Abstract
Objective:
To assess the trends in perioperative outcome of hepatectomy for hepatobiliary diseases.
Methods:
Data of 1222 consecutive patients who underwent hepatectomy for hepatobiliary diseases from July 1989 to June 2003 in a tertiary institution were collected prospectively. Perioperative outcome of patients in the first (group I) and second (group II) halves of this period was compared. Factors associated with morbidity and mortality were analyzed.
Results:
Diagnoses included hepatocellular carcinoma (n = 734), other liver cancers (n = 257), extrahepatic biliary malignancies (n = 43), hepatolithiasis (n = 101), benign liver tumors (n = 61), and other diseases (n = 26). The majority of patients (61.8%) underwent major hepatectomy of ≥3 segments. The overall hospital mortality and morbidity were 4.9% and 32.4%, respectively. The number of hepatectomies increased from 402 in group I to 820 in group II, partly as a result of more liberal patient selection. Group II had more elderly patients (P = 0.006), more patients with comorbid illnesses (P = 0.001), and significantly worse liver function. Nonetheless, group II had lower blood loss (median 750 versus 1450 mL, P < 0.001), perioperative transfusion (17.3% versus 67.7%, P < 0.001), morbidity (30.0% versus 37.3%, P = 0.012), and hospital mortality (3.7% versus 7.5%, P = 0.004). On multivariate analysis, hypoalbuminemia, thrombocytopenia, elevated serum creatinine, major hepatic resection, and transfusion were the significant predictors of hospital mortality, whereas concomitant extrahepatic procedure, thrombocytopenia, and transfusion were the predictors of morbidity.
Conclusions:
Perioperative outcome has improved despite extending the indication of hepatectomy to more high-risk patients. The role of hepatectomy in the management of hepatobiliary diseases can be expanded. Reduced perioperative transfusion is the main contributory factor for improved outcome.
This study on perioperative outcome of 1222 consecutive patients with hepatectomy from July 1989 to June 2003 in a tertiary institution demonstrated significantly reduced operative morbidity and hospital mortality in the second half of the study period, despite increased proportion of patients with impaired liver function, advanced age, and comorbid illness. The results suggest that the role of hepatic resection in the management of benign and malignant hepatobiliary diseases can be extended to more high-risk patients. Reduced perioperative blood transfusion was identified to be a major factor in the improved outcome.
Evolution of surgical techniques in partial hepatectomy has enabled the procedure to be performed with operative mortality rate of less than 5% in high-volume centers in recent years.1–5 Before the 1980s, hepatic resection was typically associated with a mortality rate of greater than 10%.6–9 Better understanding of the segmental liver anatomy and refined surgical techniques in controlling hemorrhage are the 2 most important factors that have contributed to the improved perioperative outcome of hepatectomy.5,10 Another important factor is the better selection of patients in terms of liver function reserve and comorbid conditions, which helps to reduce mortality from liver failure and other severe postoperative complications such as pneumonia.3,11,12 Finally, the concentration of hepatic resection in experienced hepatobiliary centers is also a critical factor. Recent studies from the United States have demonstrated that a high hospital mortality rate of around 10% is still being observed in low-volume hospitals, whereas the hospital mortality rate in high-volume centers is less than 5%.13,14
The improved safety of hepatic resection has led to the broadening of the indications of hepatectomy in patients with normal liver.3,4 Partial hepatectomy is now performed more frequently in good-risk patients with conditions associated with normal liver, such as colorectal liver metastasis.15 The improved safety of hepatic resection has also led to a more liberal application of the procedure in patients with benign tumors.16 With the enhanced safety, some surgeons have advocated hepatic resection for some noncolorectal liver metastases even though the oncological benefit remains unclear.17,18 However, the role of hepatic resection in patients with chronic liver disease is still considered limited because of the associated increase in operative mortality and morbidity, especially when major hepatic resection is required.14,19,20 Advanced age is considered another limiting factor as several recent studies have reported that elderly patients had significantly increased operative morbidity and mortality.4,14,20 It remains to be demonstrated that the role of hepatic resection can be extended to such high-risk patients with favorable perioperative outcome. The current study analyzes the trend of perioperative outcome of 1222 consecutive patients with hepatectomy for various benign or malignant hepatobiliary diseases in a specialized hepatobiliary center over a 14-year period, with a particular reference to the prevalence of underlying risk conditions such as impaired liver function reserve, advanced age, and presence of comorbid illnesses.
PATIENTS AND METHODS
During a 14-year period from July 1, 1989, to June 30, 2003, 1222 consecutive patients underwent elective hepatic resection for benign or malignant hepatobiliary diseases at the Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, Hong Kong, China. One hundred sixty-three living donors who underwent donor hepatic resection for liver transplantation during the study period were not included in this study. The hepatectomies were performed by a surgical team specialized in hepatobiliary surgery.
Preoperative Assessment
All patients had ultrasonography and contrast computed tomography (CT) scan or magnetic resonance imaging to evaluate the liver or biliary pathology. In patients with biliary obstruction, preoperative biliary drainage of the liver remnant to be preserved was performed endoscopically or percutaneously. Assessment of liver function was based on Child's classification, liver biochemistry, and coagulation profile. In patients with hepatocellular carcinoma (HCC), indocyanine green (ICG) clearance test was routinely performed because of the high incidence of associated chronic liver disease. This test was not performed in patients with other hepatobiliary diseases associated with normal liver. Before 1997, an ICG retention at 15 minutes (ICGR-15) of 14% was considered the safety limit of major hepatic resection, defined as resection of 3 or more Couinaud liver segments, in cirrhotic patients.21 In the more recent years, we have extended the limit of ICGR-15 for major hepatic resection to 20%.22 In the recent 2 years, CT volumetry was used to aid assessment of liver function reserve, and right portal vein embolization was performed in selected patients with a small liver remnant undergoing right or extended right hepatectomy.
Operative Techniques
Patients undergoing hepatectomy were generally explored through a bilateral subcostal incision with vertical midline extension. In selected patients with a large right lobe tumor or a small tumor located at the superior and posterior part of the right liver, a thoracoabdominal approach was used.23 Since June 1994, staging laparoscopy and laparoscopic ultrasound have been used with increasing frequency to assess the extent of tumors and to assess the severity of any cirrhosis and size of liver remnant.24 After laparotomy, intraoperative ultrasound was routinely performed to detect any lesions in the contralateral lobe, any tumor invasion of portal vein or hepatic veins, and to define the relationship between the tumor and major intrahepatic vessels.
Parenchymal transection was performed using the finger-fracture technique between 1989 and 1992 and thereafter using an ultrasonic dissector. In some cases of right hepatectomy for a large tumor that made mobilization of the right lobe difficult, parenchymal transection was performed without premobilization of the liver and extrahepatic control of the right hepatic vein (anterior approach).25 The hepatic duct was isolated and ligated or sutured at the time of hepatic transection. Since 1998, we have routinely employed an endoscopic vascular linear cutter (Ethicon Endo-surgery, Cincinnati, OH) to divide hepatic veins extrahepatically or during hepatic transection. Before 1998, intermittent Pringle maneuver was used frequently.26 However, with increased experience in recent years, Pringle maneuver was used only when significant bleeding was encountered. Instead, more attention was paid to lowering of the central venous pressure to below 5 cm H2O to reduce venous bleeding during transection. Total vascular exclusion was not performed in any patients. Meticulous attention was paid to the preservation of function in the remnant liver by avoiding prolonged rotation, hypoxic injury, or venous congestion due to overloading of circulation. After transection, bile leakage test was performed using methylene blue injection via a cystic duct cannula, and any leakage site was carefully repaired with fine sutures. Blood transfusion was initiated when the hemoglobin level fell to below 8 g/dL. We did not use fresh frozen plasma during or after operation unless there was a severe bleeding tendency. Before 1998, an abdominal drain was placed almost routinely after hepatic resection. Recently, no drain was used even in patients with chronic liver disease after a randomized trial demonstrated a higher morbidity with the use of abdominal drainage.27
Postoperative Care
All patients with major hepatic resection were monitored in the intensive care unit in the immediate postoperative period. The need for postoperative mechanical ventilation was determined by the anesthetists. Pain control was provided by continuous intravenous morphine infusion if the patient needed mechanical ventilation; otherwise, pain was controlled by on demand intravenous meperidine injection before 1994 and patient-controlled intravenous infusion of morphine after 1994. A broad-spectrum antibiotic was given for at least 3 days, and intravenous albumin was given for cirrhotic patients with hypoalbuminemia. In selected patients, a Broviac catheter was inserted at the end of the operation for postoperative parenteral nutrition. A formula enriched with branched-chain amino acids and medium-chain triglyceride was given for 5 to 7 days after operation.28 Since the demonstration of the benefit of parenteral nutrition in reducing postoperative morbidity in a randomized controlled trial published in 1994,28 postoperative parenteral nutrition became a routine practice for all patients with cirrhosis undergoing major hepatic resection. Oral feeding was started when the bowel sounds returned.
Data Collection and Analysis
To analyze the trends in perioperative variables and outcome of hepatectomy in our institution over the 14-year study period, patients operated in the first half (July 1989 to June 1996, group I) were compared with those operated in the second half (July 1996 to June 2003, group II) of the study period. Pre-, intra-, and postoperative data were recorded prospectively in a computerized database established since 1989. Histopathological data including status of underlying liver (normal, chronic hepatitis, cirrhosis) were also collected. The types of hepatic resection were described according to the terminology recommended by the International Hepato-Pancreato-Biliary Association.29 Concomitant procedure was defined as any extrahepatic organ resection, portal lymphadenectomy, or extrahepatic biliary resection and reconstruction. Thoracotomy and cholecystectomy were not considered as additional extrahepatic procedures. Hospital mortality was defined as any death that occurred during the same hospital admission for the hepatic resection.
Continuous variables were expressed as median and interquartile range and compared using Mann-Whitney U test. Categorical variables were compared by the χ2 test with Yates’ correction or Fisher exact test where appropriate. Multivariate analyses of risk factors of morbidity and mortality were performed using a binary logistic regression model. All statistical analyses were performed using statistical software (SPSS 9.05 for Windows, SPSS, Inc, Chicago, IL). Results were considered significant at P < 0.05.
RESULTS
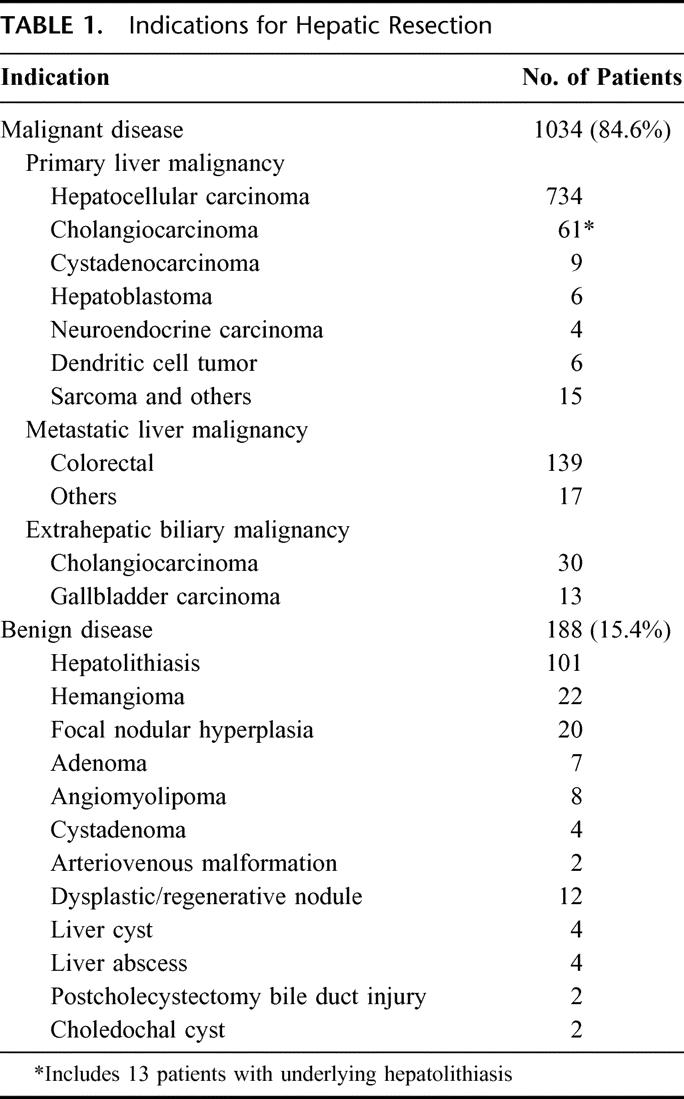
During the study period, 854 men and 368 women underwent hepatectomy for benign or malignant hepatobiliary diseases (Table 1). The most common indication was HCC, which accounted for 60% of all hepatectomies. The most common benign indication was hepatolithiasis. The number of hepatic resection had increased from 402 in the first half (group I) to 820 in the second half (group II) of the study period.
TABLE 1. Indications for Hepatic Resection
Preoperative Variables
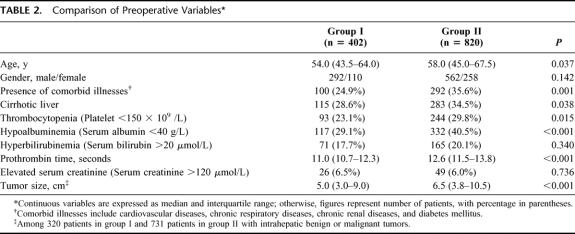
Table 2 shows a comparison of the preoperative variables between the 2 groups of patients. Group II patients were significantly older than group I patients. The proportion of patients >70 years old was higher in group II (n = 120, 14.6%) than in group I (n = 36, 9.0%) (P = 0.006). The oldest patients who underwent hepatectomy in group I and group II aged 82 and 88 years, respectively. Group II patients also had a significantly higher proportion of comorbid medical conditions. The most common comorbid condition was cardiovascular disease (n = 54 in group I, 192 in group II), followed by diabetes mellitus (n = 40 in group I, 96 in group II), chronic respiratory disease (n = 24 in group I, 58 in group II), and chronic renal disease (n = 8 in group I, 30 in group II). There was a significant increase in the prevalence of cardiovascular disease in group II compared with group I (23.4% versus 13.4%, P < 0.001). The prevalence of other comorbid conditions has also increased but the differences between the 2 groups were not statistically significant.
TABLE 2. Comparison of Preoperative Variables
There was a significantly higher proportion of cirrhosis, worse liver function in terms of serum albumin and prothrombin time, and a higher frequency of thrombocytopenia in group II. Among patients with noncirrhotic liver, 90 patients (22.4%) in group I and 236 patients (28.8%) in group II had histologic evidence of chronic hepatitis. Overall, 724 of the 1222 patients (59.2%) had histologic evidence of chronic liver disease. The tumor size of those patients with intrahepatic tumors was significantly larger in group II compared with group I.
Operative Variables
Of the 1222 patients, 755 (61.8%) underwent major hepatic resection, whereas the other 467 patients (38.2%) underwent minor hepatic resection of 2 or fewer segments. Table 3 depicts the frequencies of various types of hepatectomy. Most of the hepatectomies were anatomic resections, with only 15.3% being nonanatomical resections. Extended right or left hepatectomy was performed in a substantial proportion of patients (n = 284, 23.2%). In group II, 16 patients who had right or extended right hepatectomy received right portal vein embolization before surgery. Concomitant extrahepatic procedures were performed in 319 patients (26.1%). These procedures included partial excision of the diaphragm (n = 111), hepaticojejunostomy with or without excision of the bile duct (n = 153), portal lymphadenectomy (n = 47), pancreaticoduodenectomy (n = 2), resection of stomach (n = 16), colon (n = 15), adrenal gland (n = 16), kidney (n = 4), lung (n = 4), chest wall (n = 1), and distal pancreas (n = 1). Some patients had more than 1 extrahepatic procedure. Additional ablation of liver tumors in the contralateral lobe was performed in 25 patients, including cryotherapy in 2 patients in group I and radiofrequency ablation in 23 patients in group II.
TABLE 3. Types of Hepatic Resection Among 1222 Patients
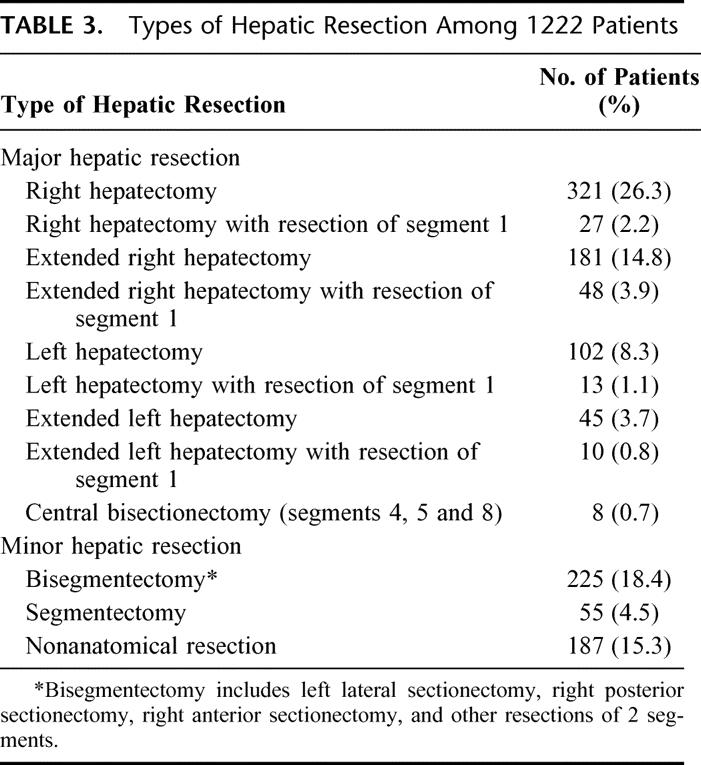
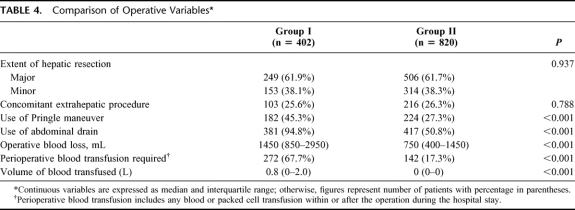
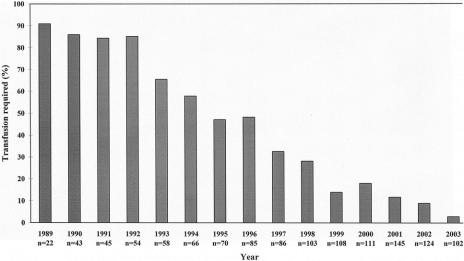
Table 4 shows a comparison of operative variables between the 2 groups. There were no significant differences in the proportion of major hepatic resections or concomitant extrahepatic procedures. The use of Pringle maneuver was significantly reduced in group II, and an increased proportion of patients in group II did not have any abdominal drainage after hepatectomy. There was also a significant reduction of blood loss and blood transfusion. Figure 1 shows the trend of perioperative blood transfusion year by year. The blood transfusion rate has been reduced from 90.9% among 22 patients operated between July and December 1989 to 2.9% among 102 patients operated between January and June 2003.
TABLE 4. Comparison of Operative Variables
FIGURE 1. Trend of perioperative blood transfusion requirement year by year over the study period. For the year 1989, only patients operated from July to December were included, and for the year 2003, only patients operated from January to June were included.
Perioperative Outcome
The overall morbidity, 30-day operative mortality, and hospital mortality of the 1222 patients were 32.4% (n = 396), 3.2% (n = 39), and 4.9% (n = 60), respectively. The hospital mortality rate was higher after hepatic resection for malignant disease than after hepatic resection for benign disease (5.5% versus 1.6%, P = 0.022), and the hospital mortality rate in cirrhotic patients was higher than that in noncirrhotic patients (6.8% versus 4.0%, P = 0.035). When stratified according to the type of hepatectomy, the highest hospital mortality was observed in patients with extended right hepatectomy (8.7%, 20/229), followed by right hepatectomy (6.6%, 23/348). In contrast, the hospital mortality rates of segmentectomy and nonanatomical resection were only 1.8% (1/55) and 2.1% (4/187), respectively.
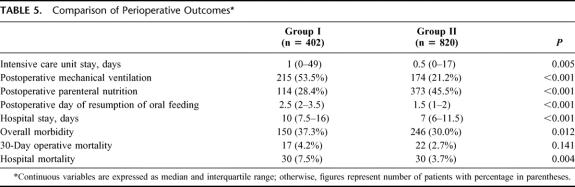
Table 5 shows a comparison of the perioperative outcome between the 2 groups. Group II had significantly reduced intensive care unit stay, use of postoperative mechanical ventilation, hospital stay, morbidity, and hospital mortality rates. The hospital mortality has been reduced from 7.5% to 3.7%, and the morbidity rate has been reduced from 37.3% to 30.0%. When stratified according to the extent of resection, there was a significant reduction in the hospital mortality after major hepatic resection from 9.6% (24/249) in group I to 4.7% in group II (24/506) (P = 0.010), and the morbidity rate was reduced from 45.8% (114/249) to 31.2% (158/506) (P < 0.001). Among patients with minor hepatic resection, there was also a reduction in hospital mortality rate from 3.9% (6/153) to 1.9% (6/314), but the difference was not statistically significant (P = 0.220). The morbidity rate after minor hepatic resection was not significantly different between the 2 groups (23.5% versus 28.0%, P = 0.302).
TABLE 5. Comparison of Perioperative Outcomes
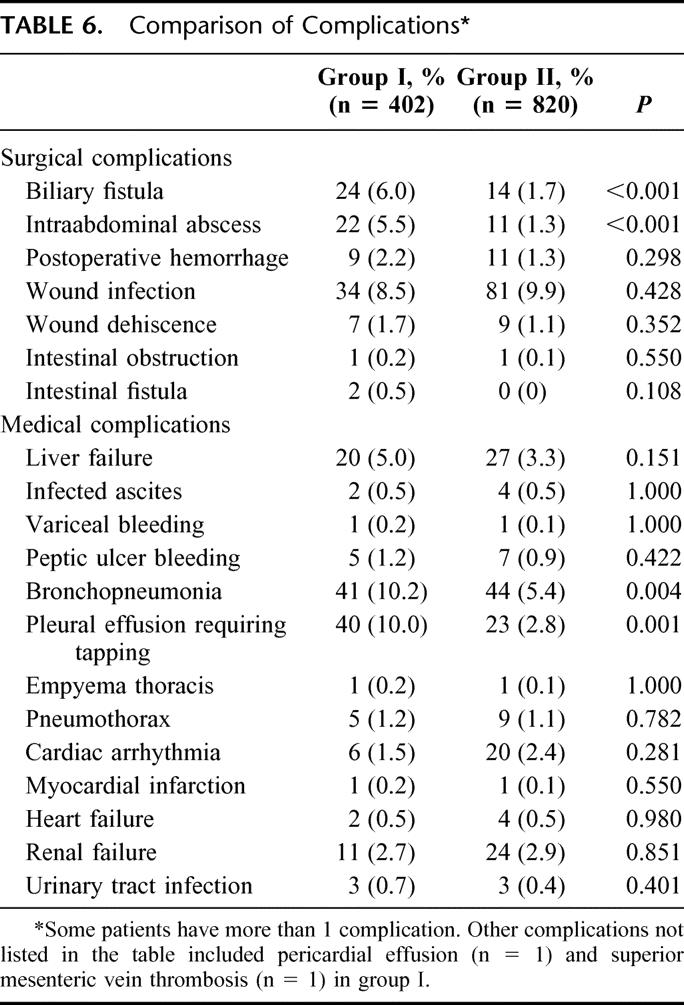
Table 6 shows a comparison of specific complications between the 2 groups. Among the surgical complications, there was a significant reduction in the rates of biliary fistula and intraabdominal abscess in group II. Among the medical complications, group II had significantly reduced rates of bronchopneumonia and pleural effusion requiring tapping. The rates of other complications were not significantly different between the 2 groups. The rate of liver failure in the 2 groups was similar despite the worse liver function in group II.
TABLE 6. Comparison of Complications
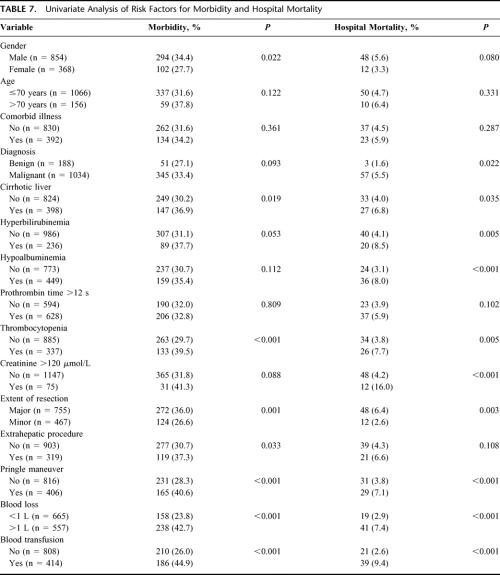
Risk Factors for Morbidity and Hospital Mortality
Table 7 shows the influence of preoperative and operative variables on morbidity and hospital mortality of the 1222 patients by univariate analysis. Male gender, cirrhotic liver, thrombocytopenia, major hepatic resection, concomitant extrahepatic procedure, use of Pringle maneuver, blood loss >1 L, and perioperative blood transfusion were associated with increased morbidity, whereas malignant disease, cirrhotic liver, hyperbilirubinemia, hypoalbuminemia, thrombocytopenia, elevated preoperative serum creatinine, major hepatic resection, use of Pringle maneuver, blood loss >1 L, and perioperative blood transfusion were associated with increased hospital mortality.
TABLE 7. Univariate Analysis of Risk Factors for Morbidity and Hospital Mortality
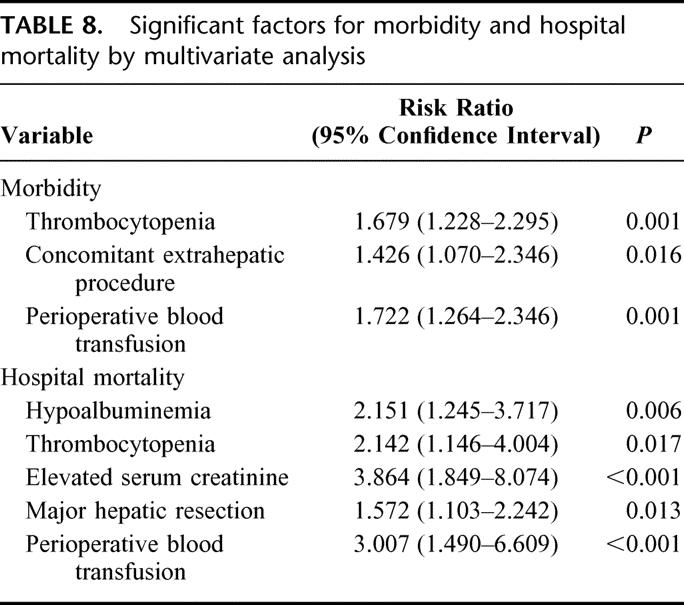
Multivariate analyses of risk factors for morbidity and hospital mortality, respectively, were performed by entering the significant factors identified in univariate analyses into a logistic regression model. The significant independent factors for morbidity were thrombocytopenia, concomitant extrahepatic procedure, and perioperative blood transfusion, and those for hospital mortality were hypoalbuminemia, thrombocytopenia, elevated serum creatinine, major hepatic resection, and perioperative blood transfusion (Table 8).
TABLE 8. Significant factors for morbidity and hospital mortality by multivariate analysis
DISCUSSION
Liver resections are increasingly performed as a standard treatment of various hepatobiliary diseases. However, there were few large contemporary studies of the perioperative outcome of hepatic resection.3–5 In a study of 747 hepatectomies in the 1990s, Belghiti et al3 demonstrated a low operative mortality rate of 1% in patients with normal liver, but the operative mortality rate was 8.7% in cirrhotic patients. Jarnagin et al4 reported a series of 1803 patients with hepatic resection in the 1990s and demonstrated a significant improvement in perioperative outcome over the study period, which was attributed to increased use of parenchymal preserving segmental resections and decrease in the number of hepatic segments resected. More recently, Imamura et al5 reported zero mortality after hepatic resection in a series of 915 patients. The authors emphasized the importance of case selection, and they adopted a strict selection in terms of liver function reserve. The current study demonstrated for the first time that improved perioperative outcome could be achieved even when the indications of hepatectomy were extended to more high-risk patients with borderline liver function reserve, advanced age, and comorbid medical illnesses.
Compared with the reported series of hepatectomy from western centers,3,4 our patient population was characterized by a high proportion of patients with HCC (60%) and hence a high proportion of patients (59.2%) with cirrhosis or chronic hepatitis. In contrast, in the study of Jarnagin et al,4 metastatic colorectal cancer accounted for 62% of the indications for hepatectomy, whereas cirrhosis or fibrosis was present in only 8.8% of the patients. Hepatic resection in cirrhotic liver is technically more challenging than resection in normal liver, with increased risk of bleeding, septic complications, and postoperative liver failure.30 Several authors have emphasized the importance of strict selection in terms of liver function reserve in ensuring favorable perioperative outcomes in patients with chronic liver disease.5,11,31 ICG clearance is a commonly used test of liver function to aid selection of patients for hepatectomy. In the study by Imamura et al,5 only patients with ICGR-15 of less than 10% were offered right hepatectomy or extended hepatectomy. The use of such a strict selection criterion was considered by the authors to be a major factor for the excellent result of zero mortality in their series. We offered major hepatectomy to patients with less favorable liver function reserve. Before 1997, major hepatic resection was offered to patients with ICGR-15 of less than 14%. In recent years, we have adopted an even more liberal selection criterion and offered right hepatectomy or extended hepatectomy to patients with ICGR-15 up to 20%.22 Our aggressive approach was partly driven by the improving survival results that we have observed in hepatic resection for HCC, even for large HCC that required extended hepatectomy.22,32 Excluding living donors with normal liver undergoing donor hepatectomy for liver transplantation, the proportion of patients with resection of 2 or more Couinaud segments was 80.2% in our series, as compared with 27.3% in the study by Imamura et al.5 In fact, the proportion of major hepatic resection was the highest in this series compared with the other 3 large series of hepatectomy recently reported in the literature.3–5
Comparing the 2 halves of the 14-year study period, the proportion of major hepatic resection has remained similar despite a significantly higher proportion of patients with cirrhosis and poorer liver function in group II. The frequency of thrombocytopenia was also higher in group II compared with group I, and this presumably reflects a higher proportion of patients with significant portal hypertension in group II. Nonetheless, the perioperative outcome in group II has improved significantly in terms of morbidity, hospital mortality, and other parameters such as intensive care unit stay and hospital stay. The liver failure rate has not increased despite the worse liver function in group II. These results supported our aggressive approach in offering hepatic resection to more patients with borderline liver function reserve. Among patients with intrahepatic tumors, there was a significant increase in tumor size in group II compared with group I, reflecting a trend of more aggressive hepatic resection for large tumors in the recent years. While major hepatic resection was a risk factor for hospital mortality, the hospital mortality of 4.7% after major hepatic resection in group II was acceptable. Cirrhosis was associated with increased morbidity and hospital mortality in the univariate analyses, but cirrhosis per se was not a significant factor in the multivariate analyses. Instead, thrombocytopenia was a significant risk factor in the multivariate analyses of both morbidity and hospital mortality, and hypoalbuminemia was another risk factor for hospital mortality. Jarnagin et al5 also found that preoperative thrombocytopenia was an independent risk factor of mortality after hepatic resection, and hypoalbuminemia was a risk factor for morbidity. In the current study, group II patients had a higher incidence of hypoalbuminemia and thrombocytopenia, yet the operative morbidity and hospital mortality had decreased. Presumably the adverse effects of hypoalbuminemia and thrombocytopenia were compensated by the improvement in surgical techniques and perioperative care.
While our experience is more concentrated on hepatic resection for HCC, a more aggressive surgical approach has also been extended to other hepatobiliary cancers. Recent studies from other groups have demonstrated improved survival with aggressive surgical approach in other hepatobiliary malignancies such as colorectal liver metastasis and hilar cholangiocarcinoma.33–35 Resection of these malignancies often requires concomitant extrahepatic procedures such as synchronous colonic resection or extrahepatic biliary resection and reconstruction. Extrahepatic biliary procedures are also required for some benign indications such as hepatolithiasis.36 While previous studies found that extrahepatic procedures increased the risk of mortality in patients undergoing hepatectomy,3,4 in our experience, extrahepatic procedures were associated with increased morbidity but not increased mortality. The capability to perform hepatectomy combined with major extrahepatic procedures safely should help to expand the role of hepatic resection to more complicated hepatobiliary diseases. Two strategies have further expanded the indications for hepatic resection. One is preoperative portal vein embolization to induce hypertrophy of liver remnant in patients with inadequate liver function reserve and small liver remnant volume. This strategy can be employed not only in patients with normal liver but also in selected patients with chronic liver disease or biliary obstruction from extrahepatic biliary malignancies.35,37 The other strategy is the use of radiofrequency ablation in combination with hepatic resection, which can be used to treat multiple bilobar malignancies when it is anatomically not feasible or too risky to encompass all the lesions by hepatic resection alone.38
In addition to the liver function reserve, advanced age and comorbid illnesses are other pre-existing host conditions that sometimes preclude patients from hepatic resection. Some previous studies have reported that advanced age was a significant risk factor for morbidity or mortality after hepatic resection,4,14,20,39 and comorbid illnesses such as cardiovascular disease and diabetes mellitus have also been reported to be significant risk factors.11,39,40 However, recent studies from our group and others have demonstrated that advanced age or comorbid illnesses may not adversely affect the outcome of hepatic resection, provided that careful preoperative assessment and meticulous perioperative management were executed.41–43 Hence, in recent years, we have become more liberal in offering hepatic resection to elderly patients and patients with comorbid illnesses. In this study, neither advanced age nor comorbid illness was associated with significantly increased morbidity or mortality. Nevertheless, we would like to emphasize that careful preoperative case selection and meticulous postoperative management in liaison with the anesthetists and the relevant specialist physicians are essential to achieve favorable outcome in elderly patients and patients with comorbid illnesses. The operative morbidity and hospital mortality have been reduced in group II compared with group I, while the proportions of elderly patients and patients with comorbid illnesses, in particular cardiovascular disease, have increased significantly. Of the medical complications, there was a significant reduction in the incidence of respiratory complications. Better postoperative pain control with the use of patient-controlled analgesia probably has played an important role in reducing respiratory complications. With the aging population worldwide, the survival benefit of hepatic resection for malignant diseases in elderly patients is expected to increase, and hence hepatic resection is going to play an increasingly important role in this group of patients. A previous study on HCC managed in our institution showed a similar survival benefit from hepatic resection in elderly patients compared with younger patients.41 While we advocate that the role of hepatectomy can be expanded in elderly patients and patients with comorbid illnesses such as diabetes mellitus and cardiovascular diseases that are under control, we caution against hepatic resection in patients with impaired renal function, because elevated serum creatinine level was a significant risk factor of mortality. A previous study on extended hepatic resection has also identified elevated serum creatinine level as a risk factor of postoperative mortality.44
Jarnagin et al4 observed that the improved perioperative outcomes of hepatic resection in their study were largely the result of a decline in the number of liver segments resected. In contrast, in our study, the overall improvement in perioperative outcome over the study period was mainly ascribed to the reduced mortality and morbidity in patients undergoing major hepatic resection. Over the study period, there have been substantial changes in the surgical technique of hepatic resection and perioperative management. It is difficult to attribute the reduced operative morbidity and hospital mortality to a specific factor. Among the risk factors of morbidity and mortality in the multivariate analyses, perioperative blood transfusion was the only risk factor that has been significantly reduced in group II compared with group I. Hence, advance in surgical technique that has led to reduced operative blood loss was likely to be an important factor for the improved outcome. Several changes in the surgical technique over the study period, including the use of ultrasonic dissection instead of the finger-fracture technique, the adoption of a low central venous pressure during hepatic transection and the use of a vascular stapler for control of hepatic veins, together with the increased experience of the surgeons, have all contributed to the reduction in operative blood loss. A notable change in the surgical technique in recent years is the reduced use of Pringle maneuver. Although we have previously demonstrated in a randomized trial that Pringle maneuver was effective in reducing blood loss during hepatic transection,26 we have also observed a time constraint in the total duration of Pringle maneuver that the liver could tolerate,45 which could sometimes lead to a hasty transection and hence more bleeding. With increased experience, we found that hepatic transection could be performed with low blood loss even without the Pringle maneuver. The absence of a time constraint from Pringle maneuver allows the surgeon to control each bleeding point precisely before continuing with the transection. Our change in attitude toward the use of Pringle maneuver was partly influenced by our experience in living donor hepatectomy, in which we never applied any portal clamping. Of the 163 living donor hepatectomies performed without Pringle maneuver in our unit during the same period, including 129 patients with right lobe donation, only 1 patient required perioperative blood transfusion, and there was no hospital mortality. In recent years, most of the hepatic resections, including those on cirrhotic liver, were performed without Pringle maneuver, and operative blood loss and the requirement for blood transfusion were on the decreasing trend. Perioperative blood transfusion could increase perioperative morbidity and mortality by causing immune suppression, and it has also been shown to adversely affect the long-term survival after hepatic resection for HCC or colorectal metastasis.46,47 The achievement of zero blood transfusion rate should be a common goal of all surgeons performing hepatic resection.
In conclusion, this study demonstrated that perioperative outcome has improved despite extending the indication of hepatectomy to more high-risk patients. Hence, the role of hepatectomy in the management of benign and malignant hepatobiliary diseases can be expanded. Reduced perioperative transfusion is the main contributory factor for the improved outcome, and further effort should be directed toward improving surgical techniques to achieve bloodless hepatic resection.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Wan Ching Yu and Ms. Yuk Kit Mak for their assistance in data collection for the prospective database.
Discussions
Dr. David M. Nagorney (Rochester, Minnesota): Dr. Poon, congratulations for showing us that major hepatic resection can be performed with less morbidity and mortality despite increases in co-morbidity and cirrhosis than in the past. Importantly, the largest gains in your series were in patients with malignancy. Again I would congratulate you and recommend that everyone peruse the article carefully to learn about the perioperative management so that we can employ these techniques that made your results so safe. I also want to commend you for extending the results of the Memorial Sloan-Kettering group that show decreased morbidity and mortality in the past. I have 3 questions.
First, the outcome of the cirrhotics improved with a decrease in operative mortality by 50% and a decrease in morbidity by 30%, but you didn't state what the Pugh-Child's class was. Did this change over time or were they similar over time?
Second, your patients were considered high risk because of cirrhosis. And because of that, a lot of people would have transplanted these patients with HCC. Do you think the degree of reduction of perioperative morbidity and mortality will affect the choice or the sequence of therapy and long-term outcome for HCC?
And finally, ablative approaches are currently the rage. Although the most attractive one is percutaneous ablation, I think the accuracy of ablation can be improved laparoscopically or at laparotomy. Do you think that your perioperative management schema can be employed as effectively in the patients with worse liver disease who are candidates for ablation as effectively as those who underwent resection?
Congratulations on your work and thank you for allowing me to comment.
Dr. J. Michael Henderson (Cleveland, Ohio): I appreciate the opportunity to comment on this paper. This group deserves a lot of credit for the outstanding work they have done over the last 2 decades in this area. This paper is really a validation of many of the other studies they have done. Randomized trials looking at drains or no drains, nutrition or no nutrition, which are further validated in this very large series. The results are outstanding, and I think you can sum it up in one word: experience. I have a couple of questions, Dr. Poon.
First in looking at your patient populations, defining who you select for surgery, indocyanine green was one of your tests, yet it did not appear as a significant test in your univariate or multivariate analyses. Why keep doing it? You have shown value in the standard tests, hypoalbuminemia and hypobilirubinemia. Is ICG of value or is it redundant? Has the use of ICG really gone?
The second point that struck me as I read the manuscript was the huge series of patients with hepatolithiasis. Was this group's morbidity different? I would imagine they might have a higher infection rate, and lumping them in with malignancy patients might alter the overall experience? Were their outcomes different?
Again congratulations on a superb paper and thank you for bringing this experience to this group.
Dr. Henry A. Pitt (Milwaukee, Wisconsin): Again, congratulations on excellent results, which are among the best in the world.
In reading your abstract and listening to your presentation I was struck by the fact that you had so few patients with biliary malignancies. My take is that you have included the intrahepatic cholangiocarcinomas, but you have not reported the gallbladder cancers and the perihilar cholangiocarcinomas. Am I correct in that presumption? If you have excluded them, why? The recent data from Memorial, as well as our recent data from Milwaukee, suggests that operative mortality is equally low in these very challenging patients. Did you also have a low mortality in patients with biliary malignancies?
Dr. Ronnie T. Poon (Hong Kong, China): Thanks to the discussants for their questions. I will answer the questions in the order they were asked.
First are the questions by Dr. Nagorney. For the first question, the severity of liver cirrhosis was similar between the 2 time periods in terms of Child-Pugh score. The majority of patients in our series had Child-Pugh score at grade A, but the proportion of grade B patients was similar between the 2 time periods.
Your second question about the role of liver resection and liver transplantation in patients with cirrhosis is very important in view of the ongoing debate on whether a Child's A cirrhotic patient with a small hepatocellular carcinoma less than 5 centimeters should receive resection or transplantation as the treatment of choice.
This study clearly demonstrates an improved perioperative outcome of hepatic resection in cirrhotic patients largely because of reduced blood loss and blood transfusions in recent years. The reduction of blood transfusion will also lead to improved long-term survival, as several studies have already demonstrated that perioperative blood transfusion has an adverse effect on long-term survival after resection of hepatocellular carcinoma probably related to an immunosuppressive effect.
In fact, a previous study from our group published in the Annals of Surgery in the year 2001 has demonstrated improving long-term survival after resection of hepatocellular carcinoma as a result of reduced perioperative blood transfusion. The 5-year survival after resection of hepatocellullar carcinoma less than 5 centimeters in Child's A cirrhotic patients in our experience was 70%, which was similar to that after transplantation.
Hence, the current study and previous studies have strengthened our belief that hepatic resection should remain the first line treatment for a Child's A cirrhotic patient with a small hepatocellular carcinoma. In our center, we reserve liver transplantation as a salvage treatment for patients who have recurrent tumor or deterioration of liver function after hepatic resection.
For your last question regarding ablative approaches, I agree with you that ablative accuracy achieved by laparoscopic or open approach may be superior to percutaneous approach. In our center, we perform radiofrequency ablation for tumors greater than 3 centimeters by laparoscopic or open approach, and our radiologists perform radiofrequency ablation for tumors less than 3 centimeters by percutaneous approach. In our experience of over 200 ablations, we did observe better complete ablation rates after ablation by surgical approach, even though the tumor size was bigger. And we employed similar preoperative management in terms of assessment of liver function and imaging in patients who require surgical ablation.
For questions asked by Dr. Henderson: for the indocyanine green clearance test, I actually did not include it in the multivariate analysis because it was performed only in a subgroup of patients with chronic liver diseases, and it was performed probably in only half of the patients. If I entered it into the multivariate analysis of the whole group, that might distort the picture because all the other variables were performed in all patients. Hence, this is the reason why it did not come out in the analysis, because it was not entered into the analysis at all. We still think that indocyanine green clearance test has significant value in the choice of cirrhotic patients with hepatocellular carcinoma for liver resection, because we all know that the Child's A category of patients with cirrhosis has a wide range of liver function and a wide range of morbidity and mortality after resection.
Regarding the hepatolithiasis, this is a specific disease of our locality. It is true that it is associated with recurrent cholangitis and a higher rate of infective complications after hepatectomy compared with hepatectomy for the other indications. However, we did not observe an increased mortality rate after hepatic resection for hepatolithiasis, and by including these patients in this series I don't think it will affect the overall mortality rate in this series.
Finally, regarding the questions by Dr. Pitt: the incidence of biliary malignancies in our locality is very low. We did offer a similar approach of aggressive hepatic resection for patients with hilar cholangiocarcinoma and also for patients with carcinoma of the gallbladder, but in our experience, of all these 1220 patients, we have only 43 extrahepatic biliary malignancies. So I think this is related to the low incidence of these malignancies in our population rather than our approach in management of these malignancies. And I agree that our series reflects probably more the role of hepatic resection in the management of intrahepatic malignancies rather than extrahepatic malignancies because of the low proportion of the latter cases.
Footnotes
Financial support: Sun C. Y. Research Foundation for Hepatobiliary and Pancreatic Surgery and Outstanding Young Researcher Award of the University of Hong Kong.
Reprints: Professor Sheung Tat Fan, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, China. E-mail: hrmsfst@hkucc.hku.hk.
REFERENCES
- 1.Fortner JG, Blumgart LH. A historic perspective of liver surgery for tumors at the end of the millennium. J Am Coll Surg. 2001;193:210–222. [DOI] [PubMed] [Google Scholar]
- 2.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. [DOI] [PubMed] [Google Scholar]
- 6.Fortner JG, MacLean BJ, Kim DK, et al. The seventies evolution in liver surgery for cancer. Cancer. 1981;47:2162–2166. [DOI] [PubMed] [Google Scholar]
- 7.Thompson HH, Tompkins RK, Longmire WP Jr. Major hepatic resection: a 25-year experience. Ann Surg. 1983;197:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turunen MJ, Huikuri K, Lempinen M. Results of 32 major hepatic resections for primary and secondary malignancies of the liver. Ann Chir Gynaecol. 1986;75:209–214. [PubMed] [Google Scholar]
- 9.Nagorney DM, van Heerden JA, Ilstrup DM, et al. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;106:740–748. [PubMed] [Google Scholar]
- 10.Heriot AG, Karanjia ND. A review of techniques for liver resection. Ann R Coll Surg Engl. 2002;84:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyagawa S, Makuuchi M, Kawasaki S, et al. Criteria for safe hepatic resection. Am J Surg. 1995;169:589–594. [DOI] [PubMed] [Google Scholar]
- 12.Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl. 2004;10(2 suppl 1):S39–45. [DOI] [PubMed] [Google Scholar]
- 13.Dimick JB, Pronovost PJ, Cowan JA Jr, et al. Postoperative complication rates after hepatic resection in Maryland hospitals. Arch Surg. 2003;138:41–46. [PubMed] [Google Scholar]
- 14.Dimick JB, Cowan JA Jr, Knol JA, et al. Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg. 2003;138:185–191. [DOI] [PubMed] [Google Scholar]
- 15.Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003;197:233–241. [DOI] [PubMed] [Google Scholar]
- 16.Kammula US, Buell JF, Labow DM, et al. Surgical management of benign tumors of the liver. Int J Gastrointest Cancer. 2001;30:141–146. [DOI] [PubMed] [Google Scholar]
- 17.Okano K, Maeba T, Ishimura K, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. 2002;235:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karavias DD, Tepetes K, Karatzas T, et al. Liver resection for metastatic non-colorectal non-neuroendocrine hepatic neoplasms. Eur J Surg Oncol. 2002;28:135–139. [DOI] [PubMed] [Google Scholar]
- 19.Das BC, Isaji S, Kawarada Y. Analysis of 100 consecutive hepatectomies: risk factors in patients with liver cirrhosis or obstructive jaundice. World J Surg. 2001;25:266–272. [DOI] [PubMed] [Google Scholar]
- 20.Alfieri S, Carriero C, Caprino P, et al. Avoiding early postoperative complications in liver surgery: a multivariate analysis of 254 patients consecutively observed. Dig Liver Dis. 2001;33:341–346. [DOI] [PubMed] [Google Scholar]
- 21.Fan ST, Lai EC, Lo CM, et al. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198–203. [DOI] [PubMed] [Google Scholar]
- 22.Poon RT, Fan ST, Lo CM, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia F, Poon RT, Fan ST, et al. Thoracoabdominal approach for right-sided hepatic resection for hepatocellular carcinoma. J Am Coll Surg. 2003;196:418–427. [DOI] [PubMed] [Google Scholar]
- 24.Lo CM, Fan ST, Liu CL, et al. Determining resectability for hepatocellular carcinoma: the role of laparoscopy and laparoscopic ultrasonography. J Hepatobiliary Pancreat Surg. 2000;7:260–264. [DOI] [PubMed] [Google Scholar]
- 25.Liu CL, Fan ST, Lo CM, et al. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CL, Fan ST, Lo CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan ST, Lo CM, Lai EC, et al. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331:1547–1552. [DOI] [PubMed] [Google Scholar]
- 29.Strasberg SM, Belghiti J, Clavien PA, et al. The Brisbane 2000 terminology of liver anatomy and resections: Terminology Committee of the International Hepato-Pancreato-Biliary Association. HPB. 2000;2:333–339. [Google Scholar]
- 30.Fan ST. Problems of hepatectomy in cirrhosis. Hepatogastroenterology. 1998;45:1288–1290. [PubMed] [Google Scholar]
- 31.Redaelli CA, Dufour JF, Wagner M, et al. Preoperative galactose elimination capacity predicts complications and survival after hepatic resection. Ann Surg. 2002;235:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000;231:743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nimura Y, Kamiya J, Kondo S, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–162. [DOI] [PubMed] [Google Scholar]
- 36.Chan DW, Poon RT, Liu CL, et al. Immediate and long-term outcomes after hepatic resection for hepatolithiasis. Surgery. 2004;135:386–393. [DOI] [PubMed] [Google Scholar]
- 37.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawlik TM, Izzo F, Cohen DS, et al. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada M, Takenaka K, Fujiwara Y, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195–198. [DOI] [PubMed] [Google Scholar]
- 40.Little SA, Jarnagin WR, DeMatteo RP, et al. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6:88–94. [DOI] [PubMed] [Google Scholar]
- 41.Poon RT, Fan ST, Lo CM, et al. Hepatocellular carcinoma in the elderly: results of surgical and nonsurgical management. Am J Gastroenterol. 1999;94:2460–2466. [DOI] [PubMed] [Google Scholar]
- 42.Poon RT, Fan ST, Wong J. Does diabetes mellitus influence the perioperative outcome or long term prognosis after resection of hepatocellular carcinoma? Am J Gastroenterol. 2002;97:1480–1488. [DOI] [PubMed] [Google Scholar]
- 43.Aldrighetti L, Arru M, Caterini R, et al. Impact of advanced age on the outcome of liver resection. World J Surg. 2003;27:1149–1154. [DOI] [PubMed] [Google Scholar]
- 44.Melendez J, Ferri E, Zwillman M, et al. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192:47–53. [DOI] [PubMed] [Google Scholar]
- 45.Man K, Fan ST, Ng IO, et al. Tolerance of the liver to intermittent Pringle maneuver in hepatectomy for liver tumors. Arch Surg. 1999;134:533–539. [DOI] [PubMed] [Google Scholar]
- 46.Fan ST, Ng IO, Poon RT, et al. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999;134:1124–1130. [DOI] [PubMed] [Google Scholar]
- 47.Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]