Abstract
Objective and Summary Background Data:
Sentinel lymph node (LN) sampling, a technique widely used to manage breast cancer and melanoma, seeks to select LNs that accurately predict regional node status and can be extensively examined to identify nodal metastatic disease not detected by standard histopathological staging. For patients with resectable colon cancer, improved identification of LN disease would significantly advance patient care by identifying patients likely to benefit from adjuvant therapy. This study, conducted by 25 surgeons at 13 institutions, examined whether sentinel node (SN) sampling accurately predicted LN status for patients with resectable colon cancer.
Methods:
SN sampling involved peritumor injection of 1% isosulfan blue, followed by identification of all LN visualized within 10 minutes. SN sampling was performed on 79 of 91 patients enrolled, followed by multilevel sectioning (MLS) of the nodes and examination by a single study pathologist.
Results:
By standard histopathology, 7 patients had primary disease that was either benign or not colon cancer and were therefore excluded from further studies. Of 72 colon cancer cases studied, 48 (66%) were node-negative and 24 (33%) contained nodal metastases. SNs were successfully located in 66 cases (92%), with an average of 2.1 nodes per patient. SNs were negative in 14 of 24 node-positive cases (58%). MLS revealed tumor in a SN in 1 of these cases, bringing the false-negative rate of SN examination to 54%.
Conclusion:
This multi-institutional study found that for patients with node-positive colon cancer, SN examination with MLS failed to predict nodal status in 54% of cases. We conclude that SN sampling with MLS, used alone, is unlikely to improve risk stratification for resectable colon cancer.
Sentinel lymph node staging seeks to improve cancer staging with the expectation that increased scrutiny of these nodes will identify patients at high risk of disease recurrence. This cancer cooperative group study indicates that sentinel node staging may not accurately predict nodal status in patients with resectable colon cancer.
The most important indicator of prognosis in potentially curable colon cancer is the presence of metastatic disease within regional lymph nodes (LN). Patients whose regional LN contain metastatic tumor are at high risk of disease recurrence and are therefore most likely to benefit from adjuvant chemotherapy. Unfortunately, more than 25% of patients whose regional LNs show no evidence of disease by conventional histopathological staging will develop recurrent disease within 5 years of surgery.1 A substantial effort is underway in the scientific community to develop methods that will identify these high-risk individuals at the time of their initial treatment, primarily so that they can be considered for adjuvant therapies. A large body of research indicates that more thorough investigation of regional LNs can detect the presence of tumor cells or products that are not identified by conventional histopathological staging. These investigation methods include multilevel sectioning (MLS) to examine a larger proportion of each individual node, immunohistochemical staining with tumor markers such as cytokeratin and carcinoembryonic antigen (CEA) to detect individual cells or cell clusters not seen with hematoxylin and eosin (H&E) staining, and polymerase chain reaction–based methods to identify tumor products such as CEA2 or mutant K-ras or p53.3 Although each of these methods of detecting micrometastatic disease (MMD) shows promise, none have yet been tested in a multicenter clinical trial to determine their relationship to disease prognosis and treatment outcome. Unfortunately, significant obstacles remain before these methods can be assessed in this manner.
One of the major obstacles to multicenter testing of the clinical significance of MMD in early stage colon cancer is the prohibitive time and expense involved in applying techniques for detection of MMD to the desired number of 12 or more regional LNs typically removed during surgery. Sentinel LN sampling is a technique that has effectively addressed this problem for patients with malignant melanoma and breast cancer. Sentinel LN sampling attempts to identify the regional LNs most likely to harbor tumor metastasis by performing LN mapping at the time of surgery to detect the nodes in the path of lymphatic drainage that are the first to receive drainage from the tumor. If these nodes, or sentinel nodes (SN), accurately predict the status of the entire nodal basin, then they can reasonably be selected for more extensive examination to detect MMD. This strategy has been validated for breast cancer and malignant melanoma; studies conducted in hundreds of patients show that SN localization can identify a subset of LN able to predict overall nodal status in 95% or more of cases.4,5
A number of studies have sought to determine the applicability of SN sampling to colon cancer staging.6–10 Several of these studies suggest that SN sampling can be applied to colon cancer with a high level of accuracy for characterization of overall nodal status. In addition, some of these studies indicate that SN sampling can identify LNs that directly receive drainage from a colon cancer, but are located outside the conventional margins of regional lymphadenectomy.6,9 The present effort, Cancer and Leukemia Group B (CALGB) Protocol 80001, was designed as a feasibility study to determine whether SN sampling for colon cancer could achieve similar results in a multi-institutional cooperative group setting.
MATERIALS AND METHODS
Clinical Characteristics
Study participants were enrolled at 13 academic medical centers in the United States. Patients were eligible for enrollment if they had a clinical or pathologic diagnosis of invasive colon cancer for which surgery with curative intent was planned. Patients with a previous history of colon surgery other than appendectomy were excluded. Informed consent was obtained prior to surgery, which was conducted according to an institutional review board–approved study protocol (CALGB Protocol 80001).
Surgical Procedure
Enrolled patients underwent exploration of the abdomen at the beginning of their surgical procedure. If, during this exploration, the patient was found to have distant metastatic disease, the SN procedure was not performed and the patient was withdrawn from the study. Patients undergoing surgical resection of a potentially curable stage I, II, or III colon cancer were evaluated with the SN technique in addition to standard resection of their tumor. After identification of the tumor site within the large bowel, 1 mL of 1% isosulfan blue was instilled circumferentially into subserosal surface of the bowel immediately adjacent to the base of the tumor with a 22-gauge needle. The mesentery was then inspected visually to determine the location of the SNs, which were identified by uptake of the blue dye within the first 10 minutes of injection. Figure 1 provides an example of SN visualized in the mesentery of the colon after injection of isosulfan blue at the tumor site. These nodes were tagged with a suture for later identification and removal by the study pathologist. The surgeon also determined whether a SN was identified outside the conventional margins of resection for the cancer. The remainder of the colectomy proceeded as usual. Immediately after the colectomy, the surgeon completed a Sentinel Node Procedure Form, documenting the exact location(s) of the sentinel LNs.
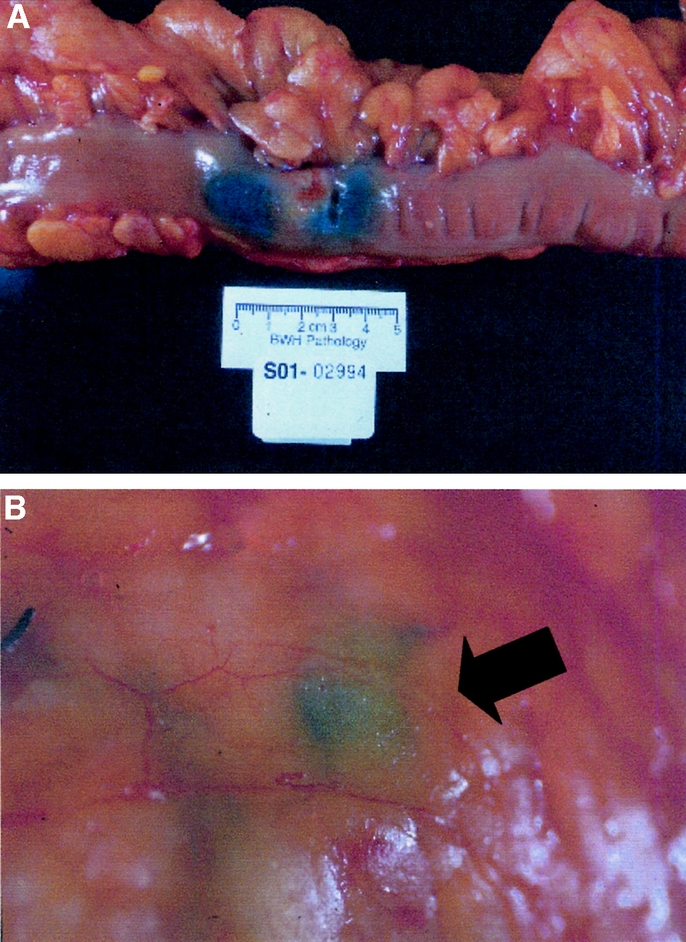
FIGURE 1. Localization of sentinel LN. After abdominal exploration to rule-out the presence of distant metastases, the colonic segment to be resected is mobilized and 1 mL of 1% isosulfan blue is infiltrated in the subserosa adjacent to the tumor (top). The arrow indicates the location of blue-stained SN appearing in the mesentery within 10 minutes of dye injection (bottom).
Each of the 25 surgeons participating in this study were proficient in SN localization for breast cancer and malignant melanoma. In addition, before enrolling a patient on the study, each surgeon was coached by a single surgeon (B.M.) who was familiar with the SN localization technique for colon cancer. In addition, operative reports and pathology reports from all cases were reviewed centrally to confirm that the study procedure met all patient eligibility and procedural requirements.
Specimen Processing and Examination
All tissues removed at surgery, including the SNs, underwent standard histopathologic diagnosis as currently performed by the departments of pathology at the treating institutions. All treatment decisions were based upon the results of standard histopathologic staging. Paraffin-embedded blocks containing SNs, non-SNs, primary tumor, and normal intestinal mucosa were collected and sent to the CALGB Pathology Coordinating Office at Ohio State University. After arrival at the Pathology Coordinating Office, sections of tumor were prepared with H&E stain. A diagnosis of invasive colon cancer was confirmed on examination of these sections by a single study pathologist (C.C.). In addition, SNs and non-SNs were sectioned at multiple levels, with a distance of 75 μm between levels. A single H&E-stained section was examined at each of 5 different levels per node. These sections were all evaluated by a single study pathologist (C.C.), who was blinded to the results reported by the treating institution.
RESULTS
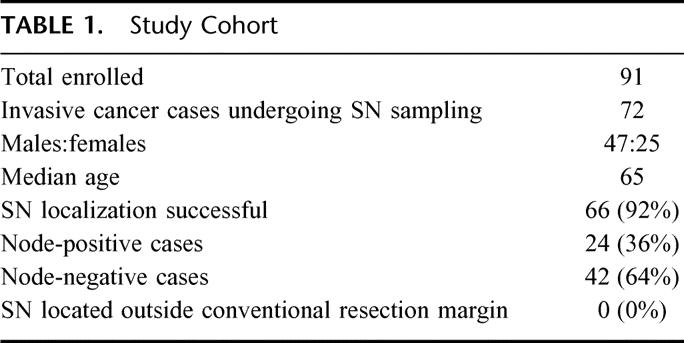
CALGB Protocol 80001 enrolled a total of 91 patients at 13 member institutions. A total of 25 surgeons participated in this study. The SN localization procedure was performed on 79 patients. Twelve patients did not undergo SN localization for the following reasons: metastatic cancer present at laparotomy,4 primary tumor other than colon cancer,2 no residual tumor after colonoscopic resection,2 surgeon trained in SN sampling technique unavailable at time of surgery,2 no dye available,1 and prior tattoo prevented visualization of dye.1 In addition, 5 patients who underwent SN sampling were found to have noninvasive disease upon final histopathology, including 4 cases in which a large adenoma was resected, and 1 case in which a mass suspected to be malignant was found to be caused by diverticulitis. A total of 72 patients with invasive colon cancer underwent SN sampling. Of these 72 patients, 11 had T1 lesions, 14 were classified as T2, 39 were classified as T3, and 8 had sufficient extension to qualify as T4. Regional LN involvement by tumor was identified in 24 patients (33%), and 48 cases (67%) were node-negative. Primary tumors were located in the following sites: cecum (15%), ascending colon (22%), transverse colon (7%), descending colon (10%), and sigmoid (46%). Additional cohort characteristics are presented in Table 1.
TABLE 1. Study Cohort
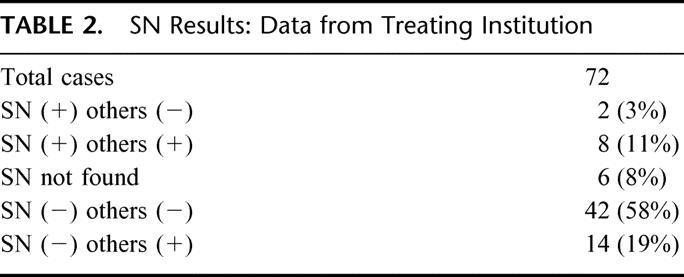
A total of 1244 LN were removed from the 72 patients undergoing SN sampling, for an average of 17.3 nodes per patient. An average of 2.1 SN per patient were identified in the 66 patients for which localization was successful. Results of conventional histopathological staging performed at each treating institution are presented in Table 2.
TABLE 2. SN Results: Data from Treating Institution
Examination of sections from multiple levels within LN can increase the detection of metastatic disease.11 All SN and a subset of non-SN were therefore subjected to MLS, followed by staining with H&E. All of these slides were reviewed and scored by a single study pathologist (C.C.). A total of 72 SN and 97 non-SN were examined in this manner. In one SN, MLS revealed the presence of metastatic disease not previously identified. This metastasis was not a micrometastasis, but rather a tumor nodule of >200 μm in size. This occurred in a case where the SNs were originally characterized as node-negative, but metastases were found in the non-SNs. MLS therefore resulted in a change in the false-negative rate for SN examination, from 58% to 54%, but did not change the nodal status of the patient. MLS also revealed tumor in one non-SN that was previously designated free of disease. This occurred in a case where the treating institution diagnosed metastatic disease in 2 of 2 SN and 1 of 17 non-SN; therefore, the MLS result did not alter the nodal status of the patient. Results of SN examination for the 66 patients who underwent successful SN localization followed by central pathology review and MLS are presented in Table 3.
TABLE 3. Results of SN Examination

Because the success of SN localization depends upon the degree of the surgeon's familiarity with the technique, we determined the relationship between successful localization of predictive SN and surgical volume. Of the 25 participating surgeons, 2 performed 10 or more SN localizations, 5 performed 5 to 9 cases each, and 18 performed fewer than 5 SN localizations. Cases considered SN localization failures were those that either failed to identify a SN (n = 6) or for which the SN did not predict the status of the nodal basin (n = 13). Surgeons who performed 10 or more SN localizations identified predictive nodes 67% of the time (7 failures/21 patients). In comparison, surgeons performing fewer than 5 cases each successfully identified predictive SN in 70% of cases (7 failures/20 patients). The highest rate of success (84%) was achieved by surgeons who performed from 5 to 9 cases each (5 failures/31 patients). These data suggest that surgeons performing more SN procedures do not have greater success in SN localization.
DISCUSSION
This study confirms that for resectable colon cancer the SN localization technique can be successfully accomplished in a multi-institutional setting. With conventional histopathological LN staging, however, we find that the SN procedure is not as accurate for colon cancer as it is for breast cancer or malignant melanoma. Our results are consistent with those of 3 additional reported studies on standard histopathological examination of a single H&E-stained section to detect LN metastases.8,12,13 The proportion of node-positive cases for which SN did not predict overall nodal status ranged from 18% to 60% in these studies.
Published reports from single institutions8,12–19 and multi-institutional studies6,9 have reached different conclusions. In a multi-institutional study including 203 colorectal cancer patients, Saha et al reported successful SN localization in over 97% of cases.9 These investigators identified an average of 1.8 SN and 14.7 total LN per patient, which is comparable to our study. Of the patients with node-positive disease, SN failed to predict nodal status in only 8 of 81 cases (11%). In that study, however, some SN that were classified as negative by conventional examination with H&E staining were scored positive if they contained either micrometastases (defined as tumor <200 μm in diameter) or single tumor cells visible only after cytokeratin immunohistochemical staining. Because the clinical significance of LN micrometastases—particularly those identified solely by immunohistochemical staining—is unknown, we did not include nodes containing this level of disease among those scored as positive. Our definition of SN positivity is therefore different from that used in the trials described above.
We reassessed the data provided by Saha et al to allow a more direct comparison of their results with our study. LNs scored as positive only on the basis of micrometastases or cytokeratin positivity were rescored as negative. This classification increased the false-negative rate for SN analysis to 15% (8/54) in the study by Saha et al. Because the same definition of positivity was not applied to the non-SNs, it is difficult to know whether this corrected figure can be directly compared with that obtained in our study. Similar arguments can be applied to several other single-institution studies of SN sampling for colon cancer (Table 4).
TABLE 4. Comparison of Current Study With Other Published Work

Approximately 35% of patients with positive LN show no evidence of tumor recurrence 5 years after surgery with curative intent and 6 months of adjuvant chemotherapy,20 and recent studies indicate that treatment outcome for stage III tumors improves when high numbers of LNs are removed.21 This suggests that removal of nodal disease prevents disease progression. One rationale for performing SN sampling in colon cancer is that clinically relevant SN may be found outside the conventional margins of resection for regional lymphadenectomy. Published reports indicate that a nonconventional location for SN is observed in from 6% to 8% of cases.6,9,14 Some authors have suggested that this aberrant lymphatic drainage is produced when bulky metastatic disease is present in the conventional regional nodes, forcing lymphatic flow to outside locations. In our study of 66 cases in which SN localization was successful, none contained SN located outside conventional lymphadenectomy margins. Our data, therefore, indicate that SN identification is not likely to alter the extent of lymphadenectomy for resectable colon cancer.
SN sampling for colon cancer offers clinical utility when it either alters the nature of the surgical procedure or leads to the correct selection of LN to study micrometastatic disease. We find no evidence that SN identification alters the extent of lymphadenectomy performed, because none of the SN isolated in our series were located outside of the conventional margins of resection. This multi-institutional study found that for patients with node-positive colon cancer, SN examination failed to predict nodal status in 54% of cases. This leads us to believe that SN may under-report MMD to a similar extent. We conclude that SN sampling in patients with colon cancer is not useful as a means of determining the optimal extent of lymphadenectomy or facilitating the examination of micrometastatic disease.
The research for CALGB 80001 was supported in part by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, chairman) and (CA59594) to the CALGB Surgery Committee (David J. Sugarbaker, chairman).
Discussions
Dr. W. Douglas Wong (New York, New York): I would like to congratulate Dr. Bertagnolli and the CALGB cooperative group on this important study and to thank them for the opportunity to review their paper and discuss it at this meeting. The results of this study certainly make a strong case that the sentinel lymph node procedure using standard histopathological lymph node staging is not a reliable representation of the entire lymph node status of the entire nodal basin for a given colon cancer.
For those of us who have been somewhat skeptical regarding the clinical utility of sentinel of node mapping for colon cancer, this study provides added evidence to maintain a cautionary approach to this conceptually intriguing paradigm. Lymphatic mapping in melanoma and breast is based on the reproducibility and orderly drainage of the lymphatics from the primary tumor initially to a sentinel node. However, in colon cancer this concept may not be reliable. The lymphatic drainage of a colon cancer can be likened more to a watershed than a more direct line as in skin and breast. Hence the lymphatic drainage could be to one or the other side of the mesentery, or either proximal or distal to the tumor or even to the center of a fat mesentery in obese patients where identification may be difficult. In addition, there is evidence in the literature that the success of sentinel lymph node identification differs between right and left colon cancers, suggesting that the complexity and variability of the lymphatic drainage may be a major factor.
This leads me to my first question. In your study you state that there was no difference in the success of the localization—at least in the manuscript you say—there was no difference in the success of the localization amongst participating surgeons based on the number of cases they contributed. All surgeons apparently were experienced in sentinel lymph node techniques for melanoma and breast and were tutored by one study participant in lymph node mapping for colon cancer. I think it was a relatively small number of cases, however, even in the high volume and the low volume surgeons that participated. And furthermore, I am not so sure that the experience from breast and melanoma is so easily transferable to that for colon, and I wonder if more emphasis on volume standardization and education would have altered the results to some extent. In the breast world it is my understanding that a minimum of 20 cases is recommended to become adequately trained and adept at this technique. My question is, would such standardization, volume, and education have potentially altered the results? I would be interested in your thoughts in that regard.
My second question pertains to other potential reasons for the high false negative results. There is some evidence that more locally advanced colon cancers may have a higher false negative rate by blocking lymphatics and altering the routing of tumor cells. Did you look at whether there was a difference in your more advanced lesions compared to the early stage lesions in your series to see if there was a difference? And along a similar line, did you evaluate whether there was a difference in the false negative rate in left-sided tumors compared to right-sided lesions?
Again let me congratulate you on a very nice study. I enjoyed reading your paper and hearing your presentation, and I look forward to your answers.
Dr. Monica Bertagnolli (Boston, Massachusetts): In our paper, we addressed the issue of procedure volume, as we were concerned that it might be one of the differences in our study compared to published reports from very dedicated groups of clinicians who have performed this procedure with more success. We did our best to standardize the technique among surgeons, and our results show no difference in success rate between the high and low volume surgeons. The numbers of cases per surgeon were small, therefore only more numbers and more investment of time would answer your question properly. One of the reasons for pursuing a cooperative group trial is to test the generalizability of the result to a larger, more heterogenous group of clinicians. Our results reflect these conditions.
As far as the high false negative results, we did examine the relationship between node positivity and primary tumor T-stage. Unfortunately, the numbers were small, and within T-stage categories we saw no difference between early and advanced tumors. Finally, one-third of our patients had right-sided tumors versus two-thirds on the left. We saw no difference in ability to locate sentinel nodes from these 2 locations. We deliberately excluded from eligibility any patient with a tumor below the peritoneal reflection.
Dr. Frederick L. Greene (Charlotte, North Carolina): As chairman of the AJCC, I want to congratulate you on this study. Of course, we have the oversight for TNM staging. And I want to thank you for making sure that you clarified the isolated tumor cell. There has been a lot of confusion because isolated tumor cells less than 0.2 millimeters can be seen by H&E or immunohistochemistry. So thank you.
My quick question is, we stratified stage II into IIA and IIB. IIA, of course, is T3N0, IIB is T4N0. In your studies did you see any differentiation between IIA and ILB relative to your studies of the nodes?
Dr. Monica Bertagnolli (Boston, Massachusetts): We did not see a difference, and I suspect again that this is due to the small number of patients we had in the T4 category. When we begin looking in a more detailed fashion at the micrometastatic disease in these patients, perhaps we will see some correlations, as we will have a greater overall number of nodes to examine. Hopefully we can use these data examine the relationship between micrometastatic disease and the standard prognostic variables.
Dr. Stanley P. L. Leong (San Francisco, California): I would like to congratulate Dr. Bertagnolli and her group for an excellent prospective study with multicenter groups to define the accuracy of sentinel lymph nodes in colon cancer.
I am sure you are aware of Dr. Saha's work, who is a champion and pioneer in this field (Saha S. et al. Ann Surg Oncol. 2001 Oct;8(9 Suppl);94S-98S). His study indicates that the false negative rate for harvesting sentinel lymph nodes in colon cancer is relatively low. Therefore, I am struck by the discrepancy between your study and Dr. Saha's. You have mentioned some of the reasons, such as the potential learning curve issue and the low patient number in your study. Since now you feel that indeed the patient number is relatively small, would you make such a firm statement that the sentinel lymph node approach should not be used in colon cancer? Further, even if your concordancy rate is 54%, you have accurately staged at least 54% of the patients. The concordancy rate in colon cancer may not be that critical since you will do a formal colectomy any way in contrast to melanoma and breast cancer in which a formal lymph node dissection is usually not performed if the SLN is negative. Thank you.
Dr. Monica Bertagnolli (Boston, Massachusetts): Thank you for the question, and I am glad that you brought up a comparison of our work with that done by others. Dr. Saha and Dr. Bilchik have both led multicenter trials of sentinel node sampling for colon cancer. Comparing their data to ours is a bit like comparing apples and oranges, however. In their work, the sentinel lymph nodes were examined by multilevel sectioning and cytokeratin staining. Lymph nodes were designated positive in these studies if they contained micrometastatic disease as I've defined it today. In addition, only the sentinel nodes were examined by these more intensive methods, and this increased scrutiny resulted in elevation of the success rate of their sentinel node sampling. We have attempted to correlate their data with ours by taking out those nodes what we would have called negative, and this lowers the specificity of their technique. This re-assignment still does not resolve the differences between our studies, as it lowers their false negative rate to approximately 20%, whereas we have an almost 50% false negative value. Another difference could be in surgeon technique and experience, because the groups led by Drs. Saha and Bilchik are highly experienced in sentinel node sampling for this disease. Finally, inherent in a cooperative group study are additional variables, such as differences among pathology departments and basic surgical techniques among our more heterogenous group of investigators.
Dr. Merrick I. Ross (Houston, Texas): This was a very nicely presented paper and I congratulate you on that. I also want to thank you for confirming what we found in a single institutional study of a 48% false negative rate in primary colon cancer. We also had a very difficult time trying to reconcile our results with those from Drs. Shaha and Bilchik.
Also I want you to know we just completed a study where we did not decrease the false negative rate at all with the use of a more sensitive technique. Other studies have tried to reduce their false negative rates by doing immunohistochemistry. If you have to use immunohistochemistry to find a positive sentinel node, then you have to question whether or not the sentinel node concept actually applies in this particular tumor system.
Where I do want to challenge you is in your last conclusion where you said that you are going to apply this to the node negative patients and look at immunohistochemistry and other markers. You just kind of proved to yourself that this may not be an accurate way to identify the lymph nodes. Why spend time and money trying to look more carefully where you may be looking at the wrong nodes?
Dr. Monica Bertagnolli (Boston, Massachusetts): The answer is that what you suggest is not what we are trying to do. We are still very interested in the definition of micrometastatic disease in colon cancer, because this certainly appears to be clinically relevant in other tumors. What our current work seems to be telling us is that we can't take a shortcut. In other words, we may not be able to examine only sentinel nodes and use these results for clinical studies of micrometastatic disease. It is possible that we are going to have to examine the entire nodal basin. Hopefully, studies currently in progress with this cohort will shed more light on this issue.
Footnotes
The following institutions participated in this study: CALGB Statistical Office, Durham, NC (Grant CA33601 to Stephen George, PhD); Christiana Care Health Services, Inc., CCOP, Wilmington, DE (Grant CA45418 to Stephen Grubbs, MD); Dana Farber Cancer Institute, Boston, MA (CA32291 to George P. Canellos, MD); Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH (CA04326 to Marc Ernstoff, MD); SUNY Upstate Medical University, Syracuse, NY (Grant CA21060 to Stephen L. Graziano, MD); The Ohio State University, Columbus, OH (Grant CA77658 to Clara D. Bloomfield, MD); University of Iowa, Iowa City, IA (Grant CA47642 to Gerald Clamon, MD); University of Massachusetts Medical Center, Worcester, MA (Grant CA37135 to Mary Ellen Taplin, MD);University of Missouri/Ellis Fischel Cancer Center, Columbia, MO (Grant CA12046 to Michael C. Perry, MD); University of Nebraska Medical Center, Omaha, NE (Grant CA77298 to Anne Kessinger, MD); Vermont Cancer Center, Burlington, VT (Grant CA77406 to Hyman B. Muss, MD); Washington University School of Medicine, St. Louis, MO (Grant CA77440 to Nancy Bartlett, MD); and the Western Pennsylvania Cancer Institute, Pittsburgh, PA (Richard K. Shadduck, MD).
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Corresponding author: Monica M. Bertagnolli, MD, Brigham and Women's Hospital, 75 Francis Street, Boston, MA 02115. E-mail: mbertagnolli@partners.org.
Reprints will not be available from the authors.
REFERENCES
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. [DOI] [PubMed] [Google Scholar]
- 2.Liefers G-J, Cleton-Jansen A-M, van de Velde CJH, et al. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998;339:223–228. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Cespedes M, Esteller M, Hibi K, et al. Molecular detection of neoplastic cells in lymph nodes of metastatic colorectal cancer patients predicts recurrence. Clin Cancer Res. 1999;5:2450–2454. [PubMed] [Google Scholar]
- 4.Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilchik AJ, Saha S, Wiese D, et al. Molecular staging of early colon cancer on the basis of sentinel node analysis: a multicenter phase II trial. J Clin Oncol. 2001;19:1128–1136. [DOI] [PubMed] [Google Scholar]
- 7.Bilchik AJ, Nora DT, Sobin LH, et al. Effect of lymphatic mapping on the new tumor-node-metastasis classification for colorectal cancer. J Clin Oncol. 2003;21:668–672. [DOI] [PubMed] [Google Scholar]
- 8.Merrie AEH, van Rij AM, Phillips LV, et al. Diagnostic use of the sentinel node in colon cancer. Dis Colon Rectum. 2001;44:410–417. [DOI] [PubMed] [Google Scholar]
- 9.Saha S, Bilchik A, Wiese D, et al. Ultrastaging of colorectal cancer by sentinel lymph node mapping technique: a multicenter trial. Ann Surg Oncol. 2001;8(suppl):94S–98S. [PubMed] [Google Scholar]
- 10.Saha S, Dan AG, Berman B, et al. Lymphazurin 1% versus 99mTc sulfur colloid for lymphatic mapping in colorectal tumors: a comparative analysis. Ann Surg Oncol. 2004;11:21–26. [DOI] [PubMed] [Google Scholar]
- 11.Tschmelitsch J, Klimstra DS, Cohen AM. Lymph node micrometastases do not predict relapse in stage II colon cancer. Ann Surg Oncol. 2000;7:601–608. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa Y, Watenabe M, Hasegawa H, et al. Sentinel node mapping for colorectal cancer with radioactive tracer. Dis Colon Rectum. 2002;45:1476–1480. [DOI] [PubMed] [Google Scholar]
- 13.Joosten JJ, Strobbe LJ, Wauters CA, et al. Intraoperative lymphatic mapping and the sentinel node concept in colorectal carcinoma. Br J Surg. 1999;86:482–486. [DOI] [PubMed] [Google Scholar]
- 14.Bilchik AJ, Saha S, Tsioulias GJ, et al. Aberrant drainage and missed micrometastases: the value of lymphatic mapping and focused analysis of sentinel lymph nodes in gastrointestinal neoplasms. Ann Surg Oncol. 2001;8(suppl):82S–85S. [PubMed] [Google Scholar]
- 15.Wood TF, Saha S, Morton DL, et al. Validation of lymphatic mapping in colorectal cancer: in vivo, ex vivo, and laparoscopic techniques. Ann Surg Oncol. 2001;8:150–157. [DOI] [PubMed] [Google Scholar]
- 16.Evangelista W, Satolli MA, Malossi A, et al. Sentinel lymph node mapping in colorectal cancer: a feasibility study. Tumor. 2002;88:37–40. [PubMed] [Google Scholar]
- 17.Paramo JC, Summerall J, Poppiti R, et al. Valideation of sentinel node mapping in patients with colon cancer. Ann Surg Oncol. 2002;9:550–554. [DOI] [PubMed] [Google Scholar]
- 18.Tsioulias GJ, Wood TF, Morton DL, et al. Lymphatic mapping and focused analysis of sentinel lymph nodes upstage gastrointestinal neoplasms. Arch Surg. 2000;135:926–932. [DOI] [PubMed] [Google Scholar]
- 19.Bendavid Y, Latulippe JF, Younan RJ, et al. Phase I study on sentinel lymph node mapping in colon cancer: a preliminary report. J Surg Oncol. 2002;79:81–84. [DOI] [PubMed] [Google Scholar]
- 20.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22. [DOI] [PubMed] [Google Scholar]
- 21.Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–2919. [DOI] [PubMed] [Google Scholar]


