Abstract
Introduction:
We hypothesized botulinum toxin (BT) infiltration of the chest wall musculature after mastectomy would create a prolonged inhibition of muscle spasm and postoperative pain, facilitating tissue expander reconstruction.
Methods:
An Institutional Review Board (IRB)-approved prospective study was conducted of all patients undergoing mastectomy with tissue expander placement during a 2-year period. Study patients versus controls had 100 units of diluted BT injected into the pectoralis major, serratus anterior, and rectus abdominis insertion. Pain was scored using a visual analog scale of 0 to 10. Wilcoxon rank sum test was used for continuous variables and the χ2 test for nominal level data to test for significance.
Results:
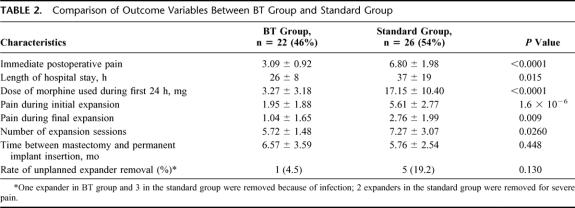
Forty-eight patients were entered into the study; 22 (46%) with and 26 (54%) without BT infiltration. Groups were comparable in terms of age (55 ± 11 years versus 52 ± 10 years; P = 0.46), bilateral procedure (59% versus 61%; P = 0.86), tumor size (2 ± 2 cm versus 2 ± 3 cm; P = 0.4), expander size and volume (429 ± 119 mL versus 510 ± 138 mL; P = 0.5). The BT group did significantly better with pain postoperatively (score of 3 ± 1 versus 7 ± 2; P < 0.0001), during initial (score of 2 ± 2 versus 6 ± 3; P = 1.6 × 10−6), and final expansion (1 ± 1 versus 3 ± 2; P = 0.009). Volume of expansion per session was greater thus expansion sessions required less in the BT group (5 ± 1 versus 7 ± 3; P = 0.025). There was a significant increase in narcotic use in control patients in the first 24 hours (17 ± 10 mg versus 3 ± 3 mg; P < 0.0001), initial as well as final expansion periods (P = 0.0123 and 0.0367, respectively). One expander in the BT group versus 5 in the control group required removal (P = 0.13). There were no BT-related complications.
Conclusion:
Muscular infiltration of botulinum toxin for mastectomy and tissue expander placement significantly reduced postoperative pain and discomfort without complications.
Intramuscular infiltration of botulinum toxin significantly reduces pain and hospital stay after mastectomy and immediate expander reconstruction.
Immediate breast reconstruction with subpectoral tissue expansion is a well-established technique that offers satisfactory cosmetic results following mastectomy for cancer. However, tissue expansion is associated with significant pain and discomfort not only in the immediate postoperative period but also during the subsequent saline expansion phase.1 Long-term arm and shoulder pain is reported to occur in 50% of patients at least 1 year out from surgery, with subpectoral implants.2 Severe pain during subpectoral expansion can result in inadequate expansion3 or surgical removal of expanders, abandoning reconstruction.4
Adjuvant radiation therapy,3 types of expanders,5 technique of insertion,6 and site of injection ports7 have been implicated in the etiology of pain following reconstruction with tissue expansion. Only recently, the spasm of pectoralis muscle has been alluded to as a cause of pain and discomfort after subpectoral tissue expansion.8–10 There have been anecdotal case reports of botulinum toxin (BT) use to paralyze the muscles for pain relief.8–10 We hypothesized that routine infiltration of pectoralis major, serratus anterior, and upper rectus abdominis muscles at the time of mastectomy and immediate reconstruction with a subpectoral tissue expander would improve postoperative pain and control spasm during tissue expansion.
METHODS
Study Design
An IRB-approved retrospective study was conducted of all patients undergoing mastectomy with tissue expander placement during a 2-year period. Study patients were nonrandomly (based on surgeon preference to use BT) selected and were subjected to infiltration of 100 units of BT (serotype A) diluted in 40 to 60 mL of normal saline, into the pectoralis major, serratus anterior, and rectus abdominis insertion. Hundred units of BT type A is a standard dose used for strabismus, glabellar injections, and dystonia. Since the effective dose depends on muscle mass and individual susceptibility, 100 U for chest wall muscles is a prudent choice, considering a much larger muscle mass. BT was ordered preoperatively with a tentative time of surgery; the freeze-dried toxin was reconstituted with normal saline in a 60-mL syringe at the start of surgery and refrigerated at 4°C till the end of mastectomy. A 20-gauge spinal needle was used after transferring the reconstituted BT to another syringe on the field, for uniform infiltration throughout the muscles (Figure 1); caution was exercised to avoid entry into the pleural space. Pregnant patients, patients with hepatorenal failure, or those with a history of hypersensitivity reactions were not subjected to BT infiltration. Patients receiving standard mastectomy with immediate insertion of tissue expanders served as controls. PMT Integra Tissue Expanders (PMT Corp., Chanhassen, MN) were used in all patients because of their specific pressure mechanics, which allow differentially more anterior expansion with intraluminal increase in pressure, effecting better projection and less chest-wall concavity. Pain was scored using a visual analog scale of 0 to 10. Postoperative analgesic requirements were recorded in the nursing notes.
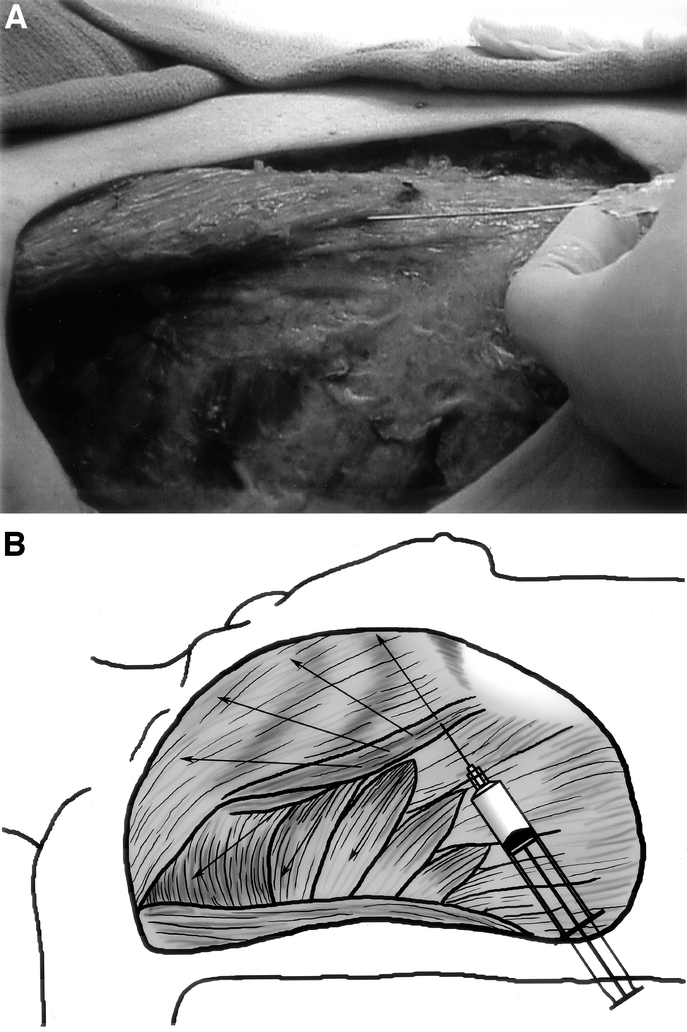
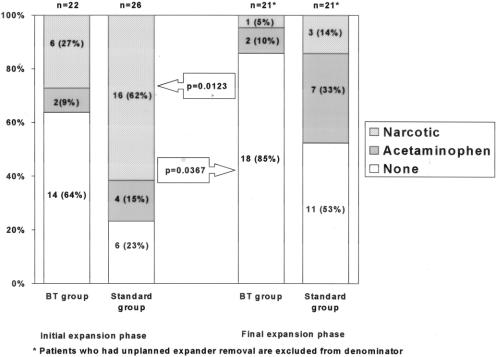
FIGURE 1. Comparison of trend of analgesic use between BT group and standard group during initial and final phases of expansion. BT group used significantly less analgesics in both the initial (BT versus standard, P = 0.01) and final (BT versus standard, P = 0.04).
Outcome measures included immediate postoperative pain, length of hospital stay, type and dose of narcotic used, pain during initial expansion, pain during final expansion, percentage of target volume achieved, and rate of unplanned expander removal due to pain.
Statistics
Data were recorded in predesigned forms and entered in spreadsheet using Microsoft Excel software. Wilcoxon rank sum test was used for comparison of continuous variables and the χ2 test for comparison of nominal level data to test for significance.
RESULTS
A total of 48 patients underwent complete reconstruction with permanent implant placement postsubpectoral tissue expansion following mastectomy and immediate insertion of tissue expander between July 2001 and November 2003. Twenty-two (46%) were subjected to BT infiltration; 26 (54%) had standard mastectomy with immediate insertion of tissue expander. Comparison of matching variables is shown in Table 1. Both groups were comparable in terms of age (55 ± 11 years versus. 52 ± 10 years; P = 0.46), bilateral procedure (59% versus 61%; P = 0.86), tumor size (2 ± 2 cm versus 2 ± 3 cm; P = 0.4), expander size (450 ± 100 mL versus 450 ± 130 mL; P = 0.72) and volume (429 ± 119 mL versus 510 ± 138 mL; P = 0.5).
TABLE 1. Comparison of Matching Variables Between BT Group and Standard Group
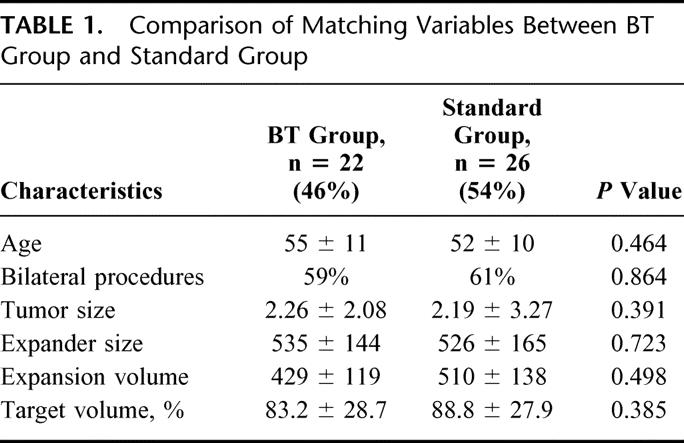
The comparison of outcome variables is shown in Table 2. BT group did significantly better with postoperative pain (score of 3 ± 1 versus 7 ± 2; P < 0.0001), pain during initial expansion (score of 2 ± 2 versus 6 ± 3; P = 1.6 × 10−6), and pain during final expansion (1 ± 1 versus 3 ± 2; P = 0.009). Volume of expansion per session was greater (90 mL versus 70 mL; P = 0.010); thus, the number of expansion sessions required in the BT group was less than the standard group (5 ± 1 versus 7 ± 3; P = 0.025). There was a significant increase in narcotic use among the standard group in the first 24 hours (17 ± 10 mg versus 3 ± 3 mg of morphine; P < 0.0001). The mean length of hospital stay was 26 ± 8 hours in BT group versus 37 ± 19 hours in standard group (P = 0.015). Since the doses of analgesics taken by patients at home were not meticulously documented, trend of using no analgesia versus acetaminophen or narcotics was analyzed for initial and final expansion periods. The increased trend towards narcotic use in patients undergoing standard operation without BT infiltration is depicted in Figure 2. Narcotic use was significantly less common in BT group during initial (P = 0.012), as well as final expansion periods (P = 0.037). One expander in the BT group versus 5 in the standard group required removal (P = 0.130). The reason for unplanned expander removal was infection in 1 BT group patient and 3 standard group patients; 2 expanders in the standard group were removed because of intractable pain. There were no complications seen with BT infiltration.
TABLE 2. Comparison of Outcome Variables Between BT Group and Standard Group
FIGURE 2. Narcotic and analgesic use in the initial and final expansion phase in the BT Vs standard group. Narcotic and analgesic use was significantly less in the BT group in both the initial and final expansion phases with a majority of patients in the BT group not requiring pain relievers (0.0123 and 0.0367, respectively).
DISCUSSION
The relevance of pectoral muscle tone with subpectoral implant insertion was first described by Hoffman and Elliot in 1987.11 They discussed that the loss of muscle tone that resulted from surgical denervation during modified radical mastectomy was advantageous during subpectoral reconstruction because the reconstructed breast projected better through a paralyzed muscle. However, the contrary concept of pectoral spasm being problematic when the muscle is not denervated was only recently suggested as a cause of pain, discomfort, and high riding of implants.8,12 Case reports of severe pectoral spasms posing challenging management issues in various surgical patients have occurred.9,10,13 The first reported case was that of severe pectoralis major spasm following sternal wound reconstruction with bilateral pectoralis major flaps based on thoracoacromial vessels.13 Bilateral neurectomies resulted in complete resolution of pain. Subsequently, 3 cases were reported where incapacitating pectoral spasm resulted from subpectoral implant placement.9,10,14 BT was used in all 3 cases, with variable success in pain management for these patients. Recently, the structural changes due to hypoxia in the pectoralis major muscle have been identified associated with expansion, and their role in the etiology of pain has been implicated.15 Focal muscle fiber degeneration with glycogen deposits and interstitial fibrosis has been seen to occur, which is attributed to muscle hypoxia resulting from spasms. Some authors have theorized that intraluminal lidocaine used in expanders slowly diffuses out of the expander and anesthetizes the muscle to effect pain relief.16,17 This concept was recently applied in the clinical setting by Pacik et al18 with use of indwelling catheters around the implant for lidocaine infiltration, reporting successful pain control in patients undergoing augmentation mammoplasty. This approach, however, requires continuous infusion of lidocaine in the postoperative period through the external catheter ports, which may risk infections, although none were reported in this series.
BT, the causative agent of botulism, a descending muscle paralysis resulting from food poisoning, was identified by Van Ermengen in 1987.19 Understanding of the pathophysiological mechanism of action of BT20 laid the foundation for its development as a therapeutic tool. The first clinical application of BT was described by Scott et al21 in treating strabismus. Subsequently, of the 8 serotypes of BT (A, B, C1, C2, and D-G), type A has found wide applications as a neuromuscular blocker in neurology, gastroenterology, ophthalmology, and orthopedics.22–24 The underlying mechanism of action involves the blockade of presynaptic acetylcholine release by binding to specific cell-surface receptors (Fig. 3). 25 The toxin receptor complex is internalized by endocytosis into nerve terminals, where it cleaves the essential protein involved in exocytosis of acetylcholine.19 The activated proteins interpose between the acetylcholine vesicle and the axon cell membrane, thereby inhibiting exocytosis, resulting in the depletion of acetylcholine in the synaptic cleft. Muscle fibers become functionally denervated, resulting in flaccid paralysis. The onset of action is between 6 and 36 hours after exposure, with maximal effect up to 7 to 14 days. The nonfunctional neuromuscular junctions are eventually replaced by new axonal sprouts, and muscle function is restored in 3 to 6 months.21 These pharmacodynamics work in the favor of easier expansion; typical expansion starts within a couple weeks of surgery when BT is at maximal effect and is completed by the time the new neuromuscular junctions regenerate. Moreover, the achievement of target volume during this phase of BT in effect allows esthetically better projection of expander and subsequent permanent implant. However, the clinical dose standardization is based on the calculation in units, 1 U being the lethal dose of toxin causing death in 50% of a group of 18- to 20-g female Swiss-Webster mice. The lethal dose of toxin causing death in 50% of humans has been estimated to be between 2800 and 3500 U. Clinically effective dose is dictated by the muscle mass and individual susceptibility.24 We used a dose 100 U for chest wall muscles as a cautious approach because it is a clinically effective dose for very small ocular muscles. Therefore, a much higher dose for the larger muscles like pectoralis major can probably be safely used; but considering the cost constraints and the fact that higher doses may be associated with increased risk of neutralizing antibody formation,24 we selected the strategy of lowest effective dose. It is not surprising that we observed significant pain control but did not encounter complete flaccid paralysis or weakness in the arm. As the clinical use of BT is expanding, the question of major nerve damage resulting from direct infiltration has been addressed. Lu et al26 prospectively evaluated the nerve damage associated with intra and extrafascicular injections and reported that even direct intraneural injection of BT does not cause any nerve damage.

FIGURE 3. Mechanism of action of botulinum toxin at the cholinergic nerve terminal. The toxin irreversibly binds to the high-affinity receptors and is endocytosed; after lysis, it activates specific proteins that inhibit fusion of acetylcholine (ACh) vesicles with the cell membrane. Deficiency of Ach in the synaptic cleft causes flaccid muscle paralysis.
We hypothesized that BT infiltration of chest wall musculature at the time mastectomy and insertion of subpectoral tissue expander would partially paralyze the muscles and reduce the postoperative pain caused by muscle spasm, allowing for easier and comfortable tissue expansion. Since good pain control is likely to achieve quicker expansion and insertion of permanent implant, we expected a shorter interval between mastectomy and permanent implant placement in the BT group, considering lesser number of sessions required to achieve the target volume; it was not the case in the current study. This resulted from patient's preference of final operation to be held at a convenient time despite completion of expansion. Pusic and Cordiero27 have reported on rapid intraoperative expansion of tissue expander which is an attractive technique; however, the use of narcotics and level of pain are not reported. Also, it is intuitively appropriate to expect that temporary paralysis of muscles may interrupt the cycle of spasm-hypoxia-pain-spasm and possible subsequent muscle damage.16 We used BT type A (the most widely used BT) marketed as Botox (Allergan, Irvine, CA), in this study. Significant reduction in pain was observed in the current study not only in the immediate postoperative phase but throughout tissue expansion (Table 2 and Fig. 2). The current study did not randomize patients to BT and standard groups because the use of toxin was initiated by a single surgeon to assess feasibility; however, the results were so dramatically different that we felt it would be unethical to withhold BT from control patients.
Other methods of reconstruction such as transverse rectus abdominis myocutaneous flaps have the advantage of single operation and excellent cosmetic outcome but a higher complication rate. Expander reconstruction may be associated with problems of thin skin envelope, high-riding implants, and requiring a second surgery, but it is technically much simpler and is a preferred method by 70% of plastic surgeons in the country. The results of this study can be potentially beneficial for most surgeons employing this method.
In conclusion, the current study demonstrates that intramuscular infiltration of BT type A is safe and significantly reduces postoperative spasm, pain, and length of stay; because of a longer half-life, it does not require indwelling catheters for continuous infusion, and most patients do well with a single intraoperative injection.
Discussions
Dr. Luis O. Vasconez (Birmingham, Alabama): Breast reconstruction with expanders is a straightforward procedure, but it is time consuming, painful, and subject to complications. Botox, a most powerful neurotoxin, has received considerable notoriety in ameliorating facial wrinkles. Of interest to general surgeons, I should indicate that Botox has effectively replaced sphincterotomy for chronic anal fissures.
The authors’ use of Botox to block the muscle action of the pectoralis major, the serratus, and the upper portion of the rectus abdominis muscle at the time of immediate reconstruction with an expander is innovative and logical. Their prospective and controlled study is well designed, and the data is accurately obtained. The results confirmed the authors’ hypothesis which includes: a decrease of pain during expansion, a decrease in the time to complete the expansion, as well as a decrease in the hospitalization.
I do have some concerns and some questions which I would like the authors to address. First, the authors indicate that the patients were stratified between treatment and control groups based on surgeon's preference. While many data different between the 2 groups and are statistically significant, could it be impacted by surgeon specific surgical techniques or pain management preferences?
The dosage of Botox is relatively small, 100 units, which is not unusual for us to use in paralyzing the facial musculature. Have the authors used a larger dose to obtain “more lasting flaccidity to the muscle”?
Concerning pain—there was a noted improvement as early as the first day. Although the authors indicated that Botox begins to work within 6 hours after administration, maximum effect is between 7 to 14 days. Would motor function examination or electromyographic studies demonstrate an early onset consistent with their clinical findings?
The decrease of hospitalization from 36 to 26 hours could have been impacted by the improvement in pain control, but other factors may have been involved. If indeed Botox is taking effect after 24 hours, how would it affect hospital length of stay?
The authors were able to deliver larger volumes with each session of expansion and it took a cumulative expansion rate of 16 weeks in the treatment group and 21 weeks in the control group. This finding should be balanced by the utilization of rapid expansion protocols for breast reconstruction which are well documented in the literature. In those protocols, expansion with an average of 4.7 office visits over a six-week period permitted inflation of a mean volume of 678 cc with a complication rate of 4%. Unfortunately, pain was not mentioned in those studies.
A problem which I have found with subpectoral tissue expansion is the concomitant concavity of the chest wall due to the forces of expansion which are exerted in every direction. I wondered if the authors have noted any decrease of this concavity by the relaxation of the overlying muscle? This would be a most important finding.
It was clear to me that the complications of breast reconstruction with expanders were not abolished and remained at a relatively high rate, particularly in the control group where at least five expanders had to be removed.
At our institution, most of our breast reconstructions are performed with autologous tissue, specifically the TRAM flap. In cases where implants are used, we transpose the latissimus dorsi myocutaneous flap, which provides us with additional muscle coverage, particularly for the lower portion of the implant, which is expanded to the desired maximum volume at the same operation. Nonetheless, the authors’ innovative use of Botox to paralyze the overlying muscle appears to be a very worthwhile concept, and I, for one, will try to study it in our group of patients where the latissimus dorsi has been transposed.
Thank you for the opportunity to discuss the paper.
Dr. Rakhshanda Layeeque (Little Rock, Arkansas): I would like to thank Dr. Kelly and the Association for giving me the privilege of closing on our paper. I also thank Dr. Vasconez for reviewing our manuscript and bringing out some very important points.
The first question was about randomization; we understand that the Gold standard to prove the efficacy of any new therapeutic strategy is to perform a randomized control trial. This study was not randomized because a single surgeon started using Botox to assess the feasibility of the technique; the results of these initial patients were exceptional and about the same time we had to remove some expanders from standard patients because of severe pain. With our initial results, we felt it would be unethical to withhold this strategy from other patients; over time this approach became generalized in our department. Standard patients in the study are therefore concurrent patients treated without Botox.
However, we do believe that the differences in pain control and hospital stay are attributable to Botox and not the differences in surgical postoperative management, for 2 reasons—the reconstructive part of surgery, ie, raising muscle flaps and subpectoral implant insertion was performed by a single plastic surgeon, and the postoperative pain management is achieved by a standard patient controlled analgesia protocol which is uniform across entire division.
Admittedly, the dose of 100 units is very small. We started with that dose because it is the standard dose for much smaller facial musculature giving us a reasonable safety margin since the paralytic dose is a function of muscle bulk and we did not want to paralyze these muscles; the objective was to dampen the hyper-reactivity and prevent painful spasm. The cost is going to be the main limiting factor for increasing the dose; it costs $800 per vial to the hospital and the patient is charged $2,500 per vial containing 100 units.
The data about onset of action at 6–36 hours has been reported from pharmaco-dynamic studies after exposure to clostridium botulinum. Since therapeutic botulism is achieved through pre-freeze-dried toxin, the onset is actually instantaneous; we have noticed the immediate attenuation of muscle reactivity to the electro-cautry. On the other occasions, when Botox has been used in the office setting, patients report immediate pain relief. It is the immediate effect that is reflected in our study by significantly shorter length of stay considering that the rest of the postoperative care was same except diazepam which was used to control spasm in the standard group.
The accelerated expansion reported by the Sloan-Kettering group is certainly a very efficient way of subpectoral expansion; however the authors have not reported on the postoperative pain and use of narcotics in these patients. We believe that Botox may actually be advantageous in accelerated expansion technique. We do not perform accelerated expansion because of severe inflammation that may set in and cause long-term contractures.
We did not observe the concavity of the chest wall after tissue expansion in either group, although we did not look at this complication as an outcome measure. This may be because we use PMT expanders which are specifically designed to exert expansion forces anteriorly to effect better projection. McGhan expanders have centrifugally uniform pressure forces that may cause concavity of the chest wall.
Lastly, TRAM flaps certainly have an excellent cosmetic outcome, but they are technically demanding, have a higher complication rate and require longer hospital stay. That is the reason why 70% of surgeons across the nation choose subpectoral implants rather than TRAM flaps.
Dr. David W. Easter (San Diego, California): An interesting and potentially provocative study. Can you help clarify some of my concerns? Did you have any real controls? Meaning, did you inject saline instead of Botox in that control arm and hence control for just the saline injection? Second, to control for observer bias. I would like to believe there was no muscle twitching, but did you have any objective measurements of either muscle twitch before or after or muscle strength after? And the third was patient controls, power of suggestion. Were the patients aware of which type of treatment they got?
Dr. Rakhshanda Layeeque (Little Rock, Arkansas): Regarding the use of placebo or saline expansion for comparison: since it was not a randomized trial, the standard group did not receive any placebo infiltration. The patients were not blinded because Botox infiltration was under consent; however the nurses managing the postoperative pain and charting scores were blinded to the use of Botox.
Attenuation of muscle twitching was an observation; we did not perform any electro-myographic studies as we were not using a paralytic dose. We have not observed any muscle dysfunction in these patients during the follow-up office visits. In fact, we have observed anecdotally, that patients have a better range of motion at the shoulder compared with standard post-mastectomy patients.
The point of permanent damage with Botox needs to be clarified; the toxin binds irreversibly to the axon terminal rendering the neuromuscular junction non-functional. There is no nerve damage as shown by electron microscopic studies on animals. New axonal sprouts regenerate from the main axon and new neuromuscular junctions are formed by 6 months restoring the function of a given neuromuscular unit. A very large dose would be needed to inhibit every neuromuscular unit in the Pectoralis muscle and cause flaccid paralysis.
Dr. Keith A. Kelly (Scottsdale, Arizona): Was it the Botox that caused damage to the muscle of the chest wall after operation? As you followed your patients through the weeks and months after operation, did you note any disability with arm motion or other muscular disabilities that you wouldn't find in a mastectomy patient who had not had Botox? It seems likely some mastectomy patients would get such complications without having had Botox. Thus you need to control for this.
Dr. Rakhshanda Layeeque (Little Rock, Arkansas): As I mentioned, we haven't seen any clinically significantly different range of motion. In fact the range of motion may be slightly better because of the lack of contracture and pain that they have in the pectoral spasm situations. But motor dysfunction we have not observed. But as I mentioned, we haven't done any objective study to look at each group of muscles per se. So that we haven't seen yet.
In regards to the question about permanent damage, yes, Botox is an irreversible toxin which will, the neuromuscular junction to which it is attached on the first part, that is a permanent damage. But the adrenals try to regenerate. And that is how it is with those related responses. And the pectoralis major muscle, since it is much larger and the dose we are using is much smaller, that is, I think, the explanation of why we do not see significant motor dysfunction.
Footnotes
Rakhshanda Layeeque and Julie Kepple are supported by the Susan G. Komen Breast Cancer Clinical Fellowship and the Virginia Clinton Kelley/Fashion Footwear Association of New York Breast Cancer Research Fellowship.
Reprints: V. Suzanne Klimberg, MD, Department of Surgery, Division of Breast Surgical Oncology, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 725, Little Rock, AR 72205. E-mail: klimbergsuzanne@uams.edu.
REFERENCES
- 1.Rimareix F, Masson J, Couturaud B, et al. Breast reconstruction by inflatable anatomical implant: retrospective study of 65 cases. Ann Chirurgie Plastique Esthétique. 1999;44:239–245. [PubMed] [Google Scholar]
- 2.Wallace MS, Wallace AM, Lee J, et al. Pain after breast surgery: a survey of 282 women. Pain. 1996;66:195–205. [DOI] [PubMed] [Google Scholar]
- 3.Fodor J, Gulyas G, Polgar C, et al. Radiotherapy and delayed breast reconstruction with implant: examination of compatibility. Magyar Oncologia. 2002;46:323–326. [PubMed] [Google Scholar]
- 4.Disa JJ, Ad-El DD, Cohen SM, et al. The premature removal of tissue expanders in breast reconstruction. Plast Reconstr Surg. 1999;104:1662–1665. [DOI] [PubMed] [Google Scholar]
- 5.May JW Jr, Bucky LP, Sohoni S, et al. Smooth versus textured expander implants: a double-blind study of capsule quality and discomfort in simultaneous bilateral breast reconstruction patients. Ann Plast Surg. 1994;32:225–232. [DOI] [PubMed] [Google Scholar]
- 6.Huang TT. Breast and subscapular pain following submuscular placement of breast prostheses. Plast Reconstr Surg. 1990;86:275–280. [DOI] [PubMed] [Google Scholar]
- 7.Sarker SK, Faliakou EC, Gui GP. Surgical technique: submuscular placement of breast implant ports with delayed expansion following ultrasound localization. Eur J Surg Oncol. 2001;27:212–213. [DOI] [PubMed] [Google Scholar]
- 8.Hoefflin SM. Botox alternatives. Plast Reconstr Surg. 1998;101:865. [DOI] [PubMed] [Google Scholar]
- 9.Richards A, Ritz M, Donahoe S, et al. Botox for contraction of pectoral muscles. Plast Reconstr Surg. 2001;108:270–271. [DOI] [PubMed] [Google Scholar]
- 10.Senior M. Botox and the management of pectoral spasm after subpectoral implant insertion. Plast Reconstr Surg. 2000;106:224. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman GW, Elliot LF. The anatomy of the pectoral nerves and its significance to the general and plastic surgeon. Ann Surg. 1987;205:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edney JA. Breast cancer-treatment for the future based on lessons from the past. Am J Surg. 2002;184:477–483. [DOI] [PubMed] [Google Scholar]
- 13.Mast BA. Painful pectoralis major myospasm as a result of sternal wound reconstruction: complete resolution with bilateral pectoral neurectomies. Plast Reconstr Surg. 1999;104:798–800. [DOI] [PubMed] [Google Scholar]
- 14.Wong L. Pectoralis major myospasm resulting from a subpectoral implant. Plast Reconstr Surg. 2000;105:1571. [DOI] [PubMed] [Google Scholar]
- 15.Gur E, Hanna W, Andrighetti L, et al. Light and electron microscopic evaluation of the pectoralis major muscle following tissue expansion for breast reconstruction. Plast Reconstr Surg. 1998;102:1046–1051. [DOI] [PubMed] [Google Scholar]
- 16.Berman DE, Lettieri J, Herold DA, et al. Lidocaine diffusion in five tissue expanders: an in-vitro and in-vivo study. Ann Plast Surg. 1991;27:312–315. [DOI] [PubMed] [Google Scholar]
- 17.Durby LD, Sinow JD, Bowers LD, et al. Quantitative analysis of lidocaine hydrochloride delivery by diffusion across tissue expander membranes. Plast Reconstr Surg. 1992;89:900–907. [PubMed] [Google Scholar]
- 18.Pacik PT, Werner C, Jackson N, et al. Pain control in augmentation mammoplasty: the use of indwelling catheters in 200 consecutive patients. Plast Reconstr Surg. 2003;111:2090–2096. [DOI] [PubMed] [Google Scholar]
- 19.Van Ermengen E. Uber einen neuen anaeroben Bacillus und seine Beziehungen zum Botulismus. Z Hyg Infektionskrankh. 1897;26:1–56. [PubMed] [Google Scholar]
- 20.Burgen ASV, Dickens F, Zatman LJ. The action of botulinum toxin on the neuromuscular junction. J Physiol. 1949;109:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott AB, Rosenbaum A, Collins CC. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol. 1973;12:924–927. [PubMed] [Google Scholar]
- 22.Brin MF. Botulinum toxin: new and expanded indications. Eur J Neurol. 1997;4(suppl):S59–64. [Google Scholar]
- 23.Matarasso A, Deva AK, American Society of Plastic Surgeons DATA Committee. Botulinum toxin. Plast Reconstr Surg. 2002;109:1191–1197. [DOI] [PubMed] [Google Scholar]
- 24.Munchau A, Bhatia KP. Uses of botulinum toxin injection in medicine today. BMJ. 2000;320:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humeau Y, Doussau F, Grant NJ, et al. Hoe botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82:427–446. [DOI] [PubMed] [Google Scholar]
- 26.Lu L, Atchabahian A, Mackinnon SE, et al. Nerve injection injury with botulinum toxin. Plast Reconstr Surg. 1998;101:1875–1880. [DOI] [PubMed] [Google Scholar]
- 27.Pusic AL, Cordiero PG. An accelerated approach to tissue expansion for breast reconstruction: experience with intraoperative and rapid postoperative expansion in 370 reconstructions. Plast Reconst Surg. 2003;111:1871–1875. [DOI] [PubMed] [Google Scholar]