Abstract
Objective:
This paper reports our experience of a large series of elephant trunk patients accumulated over 12 years.
Summary Background Data:
Extensive aneurysms of the ascending/arch and descending thoracic or thoracoabdominal aorta are significant surgical problems that have potential for great morbidity. We adopted a staged approach known as the elephant trunk procedure in 1991, and we have used it with some modifications since that time.
Methods:
Between February 1991 and December 2003, we performed 1660 operations for ascending/arch or descending thoracic/thoracoabdominal aortic aneurysms. Of these, 321 operations were performed in 218 patients for extensive aneurysms with the elephant trunk technique. We performed 218 ascending/arch repairs and 103 descending thoracic or thoracoabdominal aortic replacements.
Results:
In 218 ascending/arch repairs, strokes occurred in 3 of 218 (2.7%) patients, with 1 of 187 (0.5%) in the retrograde cerebral perfusion group and 2 of 31 (6.5%) in the no-retrograde cerebral perfusion group (odds ratio 0.08, P < 0.009). Thirty-day mortality for this group was 19 of 218 (8.7%). Among 199 recovering patients after stage 1 repair, 4 of 199 (2%) died during the 30-day to 6-week interval between stages. After stage 2 repair, 0 of 103 patients experienced immediate neurologic deficit, and 10 of 103 (9.7%) died within 30 days of surgery. Actuarial survival after completed stage 2 was 71% at 5 years.
Conclusion:
Despite extreme underlying disease, long-term survival is excellent in patients with extensive aneurysms when both stages of repair are completed. To prevent rupture, the second stage should be completed as soon as the patient's condition permits, preferably within 6 weeks.
We report our experience of the staged approach to extensive aortic aneurysm repair known as the elephant trunk technique. Over 12 years, we have performed 321 elephant trunk operations, the largest such series on record. Despite extreme underlying disease, long-term survival has been excellent for patients completing both stages of repair.
Extensive aortic aneurysms, occupying the ascending to the thoracoabdominal aorta, present a technical challenge to surgeons. The anatomy and orientation of the aorta make it extremely difficult to expose entirely through a single-incision approach. Historically, staged repair, in which the aorta was approached through separate incisions, was fraught with complications, particularly catastrophic bleeding. In 1983, however, Borst introduced the elephant trunk technique (Fig. 1), 1 which permitted the surgeon to avoid cross clamping the proximal native descending thoracic aorta in the second stage of repair and, consequently, avert problems of hemorrhage. With a few modifications, we have successfully used Borst's technique since 1991 in the repair of extensive aortic aneurysms. In the first stage, cardiopulmonary bypass, profound hypothermia, circulatory arrest, and retrograde cerebral perfusion (RCP) have provided improved protection of the brain and guarded against stroke; in the second stage, distal aortic perfusion and cerebrospinal fluid drainage have improved protection of the spinal cord. In this article, we present our rationale and approach and describe the technique as it has evolved at our center over a 12-year period.
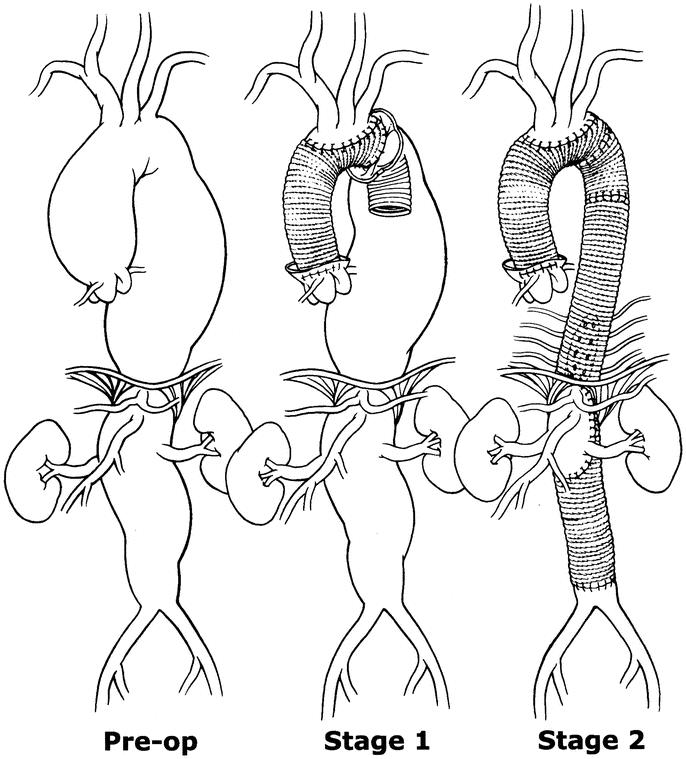
FIGURE 1. Illustration of typical extensive aortic aneurysm of the ascending/arch and thoracoabdominal aorta repaired in two stages with the elephant trunk technique.
METHODS
We performed 321 operations in 218 patients. Of these, 218 had ascending/arch operations and 103 had descending/thoracoabdominal operations. Patient demographics and operative data for both stages are displayed in Tables 1 and 2. The first stage of the elephant trunk technique is performed in a similar fashion to standard surgery of the ascending aorta and transverse arch, with the exception of the insertion of an inverted distal graft.2
TABLE 1. Determinants of 30-Day Mortality, Stage 1, Ascending/Arch
TABLE 2. Determinants of 30-Day Mortality, Stage 2, Descending/Thoracoabdominal
The patient is placed on cardiopulmonary bypass, and systemic temperature is lowered. Further cerebral protection is provided by RCP through the superior vena cava. Continuous retrograde cold-blood cardioplegia through the coronary sinus, supplemented with direct antegrade coronary ostia infusion once the aorta is opened, protects the heart. A left ventricular sump inserted through the right superior pulmonary vein prevents left ventricular distension. Transesophageal echocardiography (TEE) monitors heart function and is also used to detect the presence of potentially harmful atheromatous plaque in the ascending aorta, arch, or descending thoracic aorta. When the patient's temperature has been lowered to between 20°C and 15°C (measured nasopharyngeally) and the electroencephalogram monitoring cerebral function has become isoelectric, circulation is arrested and RCP begun. Cerebral blood flow is directly monitored by power M-mode transcranial Doppler; thus, perfusion pressure can be increased or decreased as necessary to maintain middle cerebral artery flow, eliminating the previous requirement to maintain a maximum cerebral flow rate of 500 m/min. The transcranial Doppler can also identify cerebral artery flow reversal.
We excise most of the lesser curvature and the 2 lateral walls of the transverse arch (Fig. 2a). The elephant trunk Dacron tube graft is inverted on itself (Fig. 2b). The distal portion descending length is not greater than 10 cm. The inverted graft is placed in the descending thoracic aorta, and the folded edge is sutured just distal or proximal to the left subclavian artery (Fig. 2c). When this distal anastomosis is completed, the inner portion of the graft is retrieved into the operative field, leaving the remainder of the graft (the elephant trunk) dangling in the descending thoracic aorta. A side hole is cut in the retrieved proximal portion of the graft, and the preserved aortic island containing the great vessels is sutured to the graft (Fig. 2d). After completion of the distal reconstruction, RCP is discontinued, and a cannula is placed into the proximal aortic graft. With the patient in the Trendelenberg position, cardiopulmonary bypass flow is initiated through the femoral cannula until all debris is evacuated through the open aortic graft. Antegrade flow is then established through the graft cannula, the graft is clamped, and proximal reconstruction is completed while the patient is systemically warmed (Fig. 2e). This may include aortic valve replacement or resuspension, or if the sinuses are dilated, aortic root replacement with the button or modified Cabrol technique. Warming is continued until the patient's core body temperature has reached 36°C. Stay in the intensive care unit averages 2 to 3 days, followed by another 5 to 10 days of hospital recovery. The patient is discharged and given an appointment to return in 4 to 6 weeks, depending on general physical and pulmonary status. On rare occasion, the patient will remain in hospital and undergo second-stage repair within a week of first-stage surgery.
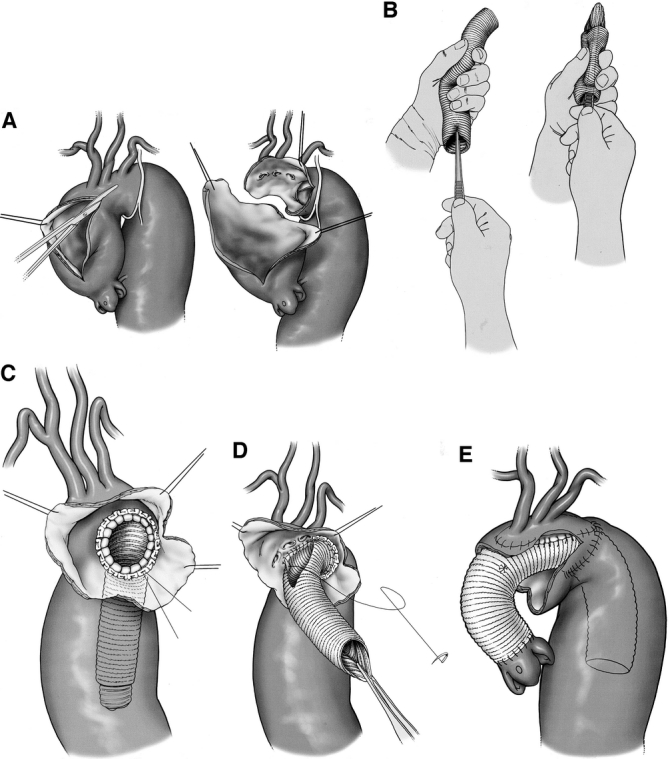
FIGURE 2. (a) Excision of the lesser curvature of the arch in stage 1; (b) inversion of elephant trunk graft; (c) placement of the inverted graft in the descending thoracic aorta; (d) distal anastomosis and attachment of great vessels to graft in stage 1 repair; (e) proximal anastomosis, stage 1 repair.
The second stage of the elephant trunk technique closely resembles standard descending thoracic or TAAA repair, for which we also use the adjuncts distal aortic perfusion and CSF drainage.3 After initiation of the pump for distal aortic perfusion, a clamp is applied distally at the mid-descending thoracic aorta. The proximal third of the descending thoracic aorta is opened without a proximal clamp. The elephant trunk portion of the graft, inserted in the descending thoracic aorta during stage 1, is grasped and clamped (Fig. 3a). The graft-to-graft anastomosis of new graft to “elephant trunk” can be performed much more quickly than graft to native aorta. Because there is no need for cross clamping of the proximal descending thoracic aorta, dissection in the area of the proximal descending aorta, transverse arch, and pulmonary artery is avoided, and the risk of massive bleeding due to injury of these vessels is minimized. Then, the intercostal anastomosis, visceral patch anastomosis, and lastly the distal anastomosis are sutured in sequential fashion (Fig. 3b and c). An illustration of an extensive aortic aneurysm before and after the first and second stages of the elephant trunk technique is shown in Fig. 4.
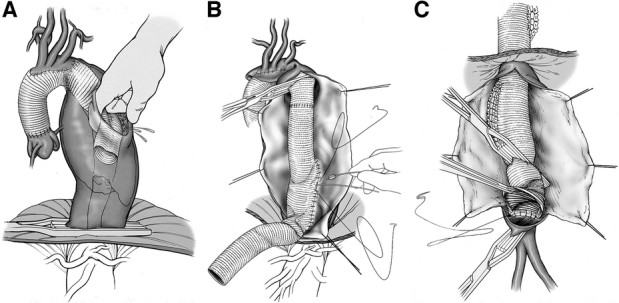
FIGURE 3. (a) Retrieval of the elephant trunk during stage 2; (b) intercostal and visceral anastomoses, stage 2; (c) distal anastomosis, stage 2.
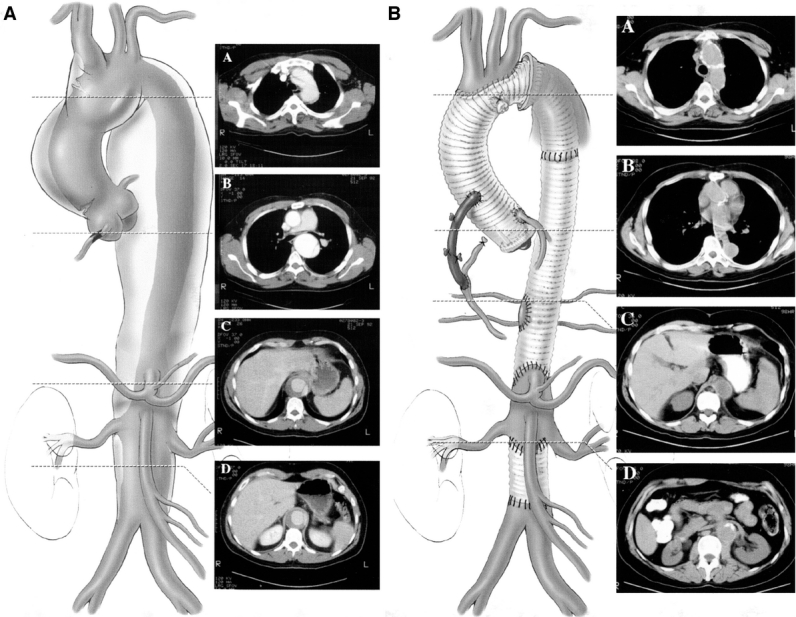
FIGURE 4. Stage 1 repair (left) and completed stage 1 and 2 repairs (right) of extensive aortic aneurysm.
STATISTICAL METHODS
Data were collected on case-report forms by our clinical research nurse and entered into a secure encrypted Microsoft Access database. Research procedures are approved by institutional review board and comply with applicable privacy statutes. Data were exported to SAS version 8.2 for processing and analysis. Univariate comparisons were made by contingency table for categorical data and by unpaired t test or Wilcoxon rank-sum as appropriate. Univariate long-term survival was estimated by the Kaplan-Meier method, and stratified comparisons were made with the log-rank statistic. Multivariable comparisons were made by regression techniques appropriate to the distribution of the dependent variable, linear, logistic, or Cox.
RESULTS
To better evaluate the results for staged repair, we divided the data into the following 4 distinct categories: (1) stage 1 surgery to 30 days postoperative, (2) the recovery interval after stage 1, from 31 days to 6 weeks postoperative, (3) stage 2 surgery to 30 days postoperative, and (4) nonreturning patients. Patients who did not undergo stage 2 repair beyond 6 weeks were considered nonreturning patients. After stage 1 ascending/arch repair, 19 of 218 (8.7%) patients died within 30 days of surgery. Three of 218 (2.7%) patients had postoperative strokes; 1 was among patients treated with RCP (1/187, 0.5%) and 2 (2/31, 6.5%) were among patients without RCP (odds ratio 0.08, P < 0.009). Of the 199 patients surviving 30 days from stage 1 ascending/arch surgery, 4 (2%) died awaiting the second stage. Ninety-eight patients did not return for stage 2 within 6 weeks of the first stage. Of these nonreturning patients, 18 (18.4%) died without completion of surgery. After stage 2 descending/thoracoabdominal repair, 10 of 103 (9.7%) patients died. No cases of paraplegia or paraparesis were observed after stage 2 surgery. Long-term survival after completion of the second stage was 71% at 5 years (Fig. 5).
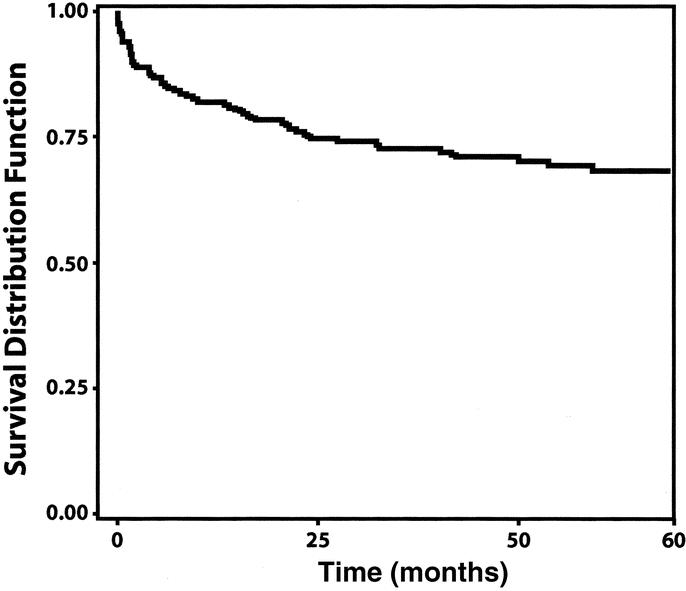
FIGURE 5. Long-term survival after completion of second-stage repair.
DISCUSSION
Extensive aneurysms of the aorta are complex surgical problems that present significant technical challenges and require an integrated approach and an experienced team. Before Borst introduced the elephant trunk technique,1 staged repair required clamping of the aneurysmal aorta at the left subclavian artery and frequently led to excessive bleeding. Bleeding could also occur from the left pulmonary artery during second-stage dissection of the descending thoracic aorta. By circumventing cross clamping of the proximal descending thoracic aorta, the elephant trunk technique resolved these problems. Crawford4 and Svensson5 made some modifications to the original elephant trunk technique, which Borst later acknowledged as improvements to his original method.6 These changes included placement of the inverted graft in the descending rather than proximal aorta and shortening the length of the free end of the graft that extends into the descending aorta to avoid kinking, obstruction, or dislodgment of emboli. Another modification evolving from Borst's original method has been the reverse elephant trunk.7–11 In a reversed elephant trunk procedure, we begin with graft replacement of the descending thoracic aorta because symptoms such as back pain or the threat of rupture demand urgent attention. The inverted graft is placed in the descending thoracic aorta and the fold sutured at the proximal descending aorta. The graft remains inverted until the second stage. The benefits of the elephant trunk technique in cases of type A dissection have also been acknowledged.12–15 After removal of the septum between the true and false lumina, insertion of the graft into the distal true lumen helps to prevent leakage at the site of the distal anastomosis and to prevent further progression of the tear. Patients treated for type A dissection without aneurysmal descending thoracic aortas or dissections that progressed into the descending aorta formed a subset of the nonreturning patients. Also part of this subset in our series were patients for whom we used the elephant trunk technique regardless of enlargement of the descending thoracic aorta, but rather to prevent clot and debris from traveling to the brain due to an atherosclerotic descending aorta.
Since our previous review of patients through the year 2000,16 we have had no additional deaths in the interval. The fact that 3 of these 4 interval deaths were caused by rupture of the unoperated aortic segment between 4 and 6 weeks after the ascending repair prompted us to make a determined effort not only to get patients to return for second-stage repair but also to return no later than 6 weeks after stage 1 repair. Advancing the second stage to the 4- to 6-week timeframe appears to be safe, in that we did not see an increase in stage 2 perioperative mortality with the reduction in interval mortality. This suggests that the interval mortality reduction is attributable to the change in protocol and not to underlying concomitant systemic disease. Because aneurysm ruptures occurred before the 4- to 6-week timeframe, this experience has also reinforced our commitment to 2-stage rather than 1-stage repair.
Our favorable results have consistently supported the use of this technique11,16,17 and reflect the outcomes of other smaller elephant trunk series.4,6,7,18 In our series, the early mortality rates after stage 1 (8.7%) and stage 2 (9.7%) were acceptable. Two of the largest series of extensive aortic aneurysms repaired in single-stage operations were necessarily limited to aneurysms extending no further than the diaphragm because of transmedial or midsternal approaches.19,20 Despite the fact that there were no high-risk type II thoracoabdominal aortic aneurysms in these series, one also reported a high (14%) incidence of paraplegia.19 Although the protective properties of hypothermia as used in these cases are well documented in surgery of the ascending aorta and transverse aortic arch, the drawbacks of lowering systemic temperature are also well known.21 In 1992, cerebral protection in the first stage of the elephant trunk technique was enhanced by the introduction of RCP.22 Second-stage graft replacement, in which we used distal aortic perfusion and cerebrospinal fluid drainage and in which 71% of patients had either type I or type II thoracoabdominal aortic aneurysms, had no instances of neurologic deficit.
Among 1660 operations for ascending/arch or descending thoracic/thoracoabdominal aortic aneurysms performed between February 1991 and December 2003, nearly 20% have required repair of more than one segment of the aorta. Despite the extensiveness of the aortic disease in these cases, the patients do remarkably well, provided that they comply with the staging protocol. Perioperative mortality after each stage is less than 8%, stroke rates are under 3% and so far immediate neurologic deficit after stage 2 is nonexistent. Our experience suggests that the second stage of the repair should be conducted as soon as practicable after the first stage and within 6 weeks whenever possible.
Discussions
Dr. John E. Connolly (Irvine, California): Thank you for the opportunity of discussing this paper and I thank Dr. Safi for sharing his manuscript with me. I would like to say a few things historically first and then ask him some questions.
I wish that Hans Borst of Germany, who is an Honorary Fellow of our Association, had been present this afternoon to hear this report of the largest series and the best results achieved with extensive aortic resection employing the elephant trunk technique that he described in 1983.
It is of particular interest to me because about 50 years ago, about a mile and a half west of here, where the Stanford Hospital was at that time, Hans was an intern in surgery. And we talked about role models earlier today, and that year his role models that I believe led him into cardiovascular surgery were Emile Holman, who was Halsted's last chief resident, and Frank Gerbode. I was his chief resident and apparently did not deter him. Subsequently, Dr. Borst returned to Germany, became a world renowned aortic surgeon, like our speaker today, whose role model I thought was Stanley Crawford, but perhaps it was also Dr. Jordan.
I would like to ask Dr. Safi a few questions. I think we all agree that dividing up these extensive operations on the aorta makes sense. I didn't quite hear how many of these patients did not come back for the second procedure. And I would like to ask whether his group does any endovascular stenting for such extensive aortic disease. There have been several reports that I am aware of now with at least early success with stenting, and I will be interested to know what he sees in the foreseeable future with endovascular surgery for thoracic aneurysms.
I also agree with him that profound hypothermia and circulatory arrest has been the best technique for major cardiovascular surgery. The key here, however, is knowing exactly what the brain temperature is throughout the arrest period. When it is done for neurosurgery, you have a thermistor probe in the frontal lobe of the brain and you know exactly what the brain and cord temperature is. And I predict that that technique will also be used for this type of operation.
I again congratulate you on the tremendous success in what is undoubtedly the most complicated type of surgery that we do today.
Dr. Hazim J. Safi (Houston, Texas): Thank you, Dr. Connolly. You know, your contribution in the treatment of aortic surgery was tremendous. When I decided to go back to the drawing board to move away from the clamp-and-go technique, you were the only one saying clamp-and-sew was not the way to go. Your contribution is well appreciated because you were the first to insist on using distal aortic perfusion. So you have a tremendous contribution to your field.
With regard to the non-returning, we had 218. One of 3 came back. I think about 98 patients did not come back because they refused the second procedure. And of those patients who did not come back, their mortality rate was 18%, and half of the deaths were related to aortic rupture. That is the natural history of a patient with an aneurysm. Unfortunately, because of new HIPPA regulations, it was difficult to use the data for this presentation.
With regard to the endovascular question, it is applicable only in the descending thoracic aorta. It cannot be applied to the thoracoabdominal aorta. Currently, we do not use this procedure in the descending thoracic aorta, but we have hired an endovascular surgeon who was trained by Dr. Greg Sicard. He will join us in July. And we are going to start a program for the endovascular repair of the descending thoracic aneurysm.
With regard to your question on brain temperature, I think the tympanic temperature is closest to brain temperature. We don’t use it, though, becaue of the risk of tympanic membrane rupture and infection. I think in the ’40s there was classified work in Canada where they used chimpanzees by cooling them to a temperature less than 10 degrees centigrade to the brain. They found some kind of damage to the brain. So, hence, we don't cool below the 12 degrees centigrade and only cool until the EEG is isoelectric.
Dr. Larry H. Hollier (New Orleans, Louisiana): Beautiful paper, suburb results. I noticed that you had no incidence of neurologic definite paraplegia or paraparesis in this, unlike the other patients who did not have a combined elephant trunk procedure, those who had the same extensive amount of replacement of the aorta separate from an elephant trunk.
We have seen the same thing. We have not seen paraplegia in those who have second stage elephant trunks, even with open repair or with the endovascular replacement. And I would like your comments or thoughts about what possible difference there might be in these patients.
Dr. Hazim J. Safi (Houston, Texas): Thank you, Dr. Hollier. Also I would like to mention your contribution in protecting the spinal cord, which is the perioperative CSF drain. We combined Dr. Connolly and Dr. Hollier’s ideas to form the way we protect the spinal cord.
Really, I don't know. If you look at the gender in the ascending aorta, it is almost a 50:50 male to female ratio, while the descending thoracic aorta is 2:1 male to female ratio, and in the abdominal aorta, it is between 5 to 9:1. We think there may be a genetic component.
And they do well. They do better than ascending; they do better than descending. Our geneticist, Dr. Milewicz, one of those involved in isolating the gene responsible for Marfan syndrome, is obtaining blood and skin samples from patients in order to determine if there is a genetic predisposition to aneurysms. But I do not have an explanation.
Dr. Richard P. Cambria (Boston, Massachusetts): Dr. Safi, great series. I believe it is the largest series of elephant trunk repairs that I know of.
I have 2 questions. First, how about those non-returners? Are these folks who said, “No more, thanks?” And how does that influence your decision about how to stage these? Do you repair the larger of the 2 first?
Most of these patients will have an area in the aortic isthmus where clamping can be carried out, so one can typically or often decide to do one or the other first. And I would echo Dr. Connolly's comments, in some patients with extensive pathology where a potential stage 2 could be done with an endovascular graft, then stage 1 of the elephant trunk affords, of course, a very nice proximal seal zone for that repair.
Dr. Hazim J. Safi (Houston, Texas): Our philosophy with patients with extensive aneurysm of the aorta, which one to repair first, is the symptomatic portion, whether descending or ascending. If they are both asymptomatic, we like to go to front because the patient tolerates this procedure and recovers better.
I think it is an intriguing method to do the endovascular treatment for the patients with descending thoracic aorta while you do the ascending portions. This is an interesting concept and we hope to implement this in the next couple of months.
Footnotes
Reprints: Hazim J. Safi, MD, Department of Cardiothoracic and Vascular Surgery, University of Texas Medical School at Houston, 6410 Fannin Street, Suite 450, Houston, TX 77030. E-mail: Hazim.J.Safi@uth.tmc.edu.
REFERENCES
- 1.Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac Cardiovasc Surg. 1983;31:37–40. [DOI] [PubMed] [Google Scholar]
- 2.Estrera AL, Miller CC 3rd, Huynh TT, et al. Replacement of the ascending and transverse aortic arch: determinants of long-term survival. Ann Thorac Surg. 2002;74:1058–1064. [DOI] [PubMed] [Google Scholar]
- 3.Estrera AL, Miller CC 3rd, Huynh TT, et al. Neurologic outcome after thoracic and thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2001;72:1225–1231. [DOI] [PubMed] [Google Scholar]
- 4.Crawford ES, Coselli JS, Svensson LG, et al. Diffuse aneurysmal disease (chronic aortic dissection, Marfan, and mega aorta syndromes) and multiple aneurysm. Treatment by subtotal and total aortic replacement emphasizing the elephant trunk operation. Ann Surg. 1990;211:521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svensson LG. Rationale and technique for replacement of the ascending aorta, arch, and distal aorta using a modified elephant trunk procedure. J Card Surg. 1992;7:301–312. [DOI] [PubMed] [Google Scholar]
- 6.Heinemann MK, Buehner B, Jurmann MJ, et al. Use of the “elephant trunk technique” in aortic surgery. Ann Thorac Surg. 1995;60:2–7. [PubMed] [Google Scholar]
- 7.Coselli JS, Buket S, Djukanovic B. Aortic arch operation: current treatment and results. Ann Thorac Surg. 1995;59:19–27. [DOI] [PubMed] [Google Scholar]
- 8.Shiiya N, Yasuda K, Murashita T, et al. Application of the reversed elephant trunk technique during total thoracoabdominal replacement in patients with Marfan's syndrome. J Card Surg. 1998;13:56–59. [DOI] [PubMed] [Google Scholar]
- 9.Carrel T, Berdat P, Kipfer B, et al. The reversed and bidirectional elephant trunk technique in the treatment of complex aortic aneurysms. J Thorac Cardiovasc Surg. 2001;122:587–591. [DOI] [PubMed] [Google Scholar]
- 10.Zanetti PP. Replacement of the entire thoracic aorta according to the reversed Elephant Trunk technique. J Cardiovasc Surg (Torino). 2001;42:397–402. [PubMed] [Google Scholar]
- 11.Estrera AL, Miller CC 3rd, Porat EE, et al. Staged repair of extensive aortic aneurysms. Ann Thorac Surg. 2002;74(suppl):S1803–S1805; discussion S1825–S1832. [DOI] [PubMed]
- 12.Ito M, Tanaka T, Tamiya Y, et al. The elephant trunk technique for type A dissection. J Card Surg. 2000;15:163–166. [DOI] [PubMed] [Google Scholar]
- 13.Ando M, Takamoto S, Okita Y, et al. Elephant trunk procedure for surgical treatment of aortic dissection. Ann Thorac Surg. 1998;66:82–87. [DOI] [PubMed] [Google Scholar]
- 14.Mori Y, Hirose H, Takagi H, et al. Aortic arch repair for Stanford type A aortic dissection with distal anastomosis to the proximal level of the distal aortic arch. J Thorac Cardiovasc Surg. 2003;126:415–419. [DOI] [PubMed] [Google Scholar]
- 15.Takahara Y, Sudo Y, Mogi K, et al. Total aortic arch grafting for acute type A dissection: analysis of residual false lumen. Ann Thorac Surg. 2002;73:450–454. [DOI] [PubMed] [Google Scholar]
- 16.Safi HJ, Miller CC 3rd, Estrera AL, et al. Staged repair of extensive aortic aneurysms: morbidity and mortality in the elephant trunk technique. Circulation. 2001;104:2938–2942. [DOI] [PubMed] [Google Scholar]
- 17.Safi HJ, Miller CC 3rd, Iliopoulos DC, et al. Staged repair of extensive aortic aneurysm: improved neurologic outcome. Ann Surg. 1997;226:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schepens MA, Dossche KM, Morshuis WJ, et al. The elephant trunk technique: operative results in 100 consecutive patients. Eur J Cardiothorac Surg. 2002;21:276–281. [DOI] [PubMed] [Google Scholar]
- 19.Beaver TM, Martin TD. Single-stage transmediastinal replacement of the ascending, arch, and descending thoracic aorta. Ann Thorac Surg. 2001;72:1232–1238. [DOI] [PubMed] [Google Scholar]
- 20.Rokkas CK, Kouchoukos NT. Single-stage extensive replacement of the thoracic aorta: the arch-first technique. J Thorac Cardiovasc Surg. 1999;117:99–105. [DOI] [PubMed] [Google Scholar]
- 21.Safi HJ, Miller CC 3rd, Subramaniam MH, et al. Thoracic and thoracoabdominal aortic aneurysm repair using cardiopulmonary bypass, profound hypothermia, and circulatory arrest via left side of the chest incision. J Vasc Surg. 1998;28:591–598. [DOI] [PubMed] [Google Scholar]
- 22.Safi HJ, Brien HW, Winter JN, et al. Brain protection via cerebral retrograde perfusion during aortic arch aneurysm repair [see comments]. Ann Thorac Surg. 1993;56:270–276. [DOI] [PubMed] [Google Scholar]