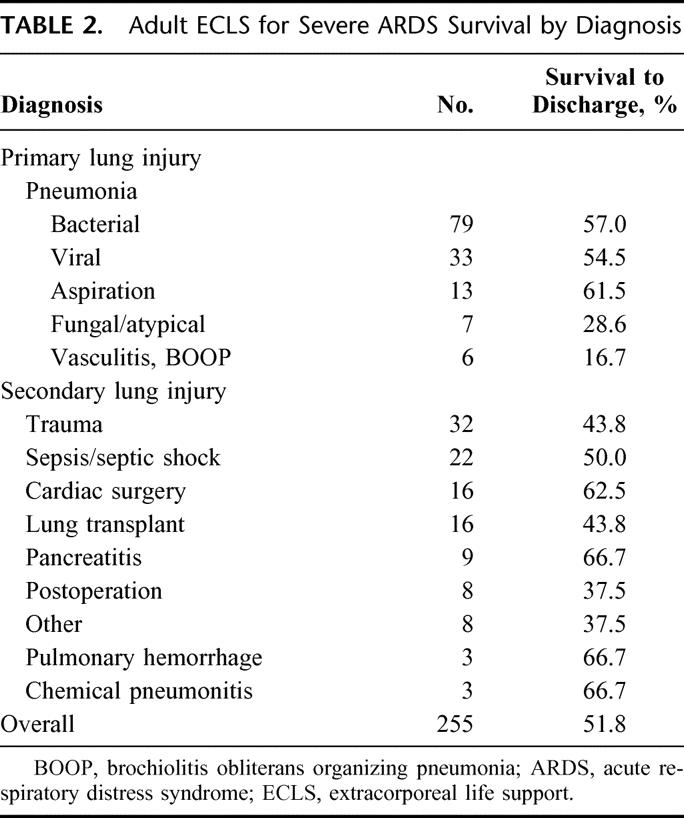
TABLE 3. Adult ECLS for Severe ARDS: Patient Characteristics
TABLE 4. Adult ECLS Mode for Severe ARDS
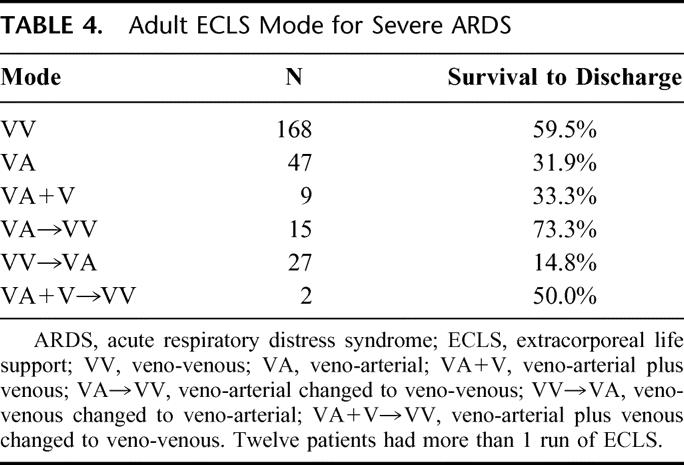
TABLE 5. Complications During Adult ECLS for Severe ARDS
TABLE 6. Data at Decannulation for Patients Weaned off ECLS
TABLE 7. Multiple Logistic Regression Analysis of Pre-ECLS Variables Influencing Outcome
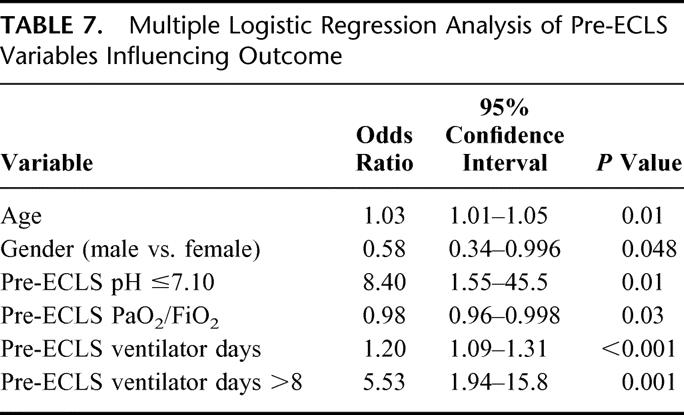
Multivariate analysis identified age, gender, pre-ECLS pH ≤7.10, pre-ELCS PaO2/FiO2 ratio, and pre-ECLS ventilator days to all be significant independent variables influencing outcome (Table 7). The logit equation calculating the probability of fatal outcome from ELCS based on these pre-ECLS variables is as follows:
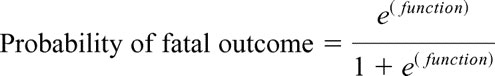 |
function = 0.18 × (pre-ECLS ventilator days) + 0.027 × (age) − 0.021 × (P/F ratio) + 2.13 × (pre-ECLS pH category) − 0.54 × (gender category) − 0.45
P/F ratio = PaO2/FiO2
pre-ECLS pH category = 0 if pre-ECLS pH >7.1 or 1 if pre-ECLS pH ≤7.1
gender category = 0 if female or 1 if male
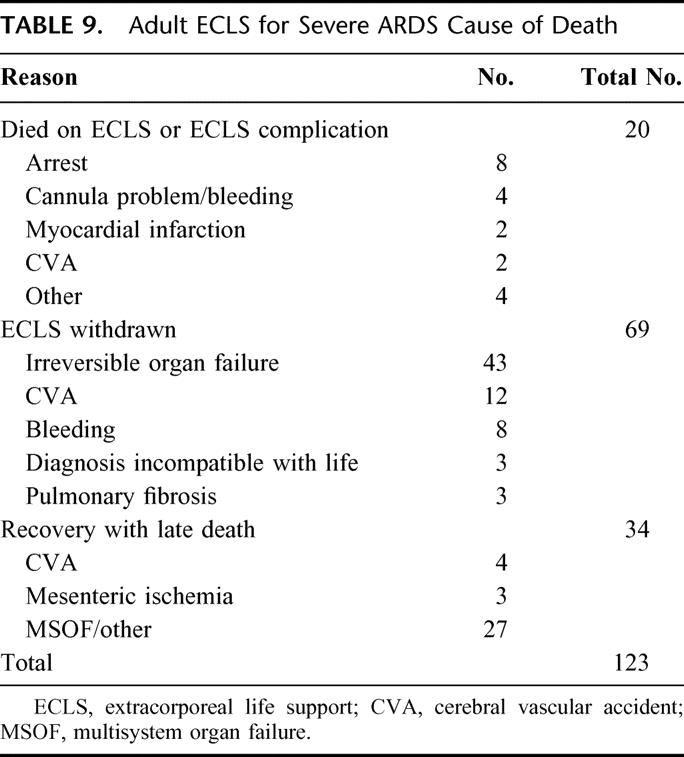
Graphical representation of the effect of pre-ECLS PaO2/FiO2 ratio on probability of fatal outcome was derived from the multiple logistic regression model and is shown in Figure 2. Graphs were also constructed for pre-ECLS ventilator days and age but are not shown. ECLS decannulation variables influencing outcome are shown in Table 8. ECLS complications that were independently associated with decreased survival, identified in multivariate analysis, included cannulation site bleeding, surgical site bleeding, cerebral infarction, renal replacement therapy, pulmonary embolism, and CPR on ELCS. In addition, all of the patients who suffered clinical brain death, ischemic or gangrenous bowel, or glucose ≤40 mg/dL died. The cause of death is shown in Table 9 for all nonsurvivors.
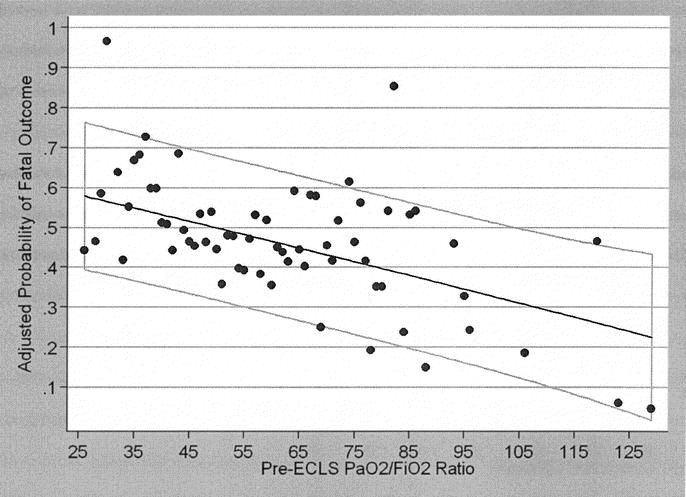
FIGURE 2. Graphical representation of the effect of pre-ECLS PaO2/FiO2 ratio on the probability of fatal outcome from ELCS, derived from the multiple logistic regression model of pre-ECLS variables found to be independent predictors of outcome. The light gray box represents the 95% confidence interval for the graph.
TABLE 8. Multiple Logistic Regression Analysis of ECLS Decannulation Variables Influencing Outcome

TABLE 9. Adult ECLS for Severe ARDS Cause of Death
Almost all surviving patients returned to normal function by 1 year postdischarge. The major abnormalities experienced are neurologic or neuromuscular disorders, including deafness and prolonged weakness or neuropathy. Pulmonary function and exercise tolerance is normal for activities of daily living in all patients, although a slight restrictive pattern is often seen on detailed pulmonary function testing. The major disability is psychologic, as is common after any life-threatening illness. Approximately 25% of patients have fear of recurrence of illness, nightmares, or even overt depression.
DISCUSSION
ARDS is characterized by profound hypoxemia caused by acute and persistent pulmonary inflammation with increased vascular permeability. The etiology of ARDS can be classified as primary lung injury (caused by bacterial, viral, or aspiration pneumonia, vasculitis, or other primary lung disease), or secondary lung injury (following shock, trauma, sepsis, pancreatitis, or other systemic conditions). Overall mortality for ARDS is currently 40% to 60% in all major series.2–7 This high mortality is caused by multiple-organ failure, sepsis, stroke, myocardial infarction, and in some cases, by progressive irreversible pulmonary failure. Within those patients with ARDS, there is a group of patients with “severe ARDS” that is characterized by A-aDO2 >600 mm Hg and/or PaO2/FiO2 ratio ≤100 on 100% oxygen despite and after optimal treatment. In some, if not all, cases, patients’ progressive pulmonary failure and multiple-organ failure is caused to an extent by the high-pressure, high-oxygen mechanical ventilation, which is necessary to keep the patients alive. These patients are candidates for extraordinary measures of life support. The patients in this series were transferred to the University of Michigan Surgical ICU because they had failed optimal conventional treatment. Most of the patients were referred from other hospitals, and some required transport on ECLS. This group of patients with severe ARDS has an 80% to 100% risk of mortality with continuing conventional treatment.2,6,20
ECLS is the standard treatment of respiratory failure in newborn and pediatric patients who fail to respond to conventional therapy. The survival rate for neonates is 88%,9 and for pediatric patients it is 71%.10 Based on our extensive experience with neonates and children, we developed a standardized protocol for the management of severe ARDS in adults which included ECLS if patients failed to respond to our treatment algorithm. Early in our experience, contraindications included duration of mechanical ventilation >5 days, age >50 years, and severe sepsis. As our experience evolved, the age and ventilator days limits progressed to 70 years and 10 days, respectively. Our previous studies have demonstrated that duration of mechanical ventilation,23 age, and pre-ECLS PaO2/FiO2 ratio18 are independent predictors of ECLS outcome. In this study, we add to the multivariate model the pre-ECLS variables of gender and pH ≤7.10 as significant independent predictors of ELCS outcome. Systemic sepsis and septic shock is now an indication,24 not a contraindication, for ECLS and has a survival of 50% in the present study. Extending indications to older patients and longer pre-ECLS duration on mechanical ventilation are two reasons why the overall survival rate has not changed significantly with time.
Our algorithm for the management of severe ARDS is based on our understanding of the pathophysiology of inhomogeneous blood and gas distribution in the edematous lung as described by Gattinoni et al25,26 Prone positioning improves oxygenation because of the effects of shifting the weight of water in the lung. Proning improves A-aDO2 promptly but must be combined with fluid-balance management to return the patient to normal dry weight to improve survival.27,28 Ventilator settings must sustain adequate gas exchange but run the risk of causing ventilator-induced lung injury by high pressure, high stretch, or high FiO2.29,30 Continuous monitoring of mixed-venous oxygen saturation, cardiac output, and pulmonary artery pressure is essential for minute-to-minute titration of FiO2, PEEP, hemoglobin, and ventilator settings with the overall goal of maintaining systemic oxygen delivery at 5 times consumption. The other centers which report a large experience with ECLS for severe respiratory failure have evolved to the same overall management protocol.14–17,31 This management protocol is continued during ECLS, with the exception that ventilator settings and FiO2 can be turned down to resting levels. High-dose corticosteroids for unresolved ARDS as proposed by Meduri et al22 were used in cases of ECLS which showed no improvement in lung function after 7 days.
During ECLS, patients progress to a condition in which native lung function would not support survival. Oxygenation and CO2 removal is accomplished through the extracorporeal circuit; otherwise, these patients would die of hypoxia and hypercarbia. We determine this by measuring oxygen and CO2 exchange across the native lung and across the ECLS circuit. This is the basis for our conclusion that >80% of these patients would have died without extracorporeal support. Furthermore, the use of ECLS allows us to study the natural history of ARDS beyond the time when patients would have died with conventional treatment. The results demonstrate that native lung recovery can occur despite total loss of lung function for a period of days or weeks. Moreover, the inevitable fibrosis which accompanies lung injury and recovery resolves over a period of a year following the episode of severe ARDS. This type of life support comes at a physiologic price. The components of the ECLS circuit can and do fail, as shown in Table 5. Physiologic complications are also common and can negatively impact outcome. Some of these complications are related to the primary disease, and some, like bleeding, are related to ECLS therapy. Nonetheless, the likelihood of surviving an otherwise fatal condition justifies the risk of serious complications.
One-hundred seventy-one of 255 patients were successfully weaned off of ECLS. Thirty-four (20%) of the patients decannulated from ECLS failed to recover completely and died before hospital discharge. The primary cause of these late deaths following ECLS continues to be infectious issues combined with multiple system organ failure. Surprisingly, despite being on anticoagulation during ECLS, development of DVT and fatal pulmonary embolism has been a cause of late deaths following decannulation. We therefore screen for DVT 5 to 10 days after ECLS is discontinued and have a low threshold for placement of a prophylactic IVC filter in patients with a positive DVT scan.
With results of 50% to 70% survival in severe ARDS, why is the technique of ECLS not used more often in the treatment of ARDS? One reason is evidence. In 1975, we participated in an NIH-sponsored study of VA ECLS for ARDS. The technique of extracorporeal support and the technique of ventilator and patient management were totally different than current practice in that study. However, the fact that only 10% of patients survived in both groups led to the general impression that ECLS had been proven ineffective in ARDS.13 Modern experience uses very different technology and a different understanding of pathophysiology of ARDS, but the 1970s trial causes some to question the evidence. Morris et al32 conducted a prospective randomized trial of extracorporeal CO2 removal in a small group of patients. That technology is very different than full-support ECLS. Neonatal ECLS has been tested in 5 prospective randomized trials, all showing major benefit both in survival and in quality life years.8,33–35 There is currently a prospective randomized trial of adult ECLS under way in the United Kingdom based in Glenfield Hospital in Leicester (the Cesar Trial).36 The design of that trial is the same as the UK neonatal ECMO trial, which was a well-conceived trial design for a life-support system which cannot be studied in the blinded fashion.35
The conduct of a prospective randomized trial of ECLS therapy is a Herculean effort and must overcome many pitfalls that are not present in classic drug trials. The learning curve for ECLS therapy usually takes 10 to 20 patients. Management of the therapy must be reproducible before a meaningful trial can be conducted. Crossover is problematic because often the conventional and experimental arms are conducted at the same center. It is difficult to deny a patient ECLS who may be dying due to family, caregiver, and institutional perspectives/bias that are rooted more in emotion than scientific fact. Indeed, a narrow window of opportunity often exists shortly after a technique is developed and mastered but before it becomes well established when a randomized clinical trial can be mounted. Other areas of controversy and concern in the conduct of ECLS trials include informed consent, trial design, and end points.37
Another reason that ECLS is not more widely practiced is the perceived cost. However, ECLS is not particularly expensive relative to other high-technology types of care. It is less expensive than organ transplant, cancer chemotherapy, or a year of hemodialysis. The actual cost of ECLS is 10% more than the total cost of conventional management of severe ARDS, but the quality-of-life year survival has been shown to be cost-beneficial.38,39 The major reason why ECLS is not more widely practiced is that it is a complex technology which requires learning and practice in the laboratory and participation of many parts of the hospital. As the technology becomes simplified and less expensive and experience is reported, we expect ECLS to be as commonplace in the adult ICU as it is in the neonatal ICU. Presently, lung transplant centers and centers which do major cardiac surgery have ECLS programs to support the occasional patient with acute cardiac or pulmonary failure following transplantation or other complex operations.40,41
It is interesting to speculate why lung recovery and improved patient survival occur with ECLS support. Certainly gas exchange is one factor, but much more important is gas exchange achieved without the problems of high-pressure, high-oxygen mechanical ventilation. Data from experimental animal29,30 and clinical series5,42,43 show that ventilator-induced lung injury also causes renal failure, liver failure, cardiac failure, and other systemic consequences. One reason for this may be elaboration of inflammatory mediators from the damaged lung when high-pressure mechanical ventilation is continued.44–46 Moreover, high-pressure mechanical ventilation injures the most normal area of the remaining native lung, resulting in the downward spiral of typical of severe ARDS. By providing life support without reliance on mechanical ventilation for gas exchange, the native lung has time to recover. Release of inflammatory mediators from the damaged native lung may be significantly decreased with extracorporeal support rather than high-pressure mechanical ventilation. Although profound hypoxemia is the prominent indication for high-mortality risk respiratory failure, “rescue therapy” to sustain gas exchange is no longer the primary goal of extracorporeal support. With this concept in mind, it is important to point out that we consider ECLS to be a technique which sustains life during recovery from lung injury and multiple organ failure.
CONCLUSIONS
ECLS can maintain life in adult patients with severe ARDS, even in the absence of significant native lung function. This allows time for lung recruitment, direct treatment of the causes of ARDS, and management of multiple-organ failure. In our series of 255 adult patients with severe ARDS who required ECLS, functional lung recovery with decannulation from ECLS occurred in 67% of patients treated, and 52% of patients ultimately survived to hospital discharge. The use of ECLS in combination with a physiologic algorithm for the management of acute respiratory failure significantly decreases mortality in severe ARDS.
ACKNOWLEDGMENTS
The successful conduct of ELCS requires a team dedicated specialists, nurses, respiratory therapists, and surgical support staff, without which this treatment would not be possible. The authors thank all of the caregivers who have participated in the treatment of these patients throughout the course of this study. We would also like to thank Karla Ahrns for her assistance in preparing the IRB approval for this project.
Discussions
Dr. Thomas M. Krummel (Stanford, California): I would like to congratulate the authors on their superb results and thank Dr. Hemmila for a very thoughtful presentation.
It is worth emphasizing the state of the art 25 years ago. In 1979, a multicenter clinical trial suggested that ECLS was worthless in adult respiratory failure. At the same time, there was a single neonatal ECMO center in the world. Twenty-five years later there are thousands of kids that have survived as a result of ECMO support. And direct responsibility goes to Dr. Bartlett and his team for that extraordinary effort. For that work, Dr. Bartlett received the ACS Jacobson Innovator Award and the Medallion for Achievement from this Society.
Fast forward now. What is the story with adults? It would appear that the current work suggests that adult ECMO, or ECLS, works. The question is can that work be translated elsewhere and do the time-honored beliefs of the failure of adult ECLS need to be reevaluated? I would like to ask 4 questions.
The old dogma was that lung injury in the adult led to fibrosis and irreversible lung failure. Is there histologic evidence that this is wrong? Has something changed? Or have the less traumatic ventilatory strategies changed that previously inevitable outcome?
Secondly, in the manuscript cost is briefly discussed. Could you elaborate on the expense and the years of human life saved?
Third, all of us in the life support community recognize the critical role of an experienced team. What are your thoughts on team training? Is there a minimum number of cases that a team must have in order to produce these kind of results?
Finally, in the spirit of Dr. Debas's comments this morning, is it time for a randomized clinical trial for adult ECLS?
I would like to thank the Association for the privilege of the floor.
Dr. Mark Hemmila (Ann Arbor, Michigan): Thank you, Dr. Krummel. With regard to your first question concerning histology and fibrosis, it is interesting that in the more recent trials with ARDS and in animal experiments we are seeing release of cytokine mediators that can lead to multisystem organ failure. Perhaps this is why our patients with severe ARDS tend to fare poorly and it is not really just irreversible pulmonary fibrosis that leads to poor outcomes. Indeed, there is only a small subset of our ECLS patients who effectively die or have support withdrawn because of pulmonary fibrosis. There is a much larger group that has died due to ongoing multisystem organ failure.
Cost is an interesting aspect of ELCS treatment. If you look at the cost of performing ECLS it appears considerable, it is labor intensive, it requires a specialist, it uses expensive equipment, and requires one-on-one nursing care. However, most of our analyses of the costs attributed to ECLS have found that it really only amounts to a 10% increase in the patient's overall hospital bill. More importantly, If you look at the patients that we treat, almost all of these people are taxpayers who go back out into their communities, back to their jobs, and back to their families. It is hard to measure that benefit against not doing ECLS therapy.
Team training is important and is certainly the most essential element of what we have at the University of Michigan. Dr. Bartlett has assembled a world-class group of nurses, ECLS specialists, respiratory technicians, and physicians who care for these patients. On one of our slides you saw that it initially took about 4 to 5 years for the mortality rate to plateau. This represents about 30–45 cases, I would say. So I think it is unfair for a center to embark on a clinical trial until they have really completed the requisite learning curve amount of cases. Part of the learning curve is figuring out and gaining experience with how you are going to cannulate these patients. There is not just one standard way to run a patient on ELCS.
And last, with regard to a clinical trial, we are very encouraged by the fact that there is currently a prospective randomized adult ELCS trial being conducted in the United Kingdom, it is the CESAR Trial, which will compare conventional mechanical ventilation methods with ELCS for adults with severe ARDS. The enrollment criteria are very similar to the criteria that we have for using ELCS in adult patients. If you look at the Leicester group who is performing the ECLS therapy in this trial, their average PaO2 to FiO2 ratio is about 65, which is about 10 higher than our average PaO2 to FiO2 ratio. Their published survival in the first 50 adult patients, prior to this trial being started, was 66%.
Dr. Joseph M. Van de Water (Macon, Georgia): Congratulations, Dr. Hemmila, on a very nice presentation of an immense amount of work with Dr. Bartlett. My questions are:
One, is the problem simply one of oxygen delivery which ultimately determines survival? If so, have you measured a significant oxygen debt in the non-survivors?
Secondly, considering that there is peripheral shunting in the inflammatory state, can you really use mixed venous oxygen content to assess oxygen utilization and to guide your therapy? In other words, your value of 60% may not be correct.
Lastly, with your highly refined techniques of resting the lungs, you have a wonderful way to study the inflammatory mediators responsible for ARDS. Have you done so? If so, have you made any progress in controlling them?
Dr. Mark Hemmila (Ann Arbor, Michigan): In regards to your first question and the oxygen debt, no, we have not actually measured an oxygen debt in our adult ECLS patients. The patients probably do have a debt when they initially go on ELCS as they are usually profoundly hypoxic and tend to have a respiratory and sometimes a metabolic acidosis. These deficits are corrected rapidly over the first few hours of ECLS therapy. After that, the patients are basically no different than patients who are on cardiopulmonary bypass. They have an effective oxygen delivery that is adequate to meet their needs and there is no ongoing oxygen debt.
Measurement of the mixed venous oxygen saturation in the venous drainage blood guides our therapy, but is not the only measurement we use. In setting the parameters for management of our patients on ECLS, we strive to keep their arterial oxygen saturation greater than 90%. We also strive to keep the ratio of oxygen delivery to consumption around 5 to 1, and to do this we monitor the oxygenization of the blood returning to the circuit.
In some of our patients who were decannulated, they are having difficulty meeting their metabolic needs and they may indeed have significant transpulmonary shunting that does not allow them to fully recover. For instance, these are patients who are bleeding or have complications while on ECLS that require them to be taken off of ELCS regardless of their current respiratory and physiologic status.
To answer your last question, we have not yet measured the cytokine profile of our adult patients on ECLS. This would be interesting, but one of the difficulties is that cytokines tend to become bound up to the plastic in the circuit and are also cleared by the dialysis filter if one is present. However, it would be interesting to do so in the future.
Dr. Anthony A. Meyer (Chapel Hill, North Carolina): I would take this opportunity to commend Dr. Bartlett and his group for their leadership in improvement of critical care of surgical patients, which, whether it is not just applied there but applied all over the country, has contributed to the increasing advances, the improved outcomes, in patients, whether they be with transplant, cancer, trauma, cardiac, or thoracic problems. I have 3 brief questions.
First of all, in the intent to treat, were there any patients who met your criteria who were not enrolled in ECLS group?
Second, in the complications of ECLS, were there any patients taken off particularly early and were any of the deaths after being taken off ECLS due to those complications?
Finally, you describe return to normal lung function. I would like to know how that was measured, since it is very important since most other studies looking at patients with severe ARDS, at least in a notable subset, have prolonged restrictive lung disease.
Dr. Mark Hemmila (Ann Arbor, Michigan): We did have incidents in which we had to take patients off of ECLS in whom we would have preferred to leave them on. Yes, these patients were included in the decannulation group, because indeed they were decannulated. Some of these patients go on to survive and some do not. We prefer to decannulate all of our patients in a controlled fashion when they have adequate lung recovery. Unfortunately, some of our patients develop complications on ECLS that preclude us waiting any longer to take them off, so, for purposes of analysis we have to include these patients as being decannulated.
With regard to measuring the patient's lung function, we periodically obtain pulmonary function tests in our patients when they come back to the clinic in follow-up. What we find is they have a mild restrictive breathing pattern but no other real deficit. None of our patients has required oxygen therapy at home, and none have required permanent mechanical ventilation. Those are our outcome criteria. Do we have exact measurements of every patient's pulmonary function? No, we do not.
Dr. Frank R. Lewis (Philadelphia, Pennsylvania): I enjoyed your presentation very much and would certainly echo the compliments to Dr. Bartlett and his team.
In your presentation, you implied that the difference in mortality between venovenous and venoarterial bypass is due to the underlying physiology of the patient which required arterial pressure support. Is it possible that the differences in outcome are due to the method itself? The volume of pulmonary blood flow is markedly different with the two systems; venovenous pulmonary flow is normal and venoarterial is markedly reduced. If that were a factor, it might explain the negative results in the randomized study in the ’70s, since all of those patients were on venoarterial bypass.
My second question is, could you mention the severity of complications that developed from the cannulation and decannulation itself? Did you see significant amounts of vascular problems or compromising extremities?
Dr. Mark Hemmila (Ann Arbor, Michigan): We do have a significant amount of complications that occur in these adults treated with ECLS. I did not present a slide on them out of respect for time, but they are covered in the manuscript. The ones that we found that happen and are also independent predictors of outcome in multivariate analysis are: bleeding, cerebral infarction, need for renal replacement therapy, pulmonary embolism, and CPR on ELCS. Interestingly, none of the mechanical complications turned out to be a real factor in predicting mortality.
For patients who are on veno-arterial ELCS, it is a very complex situation. It seems like it should not be, but it turns out to be a real dilemma how you cannulate these patients. You have 2 basic anatomic setups for placing the cannulas. One, is to place both cannulas in the neck, with one cannula in the right common carotid artery and the other in the right internal jugular vein. This provides excellent flow of oxygenated blood to the brain and heart. But it comes at a cost of potentially causing a stroke. We have found that for men the stroke rate with right common carotid artery cannulation and ligation is 10–15% and for women it is around 15–20%. This would certainly add to the mortality and morbidity of patients treated with veno-arterial ECLS.
Another means of cannulating the patient for veno-arterial support is to put the cannulas in the groin vessels. Unfortunately, groin cannulation of the femoral artery oftentimes leads to distal lower extremity ischemia and the need for a reperfusion cannula to be placed. The other difficulty is that the oxygenated blood flow is retrograde in the aorta. You can still have deoxygenated blood leaving the heart and going to the coronary arteries and brain, which are the first major branches of the aorta. Because the retrograde arterial blood flow may never reach these vital organs, we sometimes include an additional return line in the ECLS circuit which sends oxygenated blood into the right heart to offset diminished pulmonary function when we are cannulated in the groin for veno-arterial support.
In concluding, I would like to thank Dr. Bartlett for being my mentor and friend.
Dr. Robert H. Bartlett (Ann Arbor, Michigan): Apropos of the comments by Dr. Debas and Dr. Jones this morning, I will just say a brief word about clinical research and evidence based trials. In this particular entity, acute potentially fatal organ failure in which the treatment you are evaluating is mechanical support of that organ, is very difficult to do randomized trials. The problems are logistical, ethical, financial and scientific. For example, there never has been, never will be a trial of dialysis for acute renal failure or a trial of intra-aortic balloon pumping for cardiac failure.
We are quite proud of the fact that there have been 6 prospective randomized trials in ECMO in different age groups and types of population. As you heard from Dr. Hemmila, the seventh is under way—difficult to do but necessary to do, as pointed out this morning. Thank you very much.
Footnotes
Reprints: Mark R. Hemmila, MD, Department of Surgery, University of Michigan Medical Center, 1C421 University Hospital, Box 0033, 1500 E. Medical Center Drive, Ann Arbor, MI 48109-0033. E-mail: mhemmila@umich.edu.
REFERENCES
- 1.Hemmila MR, Hirschl RB. Advances in ventilatory support of the pediatric surgical patient. Curr Opin Pediatr. 1999;11:241–248. [DOI] [PubMed] [Google Scholar]
- 2.Vasilyev S, Schaap RN, Mortensen JD. Hospital survival rates of patients with acute respiratory failure in modern respiratory intensive care units: an international, multicenter, prospective survey. Chest. 1995;107:1083–1088. [DOI] [PubMed] [Google Scholar]
- 3.Weg JG, Anzueto A, Balk RA, et al. The relation of pneumothorax and other air leaks to mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:341–346. [DOI] [PubMed] [Google Scholar]
- 4.Luhr OR, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. Am J Respir Crit Care Med. 1999;159:1849–1861. [DOI] [PubMed] [Google Scholar]
- 5.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 6.Esteban A, Inmaculada A, Gordo F, et al. Prospective randomized trial comparing pressure-controlled ventilation and volume-controlled ventilation in ARDS. Chest. 2000;117:1690–1696. [DOI] [PubMed] [Google Scholar]
- 7.Bersten AD, Edibam C, Hunt T, et al. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian states. Am J Respir Crit Care Med. 2002;165:443–448. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett RH, Roloff DW, Cornell RG, et al. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;4:479–487. [PubMed] [Google Scholar]
- 9.Bartlett RH, Roloff DW, Custer JR, et al. Extracorporeal life support: the University of Michigan experience. JAMA. 2000;283:904–908. [PubMed] [Google Scholar]
- 10.Swaniker F, Kolla S, Moler F, et al. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg. 2000;35:197–202. [DOI] [PubMed] [Google Scholar]
- 11.Kolovos NS, Bratton SL, Moler FW, et al. Outcome of pediatric patients treated with extracorporeal life support after cardiac surgery. Ann Thorac Surg. 2003;76:1435–1442. [DOI] [PubMed] [Google Scholar]
- 12.Pagani F, Aaronson KD, Swaniker F, et al. The use of extracorporeal life support in adult patients with primary cardiac failure as a bridge to implantable left ventricular assist device. Ann Thorac Surg. 2001;71:S77–81. [DOI] [PubMed] [Google Scholar]
- 13.Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. JAMA. 1979;242:2193–2196. [DOI] [PubMed] [Google Scholar]
- 14.Peek GJ, Moore HM, Sosnowski AW, et al. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112:759–764. [DOI] [PubMed] [Google Scholar]
- 15.Lewandowski K, Roissant R, Pappert R, et al. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med. 1997;23:819–835. [DOI] [PubMed] [Google Scholar]
- 16.Ullrich R, Lorber C, Roder G, et al. Controlled airway pressure therapy, nitric oxide inhalation, prone position, and extracorporeal membrane oxygenation (ECMO) as components of an integrated approach to ARDS. Anesthesiology. 1999;91:1577–1586. [DOI] [PubMed] [Google Scholar]
- 17.Frenckner B, Palmer P, Linden V. Extracorporeal respiratory support and minimally invasive ventilation in severe ARDS. Minerva Anesthesiol. 2002;68:381–386. [PubMed] [Google Scholar]
- 18.Kolla S, Awad SS, Rich PB, et al. Extracorporeal support for 100 adult patients with severe respiratory failure. Ann Surg. 1997;226:544–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich PB, Awad SS, Kolla S, et al. An approach to the treatment of severe adult respiratory failure. J Crit Care. 1998;13:26–36. [DOI] [PubMed] [Google Scholar]
- 20.Bartlett RH, Morris AH, Fairley HB, et al. A prospective study of acute hypoxic respiratory failure. Chest. 1986;89:684–689. [DOI] [PubMed] [Google Scholar]
- 21.Rich PB, Awad SS, Crotti S, et al. A prospective comparison of atrio-femoral and femoro-atrial flow in adult venovenous extracorporeal life support. J Thorac Cardiovasc Surg. 1998;116:628–632. [DOI] [PubMed] [Google Scholar]
- 22.Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159–165. [DOI] [PubMed] [Google Scholar]
- 23.Pranikoff T, Hirschl RB, Steimle CN, et al. Mortality is directly related to the duration of mechanical ventilation before the initiation of extracorporeal life support for severe respiratory failure. Crit Care Med. 1997;25:28–32. [DOI] [PubMed] [Google Scholar]
- 24.Rich PB, Younger JG, Soldes OS, et al. Use of extracorporeal life support for adult patients with respiratory failure and sepsis. ASAIO J. 1998;44:263–266. [DOI] [PubMed] [Google Scholar]
- 25.Gattinoni L, Pelosi P, Vitale G, et al. Body position changes redistribute lung computed tomographic density in patients with acute respiratory failure. Anesthesiology. 1991;74:15–29. [DOI] [PubMed] [Google Scholar]
- 26.Gattinoni L, D'Andrea L, Pelosi P, et al. Regional effects and mechanism of positive end-expiratory pressure in early adult respiratory distress syndrome. JAMA. 1993;269:2122–2127. [PubMed] [Google Scholar]
- 27.Simmons RS, Berdine GG, Seidenfeld JJ, et al. Fluid balance in the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135:924–929. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg AL, Deckert RE, Bartlett RH. Outcomes associated with changes in fluid balance among patients with severe acute lung injury. Crit Care Med. 2004 (Submitted).
- 29.Kolobow T, Moretti MP, Fumagalli R. Severe impairment of lung function induced by high peak airway pressure during mechanical ventilation: an experimental study. Am Rev Respir Dis. 1987;135:312–315. [DOI] [PubMed] [Google Scholar]
- 30.Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator induced lung injury. Crit Care Med. 1993;21:131–143. [DOI] [PubMed] [Google Scholar]
- 31.Michaels AJ, Wanek SM, Dreifuss BA, et al. A protocolized approach to pulmonary failure and the role of intermittent prone positioning. J Trauma. 2002;52:1037–1047. [DOI] [PubMed] [Google Scholar]
- 32.Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure- controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305. [DOI] [PubMed] [Google Scholar]
- 33.O'Rourke PP, Crone RK, Vacanti JP, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989;84:957–963. [PubMed] [Google Scholar]
- 34.Schumacher RE, Palmer TW, Roloff DW, et al. Follow-up of infants treated with extracorporeal membrane oxygenation for newborn respiratory failure. Pediatrics. 1991;87:451–457. [PubMed] [Google Scholar]
- 35.UK Collaborative ECMO Trial Group. UK collaborative randomized trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996;348:75–82. [PubMed] [Google Scholar]
- 36.Conventional ventilation or ECMO for severe adult respiratory failure. Available at: http://www.cesar-trial.org/. Accessed April 12, 2004.
- 37.Firmin RK, Elbourne. Extracorporeal life support: how can its effectiveness be studied? In: Zwischenberger JB, Steinhorn RH, Bartlett RH, eds. ECMO Extracorporeal Cardiopulmonary Support in Critical Care. Ann Arbor: ELSO; 2000:633–644. [Google Scholar]
- 38.Schumacher RE, Roloff DW, Chapman R, et al. Extracorporeal membrane oxygenation in term newborns: a prospective cost-benefit analysis. ASAIO J. 1993;39:873–879. [PubMed] [Google Scholar]
- 39.Bartlett RH, Schumacher RE, Chapman RA. Economics of extracorporeal life support. In: Zwischenberger JB, Steinhorn RH, Bartlett RH, eds. ECMO Extracorporeal Cardiopulmonary Support in Critical Care. Ann Arbor: ELSO; 2000:659–675. [Google Scholar]
- 40.Macha M, Griffith B, Kennan R. ECMO support for adult patients with acute respiratory failure. ASAIO J. 1996;42:M841–844. [DOI] [PubMed] [Google Scholar]
- 41.Smedira NG, Moazami N, Golding CM, et al. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg. 2001;122:92–102. [DOI] [PubMed] [Google Scholar]
- 42.Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16:372–377. [DOI] [PubMed] [Google Scholar]
- 43.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1988;338:347–354. [DOI] [PubMed] [Google Scholar]
- 44.Tremblay L, Valenza F, Ribeiro SP. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slutsky AS, Tremblay LN. Multiple system organ failure: is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–1725. [DOI] [PubMed] [Google Scholar]
- 46.Rich PB, Douillet CD, Hurd H, et al. Effect of ventilatory rate on airway cytokine levels and lung injury. J Surg Res. 2003;113:139–145. [DOI] [PubMed] [Google Scholar]