Abstract
Objective:
To determine if chemotherapy offers a survival benefit to patients with large, high-grade, primary extremity liposarcoma.
Summary Background Data:
The impact of chemotherapy on the survival of patients with primary extremity soft tissue sarcoma is controversial and its effect on individual histologic subtypes is not defined.
Patient and Methods:
Two prospectively collected sarcoma databases were used to identify all patients with >5 cm, high-grade, primary extremity liposarcoma that underwent surgical treatment of cure from 1975 to 2003 (n = 245). Clinical, pathologic and treatment variables were analyzed for disease-specific survival (DSS), distant recurrence-free survival (DRFS) and local recurrence-free survival (LRFS).
Results:
Sixty-three (26%) patients were treated with ifosfamide based chemotherapy (IF), 83 (34%) with doxorubicin based chemotherapy (DOX) and 99 (40%) received no chemotherapy (NoC). To assess the impact of DOX, a contemporary cohort analysis of patients treated from 1975 to 1990 was performed. The 5 year DSS of the DOX treated patients was 64% (53%–74%) compared with 56% (51%–79%) for the NoC patients (log-rank P value = 0.28). To assess the impact of IF, a contemporary cohort analysis of patients treated from 1990 to 2003 was performed. The 5 year DSS of the IF treated patients was 92% (84%–100%) compared with 65% (51%–79%) for the NoC patients (log-rank P value = 0.0003). Independent prognostic factors for improved DSS were smaller size (HR = 0.7, P = 0.01), myxoid/round cell histologic subtype (HR = 0.3, P = 0.03) and treatment with IF (HR = 0.3, P = 0.01). The five-year DRFS of the IF treated patients was 81% (70%–92%) compared with 63% (50%–76%) for the NoC patients (log-rank P value = 0.02). The 5 year LRFS of the IF treated patients was 86% (76%–96%) compared with 87% (77%–97%) for the NoC patients (log-rank P value = 0.99).
Conclusions:
In patients with large, high-grade, primary extremity liposarcoma; DOX is not associated with improved DSS and IF is associated with an improved DSS. Treatment with IF should be considered in patients with high-risk primary extremity liposarcoma.
Two prospectively collected sarcoma databases were used to analyze the impact of chemotherapy on the survival of patients with high-risk, primary extremity liposarcoma. Doxorubicin based chemotherapy was not associated with an improved survival and ifosfamide based chemotherapy was associated with an improved survival compared to patients that received no chemotherapy.
Soft tissue sarcomas (STS) are a collection of histologically distinct neoplasms that are classified based on their type of mesenchymal tissue differentiation.1,2 Liposarcoma is the most common soft tissue sarcoma and accounts for at least 20% of all sarcomas in adults with the extremity being the most common site of primary disease.3–8 Classification of liposarcoma into 3 types, based on morphologic features and cytogenetic aberrations, is now widely accepted.9 These 3 types are: well-differentiated/de-differentiated,1 myxoid/round cell,2 and pleomorphic.3 The extent of differentiation, as reflected by histologic grade, remains the most important determinant of clinical course and of ultimate prognosis for patients with liposarcoma.9–17 Myxoid/round cell liposarcoma represents a morphologic continuum, and histologic grading is based on the extent of round cell component.10 Myxoid/round cell tumors with greater than 5% round cell component, dedifferentiated and pleomorphic liposarcoma are all considered high grade.10,11,14–19 The development of distant metastasis is the main determinant of survival for these higher grade histologic subtypes and is related to the size of the primary tumor as well as to the specific histologic subtype. For extremity liposarcoma >5 cm, distant metastasis may occur in 25% of dedifferentiated lesions, in 50% of myxoid/round cell lesions and in 75% of pleomorphic lesions.17 Although surgery and radiation therapy have achieved excellent local control and functional results for extremity liposarcoma, distant metastasis remains a difficult problem limiting survival.
Over the past 2 decades neoadjuvant/adjuvant chemotherapy has been used in an attempt to improve the outcome of patients with high-risk primary extremity STS. A meta-analysis of 14 randomized trials employing adjuvant doxorubicin based chemotherapy (DOX) found that DOX was associated with a significant (10%) improvement in recurrence-free survival but not associated with an improvement in overall survival.18 Ifosfamide based chemotherapy (IF) for patients with primary STS was introduced in the early 1990s as a promising treatment based on the responses generated in the treatment of metastatic disease.19–21 The few randomized trials performed using IF have come to differing conclusions and are limited in their impact due to small sample sizes, lack of stratification for histologic subtype, and inclusion of multiple nonextremity tumor sites.22–24
All of these studies were designed to examine impact of chemotherapy on a collection of many different histologies making it difficult to assess the potential benefit to a specific histology. Chemotherapy is now standard treatment of Ewing's sarcoma and rhabdomyosarcoma given their high response rates and improved survival with systemic therapy. For patients with primary extremity sarcoma of other histologies, such as liposarcoma, the impact of chemotherapy on survival remains controversial and has not been evaluated systematically. The objective of this study is to determine if chemotherapy offers a survival benefit to patients with high-risk primary extremity liposarcoma.
METHODS
The prospectively collected sarcoma databases from Memorial Sloan-Kettering Cancer Center (MSKCC) (1982–2003) and the University of California, Los Angeles (UCLA) (1975–2003) were used to identify all adult patients (≥16 years) with >5 cm, high-grade, deep, primary, extremity liposarcoma that underwent surgical treatment of cure. Clinical, pathologic, and treatment data were verified and analyzed with respect to local recurrence, distant recurrence and disease-specific survival. Clinical variables include age at diagnosis, sex and site. Upper extremity was defined as a tumor at, or distal to the shoulder. Lower extremity was defined as a tumor at, or distal to the groin or gluteal region.
Pathologic characteristics included histologic grade, tumor size, liposarcoma histologic subtype, and microscopic margins. Histologic grade was classified as low or high based on histologic subtype. Liposarcoma histologic subtype was assigned by the published criteria of the World Health Organization Classification of Tumors of Soft Tissue and Bone.25 Histologic subtype was classified as well-differentiated/dedifferentiated, myxoid/round cell or pleomorphic. Liposarcomas were considered high grade if they showed evidence of greater than 25% dedifferentiated morphology, greater than 5% round cell morphology, or pleomorphic morphology. Well-differentiated liposarcoma and pure myxoid liposarcoma were considered to be low-grade lesions and were not included in the present study. In patients that received neoadjuvant chemotherapy, tumor size was defined as maximum diameter measured by computed tomography or magnetic resonance imaging prior to treatment. In patients that received adjuvant chemotherapy or no chemotherapy, tumor size was defined as maximum diameter at pathologic analysis. Margin status was determined as part of the histopathologic assessment. Negative microscopic margins were defined as no tumor at the inked margin. Positive microscopic margins were defined as tumor at the inked margin.
Treatment modalities applied to the primary tumor were analyzed and included the surgical procedure, radiation therapy and neoadjuvant/adjuvant chemotherapy. All patients underwent complete surgical resection or amputation of their primary tumor at either MSKCC or UCLA. In patients treated from 1990–2003, radiation therapy was identified and included external-beam radiation or brachytherapy. Patients were grouped as either having received radiation therapy or not having received radiation therapy. Neoadjuvant/adjuvant chemotherapy was grouped into 3 treatment groups; patients who received no neoadjuvant/adjuvant chemotherapy for the primary tumor (NoC), patients who were treated with doxorubicin based chemotherapy (DOX), and patients who were treated with ifosfamide based chemotherapy (IF). The DOX treated patients were defined as patients that received doxorubicin chemotherapy (neoadjuvant or adjuvant) for the primary tumor either alone or in combination with other nonifosfamide containing regimens. IF treated patients were defined as patients that received ifosfamide chemotherapy (neoadjuvant or adjuvant) for the primary tumor either alone or in combination with other agents. Although the patients from UCLA were treated with neoadjuvant chemotherapy in a protocol manner, the treatments at both UCLA and MSKCC were nonrandomized. The neoadjuvant/adjuvant chemotherapy was ultimately administered on the basis of physician judgment and reflects the evolution of treatment at each institution.
The association of clinical, pathologic and treatment variables with disease specific survival (DSS) was examined. In performing comparisons among different groups, equivalent follow-up time is expected. Although the patients treated with NoC span the entire study period (1975–2003), the DOX patients were treated in a different decade (1975–1990) than the IF treated patients (1990–2003). A contemporary cohort of patients (n = 135, treated from 1975–1990) was used to examine the effect of DOX on DSS. During this time period, 83 patients were treated with DOX, 6 with IF and 46 received NoC. The 6 patients treated with IF were treated in 1990 and were excluded, resulting in 129 patients for the DOX cohort analysis. Since IF was first used to treat patients in 1990, a separate contemporary cohort of patients (n = 130, treated from 1990–2003) was used to examine the effect of IF on DSS, distant recurrence-free survival (DRFS) and local recurrence-free survival (LRFS). During this time period 63 patients were treated with IF, 4 with DOX and 63 received NoC. The 4 patients treated with DOX were treated in 1990 and were excluded, resulting in 126 patients for the IF cohort analysis.
Fisher exact test and Wilcoxon rank sum tests were used to examine the association of treatment with clinical and pathologic variables. DSS was defined as time from surgery date to death due to disease or to last follow-up. Time to DR was defined as time from surgery date to date of first DR or to last follow-up. Time to LR was defined as time from surgery date to date of first LR or to last follow-up. The association of the clinical, pathologic and treatment variables on these endpoints were examined using the log-rank test for categorical variables and score test for continuous variables. Two-year and 5-year estimates of DSS, DR, and LR and the corresponding 95% confidence intervals are reported after the estimates in parentheses. To examine the association of treatment on DSS, DR and LR while adjusting for important prognostic factors, treatment and those variables significant univariately at the 0.10 level were entered into a Cox proportional hazards model.
RESULTS
There were a total or 245 patients with >5 cm, high-grade, deep, primary extremity liposarcoma that underwent treatment of cure from 1975–2003. One hundred and thirty (53%) patients were treated at UCLA and 115 (47%) were treated at MSKCC. Eighty-three (34%) patients received DOX treatment from 1975 to 1990. Sixty-four (77%) received neoadjuvant treatment and 20 (23%) received adjuvant treatment. The DOX regimens used were: doxorubicin alone (n = 61, 74%), doxorubicin/methotrexate (n = 9, 11%), doxorubicin/cytoxan (n = 7, 8%), doxorubicin/cisplatin (n = 6, 7%). Ninety-nine (40%) received NoC and these patients span the entire study time from 1975 to 2003. Sixty-three (26%) patients received IF treatment from 1990 to 2003. The IF treated patients received a median of 4 cycles of ifosfamide at a median dose of 10 g/m2/cycle. Seventy-one percent (n = 45) received neoadjuvant treatment and 29% (n = 18) received adjuvant treatment. The IF regimens used were: ifosfamide/doxorubicin/cisplatin/mesna (n = 38, 60%), ifosfamide/doxorubicin/mesna (n = 20, 32%), and ifosfamide/doxorubicin/dacarabazine/mesna (n = 5, 8%).
1975–1990
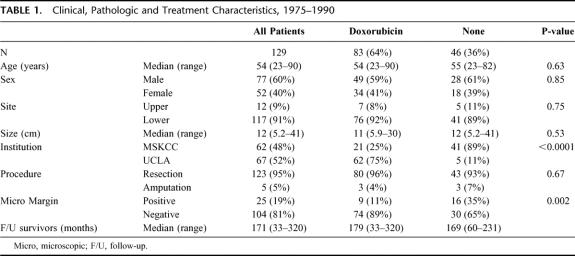
Clinical, pathologic and treatment variables of a contemporary cohort of 129 patients that underwent treatment of cure from 1975 to 1990 are listed in Table 1. Sixty-seven (52%) patients were treated at UCLA and 62 (48%) were treated at MSKCC. Eighty-three (64%) patients received DOX treatment and 46 (36%) NoC. The clinical, pathologic and treatment variables of each treatment group are also listed in Table 1. The median follow-up was 179 months (range = 33–320) for the DOX treated patients and 169 months (range = 60–231) for the NoC patients. The median tumor size was 11 cm (range = 5.9–30 cm) for the DOX treated patients and 12 cm (range = 5.2–41 cm) for the NoC patients. Seventy-five percent (n = 62) of the DOX patients were treated at UCLA and 25% (n = 21) at MSKCC. Eighty-nine percent (n = 41) of the NoC patients were treated at MSKCC and 11% (n = 5) at UCLA.
TABLE 1. Clinical, Pathologic and Treatment Characteristics, 1975–1990
Disease-Specific Survival
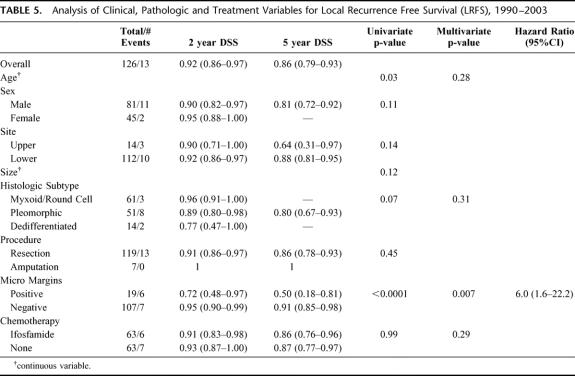
For all 129 patients, the overall DSS was 64% (56%–72%) at 5 years. Treatment with DOX was not significantly associated with DSS (P = 0.28). The five-year DSS of the DOX treated patients was 64% (53%–74%) compared with 56% (51%–79%) for the NoC patients (Fig. 1). Multivariate analysis also revealed that treatment with DOX was not associated with an improved DSS (P = 0.92).
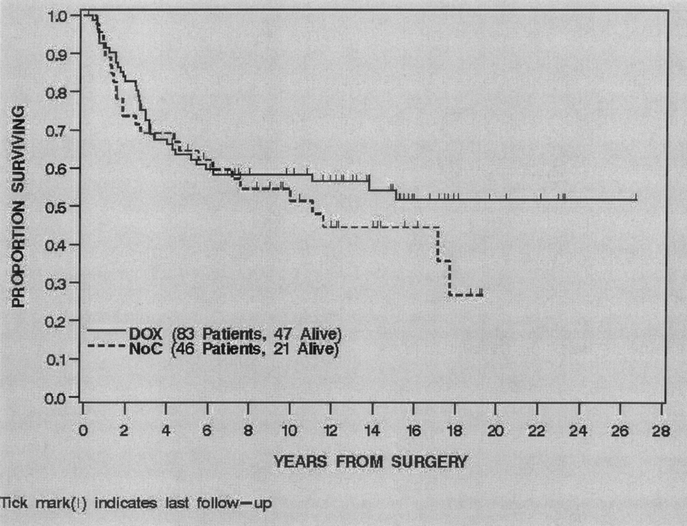
FIGURE 1. Disease specific survival by treatment, 1975–1990.
1990–2003
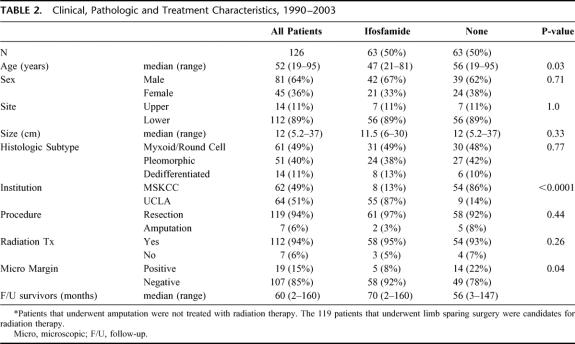
Clinical, pathologic and treatment variables of the contemporary cohort of 126 patients that underwent treatment of cure from 1990 to 2003 are listed in Table 2. Sixty-four (51%) patients were treated at UCLA and 62 (49%) were treated at MSKCC. Sixty-three (50%) patients received IF treatment and 63 (50%) received NoC. Ninety-four percent (112/119) of the patients that underwent limb-sparing surgery received adjuvant radiation therapy and 6% (7/119) did not. Of the 7 patients that did not receive radiation therapy, 4 received NoC and 3 received IF treatment.
TABLE 2. Clinical, Pathologic and Treatment Characteristics, 1990–2003
The clinical, pathologic and treatment variables of each treatment group are also listed in Table 2. The median follow-up was 70 months (range = 2–160) for the IF treated patients and 56 months (range = 3–147) for the NoC patients. The median tumor size was 11.5 cm (range = 6–30 cm) for the IF treated patients and 12cm (range = 5.2–37 cm) for the NoC patients. For the IF treated patients, the histologic subtypes were: myxoid/round cell (MR) n = 31 (49%), pleomorphic (PL) n = 24 (38%) and dedifferentiated (DD) n = 8 (13%). For the NoC patients, the histologic subtypes were: MR n = 30 (48%), PL n = 27 (42%) and DD n = 6 (10%). Eighty-seven percent (n = 55) of the IF patients were treated at UCLA and 13% (n = 8) at MSKCC. Eighty-six percent (n = 54) of the NoC patients were treated at MSKCC and 14% (n = 9) at UCLA.
Disease-Specific Survival
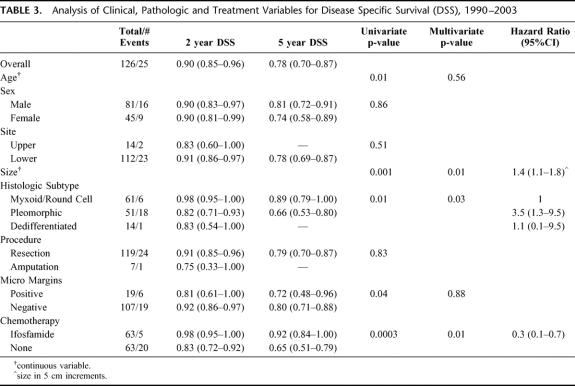
For all 126 patients, the overall DSS was 90% (85%–96%) at 2 years and 78% (70%–87%) at 5 years. Treatment with IF was significantly associated with DSS (P = 0.0003). The 2- and 5-year DSS of the IF treated patients was 98% (95%–100%) and 92% (84%–100%) compared with 83% (72%–92%) and 65% (51%–79%) for the NoC patients (Fig. 2). Univariate analysis of the clinical, pathologic and treatment variables prognostic for DSS revealed that younger age, smaller size, myxoid/round cell histologic subtype, negative microscopic margin and treatment with IF were associated with an improved DSS. Multivariate analysis revealed that smaller size (HR = 0.7 for 5–10cm compared with >10 cm, P = 0.01) myxoid/round cell histologic subtype (HR = 0.3 compared with pleomorphic, P = 0.03) and treatment with IF (HR = 0.3 compared with NoC, P = 0.01) were independently associated with an improved DSS (Table 3). The 5-year DSS of the ≤10 cm IF treated patients was 95% (86%–100%) compared with 81% (61%–100%) for the ≤10 cm NoC patients. The 5-year DSS of the >10 cm IF patients was 89% (78%–100%) compared with 58% (40%–75%) for the >10 cm NoC patients (Fig. 3). The 5-year DSS of the myxoid/round cell IF treated patients was 100% compared with 78% (57%–98%) for the myxoid/round cell NoC patients. The 5-year DSS of the pleomorphic IF treated patients was 83% (67%–98%) compared with 52% (32%–72%) for the pleomorphic NoC patients.
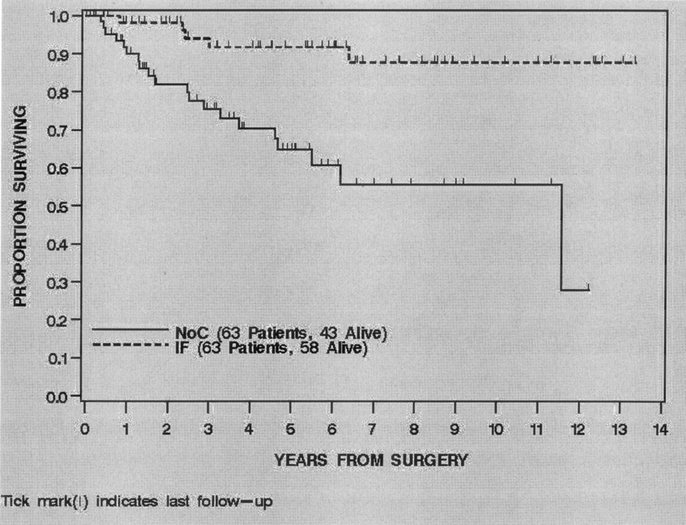
FIGURE 2. Disease specific survival by treatment, 1990–2003.
TABLE 3. Analysis of Clinical, Pathologic and Treatment Variables for Disease Specific Survival (DSS), 1990–2003
FIGURE 3. Disease specific survival by treatment and size category, 1990–2003.
Distant Recurrence
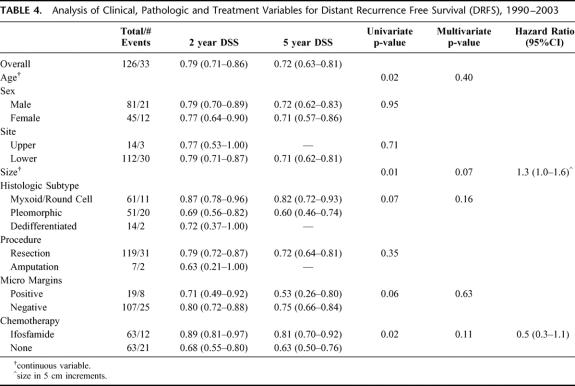
For all 126 patients, 33 (26%) developed a DR at last follow-up. Of the 33 DR's 23 (70%) were to the lung, 6 (18%) to abdomen/retroperitoneum, 4 (12%) to bone/soft tissue. The overall distant recurrence-free survival (DRFS) was 79% (71%–86%) at 2 years and 72% (63%–81%) at 5 years. Treatment with IF was significantly associated with DRFS (P = 0.02). The 2- and 5-year DRFS of the IF treated patients was 89% (81%–97%) and 81% (70%–92%) compared with 68% (55%–80%) and 63% (50%–76%) for the NoC patients (Fig. 4). Univariate analysis of the clinical, pathologic and treatment variables prognostic for DRFS revealed that younger age, smaller size and treatment with IF were associated with an improved DRFS. Multivariate analysis revealed that no variable was independently associated with an improved DRFS (Table 4).
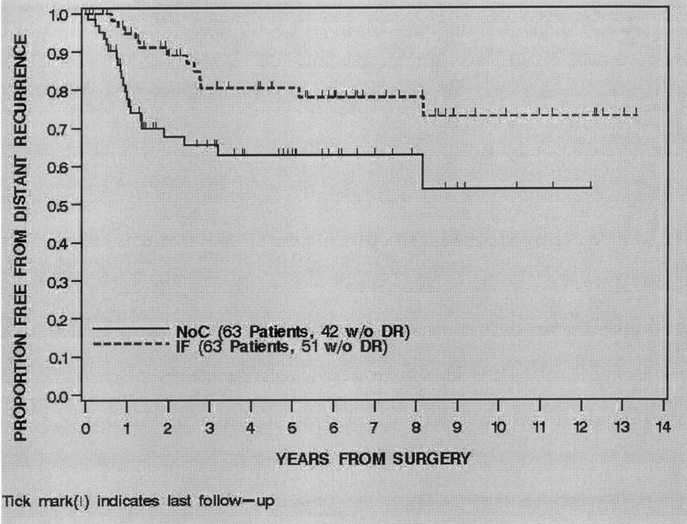
FIGURE 4. Distant recurrence free survival by treatment, 1990–2003.
TABLE 4. Analysis of Clinical, Pathologic and Treatment Variables for Distant Recurrence Free Survival (DRFS), 1990–2003
Local Recurrence
For all 126 patients, 13 (10%) developed a LR at last follow-up. The overall local recurrence-free survival (LRFS) was 92% (86%–97%) at 2 years and 86% (79%–93%) at 5 years. Treatment with IF was not associated with LRFS (P = 0.99). The 2- and 5-year LRFS of the IF treated patients was 91% (83%–98%) and 86% (76%–96%) compared with 93% (87%–100%) and 87% (77%–97%) for the NoC patients (Fig. 5). Univariate and multivariate analysis of the clinical, pathologic and treatment variables prognostic for LRFS revealed that a negative microscopic margin (HR = 0.16, P = 0.007) was the only variable independently associated with an improved LRFS (Table 5).
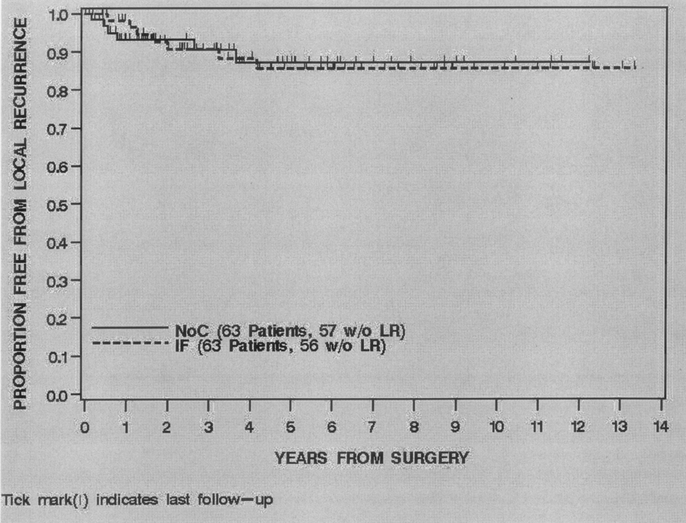
FIGURE 5. Local recurrence free survival by treatment, 1990–2003
TABLE 5. Analysis of Clinical, Pathologic and Treatment Variables for Local Recurrence Free Survival (LRFS), 1990–2003
DISCUSSION
Over the past several decades neoadjuvant/adjuvant chemotherapy has been used in an attempt to improve the outcome of patients with localized and locally advanced STS. These efforts have largely been focused on the treatment of patients with high-risk extremity STS as they have a significant risk of harboring subclinical micrometastasis at presentation. From the mid 1970s to the early 1990s doxorubicin based chemotherapy was the most widely used systemic treatment of adult STS. Its impact on patients with localized soft tissue sarcoma is well summarized by a meta-analysis of 1568 patients from 14 randomized trials comparing doxorubicin based chemotherapy to no chemotherapy.18 Although there was a significant (10%) improvement in recurrence-free survival for patients treated with doxorubicin based chemotherapy, there was only a 4% improvement in overall survival that was not statistically significant. A subset analysis of 886 patients with extremity STS found a slight (7%) but significant improvement in survival at 10 years for treated patients, however, this is subject to criticism due to a number of issues including the fact that it was an unplanned subset analysis.26
Ifosfamide based chemotherapy for patients with primary STS was introduced in the early 1990s as a promising treatment based on the responses generated in the treatment of metastatic disease.19–21 The few randomized controlled clinical trials performed using ifosfamide have come to differing conclusions and are limited in their impact due to small sample sizes, heterogeneity of histologic types and inclusion of multiple, nonextremity tumor sites.22–24 The most encouraging of these studies by Frustaci et al demonstrated a statistically significant survival benefit at 4 years in 53 patients treated with ifosfamide based chemotherapy.24 Although the 5-year overall survival benefit remains statistically significant at 7 years of follow-up, the overall survival in an intent to treat analysis is now no longer statistically significant.27 In addition, the control no chemotherapy arm had a much poorer survival than is typically found for high risk extremity lesions which may be explained by an imbalance in the 2 treatment arms with regard to histologic type and status at presentation. An additional, nonrandomized study by Eilber et al found that 125 patients with primary extremity STS who received protocol neoadjuvant ifosfamide based therapy had an increased pathologic response and improved overall survival compared with patients treated with nonifosfamide based protocols, however, the various protocols compared in this study were performed over different time periods.28 Although these studies suggest that ifosfamide based therapy offers a survival benefit to some high risk patients with primary extremity STS, the benefit may well be histology specific. Unfortunately, it is difficult to identify the potential benefit to a specific histology in studies, which group all histologic subtypes together without proper stratification.
To address some of these issues we chose to evaluate the association of chemotherapy with survival in the most common histologic subtype of STS, liposarcoma. All patients with >5 cm, high-grade, primary extremity liposarcoma (n = 245) were identified from the prospective sarcoma databases at MSKCC (1982–2003) and UCLA (1976–2003). Patients were treated with DOX (n = 83, 34%), IF (n = 63, 26%) and NoC (n = 99, 40%). Although the patients treated with NoC span the entire study period (1975–2003), the DOX patients were treated in a different decade (1975–1990) than the IF treated patients (1990–2003). To accurately assess the impact of treatment with DOX and IF, 2 separate contemporary cohort analyses were performed.
A cohort of patients treated from 1975–1990 was used to analyze the impact of DOX on DSS. Of the 129 patients identified, there was a similar number of patients treated at MSKCC (n = 62, 48%) and at UCLA (n = 67, 52%). Eighty-three (64%) patients received treatment with DOX and 46 (36%) with NoC. The clinical and pathologic characteristics of the DOX treated patients, including size, were very similar to the NoC treated patients. The major difference between these treatment groups was institutional treatment. The majority of patients from UCLA were treated with DOX (n = 62, 75%) and the majority of patients from MSKCC were treated with NoC (n = 41, 89%). With a median follow-up of over 14 years for survivors, treatment with DOX was not found to be significantly associated with DSS either on univariate or multivariate analysis.
A cohort of patients treated from 1990–2003 was used to analyze the impact of IF on DSS. Of the 126 patients identified, there was a similar number of patients treated at MSKCC (n = 62, 49%) and at UCLA (n = 64, 51%). Sixty-three (50%) patients received treatment with IF and 63 (50%) with NoC. The clinical and pathologic characteristics of the IF treated patients, including size and histologic subtype, were very similar to the NoC treated patients. Again, the major difference between these treatment groups was institutional treatment. The majority of patients from UCLA were treated with IF (n = 55, 87%) and the majority of patients from MSKCC were treated with NoC (n = 54, 86%).
With a median follow-up of 5 years for survivors, treatment with IF was found to be significantly associated with DSS (P = 0.0003). The 5-year DSS was 92% (84%–100%) in the IF treated patients and 65% (51%–79%) in the NoC patients. By multivariate analysis smaller size, myxoid/round cell histologic subtype, and treatment with IF were independently associated with an improved DSS. Patients that did not receive IF had a 3-fold increased risk of death from disease compared with patients that received IF. In addition, patients with pleomorphic liposarcoma had a 4-fold increased risk of death from disease compared with patients with the myxoid/round cell subtype.
Additional analyses were performed to determine if there was a tumor size range and/or histologic subtype that benefited most from treatment with IF. Although there appears to be a modest (14%) survival benefit at 5 years for ≤10 cm IF treated patients, there was a 31% survival benefit at 5 years for >10 cm IF treated patients. The 5-year DSS for >10 cm IF treated patients was 89% (78%–100%) compared with 58% (40%–75%) for >10 cm NoC patients (Fig. 3). Interestingly, both myxoid/round cell and pleomorphic histologic subtypes benefited from treatment with IF. There was a 22% survival benefit at 5 years for myxoid/round cell IF treated patients and a 31% survival benefit at 5 years for pleomorphic IF treated patients.
Both DR and LR were analyzed in the 126 patients treated from 1990–2003. At last follow-up, 26% developed a DR with most of DRs being in the lung (70%). As with DSS, treatment with IF was also significantly associated with DRFS (P = 0.02). However, by multivariate analysis no variable was independently associated with an improved DRFS. With 10% of the patients having developed a LR at last follow-up, treatment with IF was not associated with an improved LRFS and a negative microscopic margin was the only variable independently associated with an improved LRFS.
With no impact on local control and an improvement in DRFS, the significant improvement in DSS associated with IF is likely due to the treatment of subclinical systemic disease. Whether IF treatment has eliminated subclinical systemic disease preventing the development of distant recurrences or whether it has just significantly slowed its growth delaying the development of distant recurrences will be determined by longer follow-up. Although there was a significant improvement in DRFS for IF treated patients when examined univariately, this association did not hold up on multivariate analysis suggesting that some patients may only experience a delay in their disease process.
The obvious and most significant limitation of this study is that it is a retrospective cohort study, not a randomized trial. Although the patients from UCLA were treated with neoadjuvant chemotherapy in a protocol manner, the treatments at both UCLA and MSKCC were nonrandomized. The neoadjuvant/adjuvant chemotherapy was ultimately administered on the basis of physician judgment and reflects the evolution of treatment at each institution. The fact that a median of 8 patients per year with the most common, high risk, primary extremity soft tissue sarcoma are being treated at UCLA and MSKCC combined, demonstrates how rare these tumors are and how difficult it would be to perform a histology specific randomized trial in STS. Despite this difficulty, the importance of identifying histology specific treatment is becoming increasingly important in the current era where STS are being classified and treated based on their molecular and genetic characteristics.9,10,29–31 Until a national or international multicenter effort can be organized to accrue a sufficient numbers of patients to perform a histology specific randomized trial, the prospectively collected sarcoma databases from such institutions will provide the best data to estimate survival benefit from neoadjuvant/adjuvant systemic treatment.
In summary, this analysis of patients with high-risk, primary, extremity liposarcomas found that doxorubicin based chemotherapy was not associated with an improved DSS compared with patients that received no chemotherapy. Ifosfamide based chemotherapy was associated with an improved DSS compared with patients that received no chemotherapy. The association of ifosfamide based therapy with an improved DSS was strongest in patients with tumors >10 cm in size. Treatment with IF should be considered in patients with large, high grade, primary, extremity liposarcoma.
Discussions
Dr. Raphael E. Pollock (Houston, Texas): Thank you for the opportunity to discuss this very thought-provoking paper. The investigators have sought to shed light on a very rare tumor, liposarcoma of the extremity, using the pooled databases from two major sarcoma centers, Memorial Sloan-Kettering and UCLA.
The authors correctly allude to the difficulties in doing this—different institutions, different surgical oncologists, different pathologists, different medical oncology regimens, the rarity of the disease; indeed, different eras of treatment. But these caveats notwithstanding, the researchers still sought to determine the impact of 2 important drugs in this disease, doxorubicin and ifosfamide that are commonly used in its treatment.
This leads to 2 questions. One, it is known that the total dose of doxorubicin that can be administered to patients depends on the mode of administration. For example, nearly twice as much doxorubicin can be given to a specific patient if it is given as a 96-hour continuous infusion rather than as a 3-hour bolus given sequentially over several days.
Also it is known that the doxorubicin mediated tumor cytotoxicity appears to be a dose-related phenomenon. And for this reason, before we conclude that doxorubicin is less efficacious than ifosfamide in this disease, we have to look a little more specifically at the mode of administration and how much total doxorubicin was given to patients in the study itself. In the absence of such information, it is quite possible that what we are really observing is that an otherwise potent drug, doxorubicin, failed to demonstrate efficacy simply because it was not administered in an optimal manner.
The second question focuses on the 2 groups of treatments that were under study. All patients in the ifosfamide group also received doxorubicin whereas none of the patients in the doxorubicin group received ifosfamide. So it may be specious to analyze the patients as doxorubicin versus ifosfamide comparison and more accurate to categorize the patients as doxorubicin plus something versus doxorubicin plus ifosfamide.
If that is the case, then it is difficult to ascribe the improved disease specific survival to ifosfamide per se, and an alternative explanation could be that ifosfamide, to be effective, must be given with doxorubicin, or vice versa, ie, that the 2 drugs must be used in combination. In the absence of an ifosfamide alone or ifosfamide without doxorubicin treatment group, it is hard to draw the specific conclusion that ifosfamide is more efficacious than doxorubicin.
Dr. Frederick C. Eilber (New York, New York): As far as the doses of the doxorubicin based therapy for the earlier cohort, we did not look at that once we found that there was no impact on survival. We do know, based on a randomized trial from UCLA, that if you gave the doxorubicin preoperatively and compared that to a group that got it pre-op and postoperatively, there was no difference in outcome.
As far as there being some synergy between ifosfamide and doxorubicin, it is possible. However, when we looked at the evolution of the protocols, particularly at UCLA, the increase in survival and response rate did not occur at all until ifosfamide was added. The way I carefully defined the ifosfamide based chemotherapy group was to include the possibility that there could be doxorubicin in that group. So yes, there could be a synergy; however, it is our feeling, having carefully studied the evolution of treatment, that it is predominantly an ifosfamide effect.
Dr. Harold J. Wanebo (Providence, Rhode Island): The Eilber family and Drs. Brennan and Singer have demonstrated in a retrospective review of a prospectively acquired database at two different institutions that the disease specific survival is augmented by adjuvant ifosfamide based therapy compared to adriamycin based therapy. Although there are many questions about the study, it is a very interesting finding.
Adriamycin has been classically associated with treatment of this disease, but it is frequently combined with ifosfamide such as in the MAID combination chemotherapy. One of my questions is to ask whether there is data, perhaps from metastatic disease, to suggest that adriamycin is perhaps less active in sarcomas and is there any comparison with ifosfamide in that setting per se? Or is liposarcoma rather unique in its response to ifosfamide?
I think, as was pointed out by the other commentators, that in this study you really are comparing ifosfamide, which appears to be in combination with adriamycin versus adriamycin. You have raised an interesting question in your study of adjuvant chemotherapy for liposarcoma. Do you propose a clinical trail to really confirm this? Perhaps such a clinical trial might include one arm containing ifosfamide, perhaps in combination with adriamycin and the other arm adriamycin alone. I would be interested in your comments on that.
Dr. Frederick C. Eilber (New York, New York): I think an interesting point within this study, which I did not touch on, is that this is a rare tumor. There is a median of 8 patients per year from both institutions combined. And as the understanding of soft tissue sarcoma progresses, it is becoming clear that they are indeed very different tumors. To do a histology specific randomized trial with 8 patients a year at 2 of the busiest institutions clearly is a challenging feat. To overcome that, we chose to use these combined databases to get information about which we should and should not be treating with chemotherapy. So ideally, yes, I would love to do a randomized trial. Is it feasible? Probably not.
As far as a comparison of doxorubicin and ifosfamide in the metastatic setting, I am not aware of a head-to-head comparison. I know that the rationale for using high dose ifosfamide in the primary extremity setting was based on significant responses generated in the metastatic setting, and that these responses are seen to a greater degree in certain histologies. So we chose to focus on one histology, the most common, to see if we could come up with an answer as to whether or not there was an impact. And you see the results of that effort.
Dr. James E. Goodnight, Jr. (Sacramento, California): Given the institutions involved and the individual surgeons, this is a rather remarkable study. I can't imagine it being repeated. Having a 90% 10-year survival in high-grade, large extremity sarcomas is a remarkable feat no matter how it is achieved. That is amazing.
Given that survival was the endpoint of the study, was the incidence of resection for pulmonary metastases the same in both groups, or actually all three groups? The other question would be whether you had deaths from congestive heart failure from the use of the doxorubicin.
Dr. Frederick C. Eilber (New York, New York): There was a significant improvement in distant recurrence-free survival. I think it is a very interesting question what impact did the high dose ifosfamide have on the outcomes of patients that developed distant recurrences.
I don't actually know the answer to whether they were more resectable or not. I know that fewer patients got distant recurrences with the high dose ifosfamide and when they got a distant recurrence fewer of them were dying of their distant recurrences. Now, whether that means that ifosfamide based chemotherapy wiped out small volume micrometastatic disease and made the patients with larger volume micrometastatic disease more treatable or whether it is delaying the disease process to a point that we can't see right now is question for additional follow-up. I think that is an important point and that additional follow-up is needed in this group. But my suspicion is that ifosfamide based chemotherapy is treating micrometastatic disease and is in fact making distant recurrences more treatable when they occur.
Dr. Jonathan L. Meakins (Oxford, England): Is it possible that we are trapped by the rules of evidence that demand always level 1 evidence being exclusively a randomized control trial?
What struck me about this paper is that it is taking a very rare tumor and in a very imaginative manner trying to answer an important question about its global management. A randomized control trial simply is not possible in this setting. To get clinicians of radically different opinions about management to participate in a trial of this sort would be very difficult. So is it time to rethink some of the rules of evidence as they apply to the way in which we look after patients? This presentation is a very creative way of getting evidence otherwise unobtainable that should help us to move forward. I wonder if perhaps there aren't other study designs we should think about that would be equivalent to level 1 or 2 evidence, rather than to demand always a randomized control trial.
Dr. Frederick C. Eilber (New York, New York): I think that is a very good point and very applicable to rare tumors such as sarcoma. As I mentioned, soft tissue sarcomas are getting sub-classified as we are learning how different they are—such as gastrointestinal stromal tumor. In the future, it is going to be difficult to lump these very different tumors together.
The only way that this kind of study can be done is if the databases are kept. So if people have well-kept prospective databases such as these, then, yes, evidence like this, I think, can be used. But if you don't have databases such as these, then this cannot be done. So I think it speaks to the importance of having such databases.
Footnotes
Supported by NIH Program Project Grant P01CA47179 (MFB) and Kristen Ann Carr Fellowship (FCE).
Reprints: Samuel Singer, MD, Memorial Sloan-Kettering Cancer Center, Department of Surgery, 1275 York Avenue, New York, NY 10021. E-mail: singers@mskcc.org.
REFERENCES
- 1.Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg. 1996;33:817–872. [PubMed] [Google Scholar]
- 2.Eilber FC, Eilber FR. Soft tissue sarcoma. In: Cameron ed. Current Surgical Therapy. Seventh Edition. St. Louis, MS, Mosby 2001;1213–1218. [Google Scholar]
- 3.Linehan DC, Lewis JJ, Leung D, et al. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol. 2000;18:1637–1643. [DOI] [PubMed] [Google Scholar]
- 4.Singer S, Corson JM, Gonin R, et al. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg. 1994;219:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisters PWT, Leung DHY, Woodruff J, et al. Analysis of prognostic factors in 1041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JJ, Leung D, Casper ES, et al. Multifactorial analysis of long-term follow-up (more than 5 years) of primary extremity sarcoma. Arch Surg. 1999;134:190–194. [DOI] [PubMed] [Google Scholar]
- 7.Eilber FC, Rosen G, Nelson S, et al. High grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koea JB, Leung D, Lewis JJ, et al. Histopathologic type: an independent prognostic factor in primary soft tissue sarcoma of the extremity? Ann Surg Oncol. 2003;10:432–440. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher CM, Akerman P, Cin I, et al. Correlation between clinicopathological features and karyotype in lipomatous tumors. A report of 178 cases from the Chromosomes and Morphology (CHAMP) collaborative study group. Am J Pathol. 1996;148:623–630. [PMC free article] [PubMed] [Google Scholar]
- 10.Antonescu CR, Tschernyavsky SJ, Decuseara R, et al. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7:3977–3987. [PubMed] [Google Scholar]
- 11.Gebhard S, Coindre JM, Michels JJ, et al. Pleomorphic liposarcoma: clinicopathologic, immunohistochemical, and follow-up analysis of 63 cases: a study from the French Federation of Cancer Centers Sarcoma Group. Am J Surg Pathol. 2002;26:601–616. [DOI] [PubMed] [Google Scholar]
- 12.Chang HR, Hajdu SI, Collin C, et al. The prognostic value of histologic subtypes in primary extremity liposarcoma. Cancer. 1989;64:1514–1520. [DOI] [PubMed] [Google Scholar]
- 13.Chang HR, Gaynor J, Tan C, et al. Multifactorial analysis of survival in primary extremity liposarcoma. World J Surg. 1990;14:610–618. [DOI] [PubMed] [Google Scholar]
- 14.Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henricks WH, Chu YC, Goldblum JR, et al. Dedifferentiated liposarcoma: a clinicopathologic analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Path. 1997;21:271–281. [DOI] [PubMed] [Google Scholar]
- 16.McCormick D, Mentzel T, Beham A, et al. Dedifferentiated liposarcoma: clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994;18:1213–1223. [DOI] [PubMed] [Google Scholar]
- 17.Dei Tos, AP. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol. 2000;4:252–266. [DOI] [PubMed] [Google Scholar]
- 18.Adjuvant chemotherapy for localized resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350:1647–1654. [PubMed] [Google Scholar]
- 19.Antman KH, Ryan L, Elias A, et al. Response to ifosfamide and mesna: 124 previously treated patients with metastatic or unresectable sarcoma. J Clin Oncol. 1989;7:126–131. [DOI] [PubMed] [Google Scholar]
- 20.Antman KH. Chemotherapy of advanced sarcomas of bone and soft tissue. Semin Oncol. 1992;19:13–20. [PubMed] [Google Scholar]
- 21.Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol. 1993;11:1276–1285.8315425 [Google Scholar]
- 22.Brodowicz T, Schwameis E, Widder J, et al. Intensified adjuvant IFADIC chemotherapy for adult soft tissue sarcoma: a prospective randomized feasibility trial. Sarcoma. 2000;4:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for “high-risk” adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–1103. [DOI] [PubMed] [Google Scholar]
- 24.Frustaci S, Gherlinzoni F, DePaoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcoma of the extremity and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19:1238–1247. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher C, Unni K, Mertens F, et al. Pathology and genetics of tumors of soft tissue and bone. In: Kleihues P, Sobin L, eds. World Health Organization Classification of Tumors, Vol. 4. Lyon, France: International Agency for Research on Cancer Press;2002:427 [Google Scholar]
- 26.Verweij J, Seynaeve C. The reason for confining the use of adjuvant chemotherapy in soft tissue sarcoma to the investigational setting. Semin Radiat Oncol. 1999;9:352–359. [DOI] [PubMed] [Google Scholar]
- 27.Frustaci S, De Paoli A, Bidoli E, et al. Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology. 2003;65:80–84. [DOI] [PubMed] [Google Scholar]
- 28.Eilber FC, Rosen G, Eckardt J, et al. Treatment induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203–3209. [DOI] [PubMed] [Google Scholar]
- 29.Antonescu CR, Elahi A, Healy JH, et al. Monoclonality of multifocal myxoid liposarcoma: confirmation by analysis of TLS-CHOP or EWS-CHOP rearrangements. Clin Cancer Res. 2000;6:2788–2793. [PubMed] [Google Scholar]
- 30.Demitri GD, Fletcher CDM, Mueller, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci. 1999;96:3951–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tontonoz P, Singer S, Forman BM, et al. Terminal differentiation of human liposarcoma cells induced by ligand for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci. 1997;94:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]