Abstract
Objective:
To prospectively evaluate surgical procedures performed with palliative intent.
Summary Background Data:
There is a paucity of outcomes data necessary to allow sound surgical decision-making and informed consent for palliative procedures.
Methods:
Procedures to palliate symptoms of advanced cancer were identified prospectively from all operations performed. Patients were observed for >90 days or until death.
Resutls:
There were 1022 palliative procedures performed in 823 patients from July 2002 to June 2003. Operative (713/1022) or endoscopic (309/1022) procedures were performed for gastrointestinal obstruction (34%), neurologic symptoms (23%), pain (12%), dyspnea (9%), jaundice (7%) or other symptoms (15%). Symptom improvement or resolution within 30 days was achieved in 80% (659/823). Median duration of symptom control was 135 days. Recurrence of the primary symptom occurred in 25% (165/659) while treatment of debilitating additional symptoms was required in 29% (191/659). Palliative procedures were associated with 30-day postoperative morbidity (29%) and mortality (11%). A major postoperative complication reduced the probability of symptom improvement to 17%. Median survival was 194 days from the time of the palliative procedure and was adversely associated with poor performance status (ECOG ≥ 2 [P < 0.001] or NCI fatigue score of ≥1 [P < 0.001]), poor nutrition (albumin <3.5 [P = 0.005] or significant weight loss [P = 0.003]), and no previous cancer therapy (P = 0.002).
Conclusions:
In carefully selected patients, relief of symptoms following palliative procedures can be expected, but new or recurrent symptoms limit durability. Potential benefits are minimized by postoperative complications and are less predictable for patients with poor performance status, malnutrition and no prior cancer therapy.
Effective palliation rather than cure is often the most appropriate goal of treating advanced malignancies. Predictable relief of symptoms following palliative procedures can be expected in carefully selected patients. The recurrence or development of additional symptoms limits the durability of the intervention.
Recommendations from the Institute of Medicine and National Cancer Policy Board have stressed the need for an expanded role of palliation in the comprehensive management of cancer patients.1 Initiatives by the American College of Surgeons, summarized by the Principles Guiding Care at the End of Life, are providing essential knowledge about the current concepts and practice of palliative care that may help to overcome the lack of formal training that most surgeons have in palliative care and supplement shortcomings in the surgical peer-reviewed and textbook literature.2,3
Surgical palliation of cancer is characterized best by procedures employed with noncurative intent with the primary goal of improving symptoms caused by an advanced malignancy. The effectiveness of a palliative intervention should be judged by the presence and durability of patient acknowledged symptom resolution. When accompanied by overall improvement in quality of life, limited morbidity and mortality of therapy and modest resource utilization, its value is enhanced. Although symptom palliation may result in increased survival for the individual patient, it is inappropriate to select a palliative procedure solely based on a desire for improved duration of survival.4–6
When therapy is given with curative intent, the prolongation of life or elimination of disease often allow the potential consequences of treatment such as severe toxicity, patient discomfort, and infrequent mortality to be viewed as acceptable risks. Clinical decision-making often revolves around well-characterized surgical principles and procedures based on a robust body of literature and clinical trials. There have been few such trials concerned with surgical palliation and a paucity of data generated to build an evidence-based body of knowledge which could ultimately lead to improved surgical care of patients near the end of life.3 The goal of this study is to prospectively follow all patients undergoing a palliative operative or endoscopic procedure during a one year period to obtain some of the data that are required to guide sound clinical decisions and allow more adequate patient counseling. This comprehensive analysis also serves as a framework for future symptom, disease, and procedure specific analyses of surgical palliation.
METHODS
The medical records of all patients undergoing a surgical procedure at Memorial Sloan-Kettering Cancer Center between July 2002 and July 2003 were screened daily to designate cases as palliative or nonpalliative. Procedures performed primarily for symptom management on patients with metastatic or advanced locoregional cancer were identified. An intervention was considered palliative only when the medical record clearly documented palliative intent or when interview of the attending surgeon confirmed that it was performed to relieve specific symptoms, control pain or improve quality of life.
Patients found to have had a palliative procedure either by surgeon confirmation or explicit chart analysis were entered into a prospective database. The subjects’ demographic information, primary cancer, chief complaint, prior treatment history, ECOG level, NCI fatigue scale, hemoglobin, albumin, and type of palliative procedure, were obtained from the clinical record. Procedures performed primarily with a bronchoscope, cystoscope, or gastrointestinal endoscope were considered “endoscopic” procedures. All other cases were classified as “operative.”
Surgical complications within 30 days of operation were graded using a previously described surgical secondary events grading system in which a grade 1 complication required local or bedside care, a grade 2 complication required invasive monitoring or intravenous medication, a grade 3 complication required an operation, interventional radiology, intubation, or therapeutic endoscopy, a grade 4 complication resulted in a persistent disability or required major organ resection, and a grade 5 complication resulted in death.7 The highest severity level was recorded when a patient had more than one complication associated with a specific procedure. Grade 3 and 4 complications were considered high grade. The length of hospitalization associated with the initial palliative procedure was determined.
Following the initial palliative procedure, the presence or absence of symptoms was determined and followed over time. Symptom assessment scales, pain scores, and quality of life instruments from the patient's records were evaluated. Although these tools were used to classify all patients before surgery, they were available in approximately half of the patients during the follow-up period. In the absence of these instruments, a patient was considered to have a specific symptom if there was a documented finding on radiographic, endoscopic, laboratory, or physical examination related with the complaint. Patients were classified as having clinically significant pain if they required narcotic pain relief for >30 days, were treated by a pain specialist, or complained of pain in a location compatible with their clinical scenario on more than 2 clinic visits. Clinically significant weight loss was defined as an unplanned weight loss greater than 10 pounds over a 90-day period. Evidence of symptom resolution or the development of new symptoms was collected from the patient record. Further surgical, medical, or radiation therapy given during the follow-up period and additional procedures to manage recurrent or new symptoms were recorded. All palliative patients were followed for a minimum of 90 days or until death.
Data were analyzed using SAS statistical software (Release 5.0, SAS Institute Inc., Cary, NC). Data were expressed as percentages in the case of categorical variables and as medians in the case of continuous variables. Means were compared with the use of the Student t test and frequencies were compared by the χ2 test where appropriate. Survival curves were constructed using the Kaplan-Meier method. The univariate associates between clinical variables and survival were examined by the log-rank test. Independently associated factors were identified by proportional hazard regression analysis (Cox model). P values less than 0.05 were considered significant.
RESULTS
From July 2002 through June 2003, 1022 palliative operative or endoscopic procedures were performed at Memorial Sloan-Kettering Cancer Center. This constituted 6% of the approximately 17,000 cases performed at the institution. Procedures were performed on patients by all services in the Department of Surgery irrespective of the primary tumor type. Following the initial palliative intervention in 823 patients, an additional 109 procedures were performed for recurrent symptoms and 90 more cases were performed for the development of new symptoms. The clinical characteristics of patients at the time of their initial palliative procedure are summarized in Table 1 and primary tumor site is depicted in Figure 1. In all of these procedures, the patient had a palliative intervention deliberately proposed to address specific symptoms or improve quality of life. Before inclusion into this study, palliative intent was confirmed directly by the surgeon in 58% (480/823) of the subjects. In the remaining cases, the operative report explicitly stated that the case was performed with palliative intent to manage specific symptoms or to improve quality of life in patients with an advanced malignancy. The procedure was performed electively in 82% (676/823), urgently in 16% (128/823), and emergently in 2% (19/823). The procedures were operative in 70% (713/1022) and endoscopically based in 30% (309/1022). Following the initial palliative procedure, 47% (387/823) of the patients were given palliative chemotherapy and 17% (143/823) received radiation therapy.
TABLE 1. Patient Characteristics at Initial Palliative Procedure
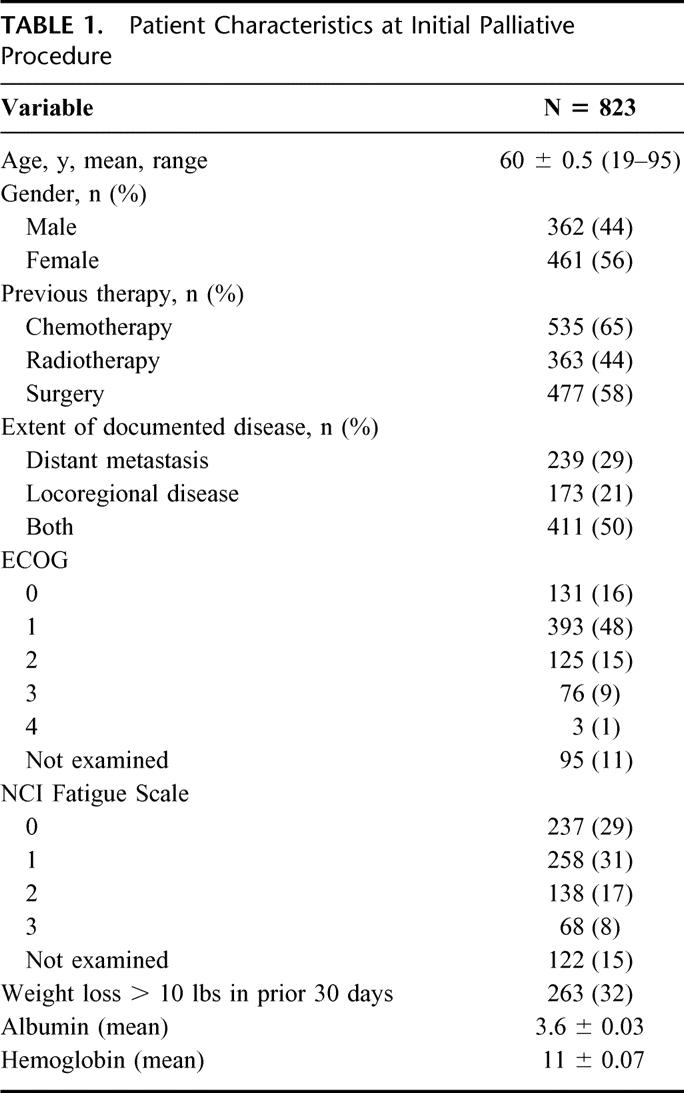
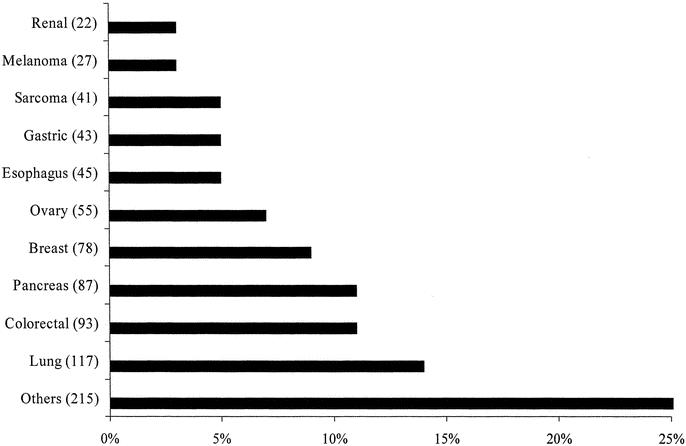
FIGURE 1. The primary tumor site of 823 patients at the time of their initial palliative procedure. “Other” group represents an additional 15 types of primary malignancies.
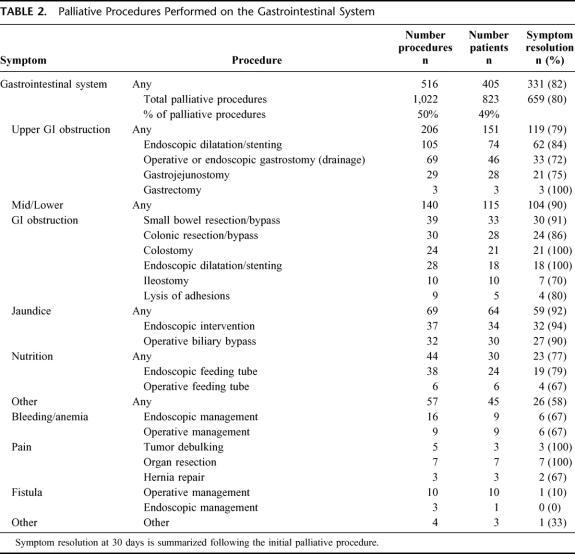
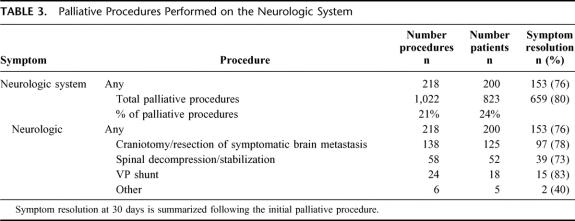
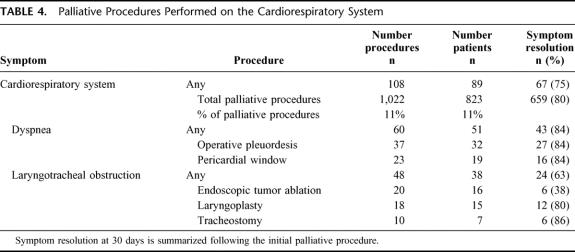
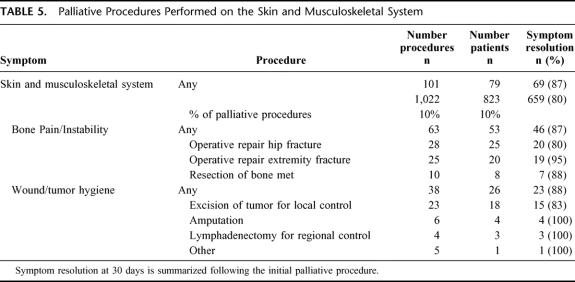
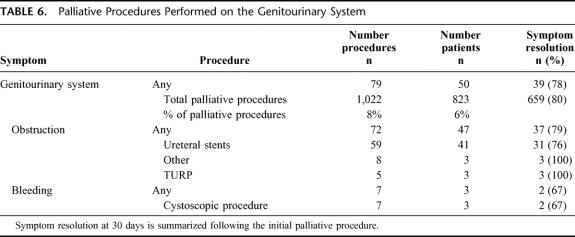
Explicit documentation of symptom improvement or resolution was noted in 80% (659/823) of the subjects. All patients who experienced symptom relief did so within 30 days of operation. System-specific outcomes are summarized in Tables 2 through 6. The initial palliative procedure was associated with symptom improvement in the gastrointestinal system for 82%, the neurologic system for 76%, the cardiorespiratory system for 75%, the skin and musculoskeletal system for 87%, and the genitourinary system for 78%. There was no difference between endoscopic or operative procedures in the frequency of symptom resolution (P = 0.20). The median duration of symptom control was 135 days. The durability of symptom relief was not associated with the type of presenting symptom (P = 0.79) or the primary malignancy (P = 0.54). As seen in Figure 2, management of a recurrent primary symptom (25%) and treatment of additional debilitating symptoms (29%) were frequently required. No signs of clinical improvement were noted in a total of 164 (20%) of the 823 patients. This group of patient was composed of:1 those who received no benefit because they died in the hospital as a result of either complications or progression of disease within 30 days the procedure;2 those who required further palliative care to manage their chief complaint prior to documented improvement; and,3 those who demonstrated no evidence of subjective improvement as documented in the medical record. In the cohort of patients who have been followed to death, symptom resolution without the development of recurrent or new symptoms was observed in 16% (52/315).
TABLE 2. Palliative Procedures Performed on the Gastrointestinal System
TABLE 3. Palliative Procedures Performed on the Neurologic System
TABLE 4. Palliative Procedures Performed on the Cardiorespiratory System
TABLE 5. Palliative Procedures Performed on the Skin and Musculoskeletal System
TABLE 6. Palliative Procedures Performed on the Genitourinary System
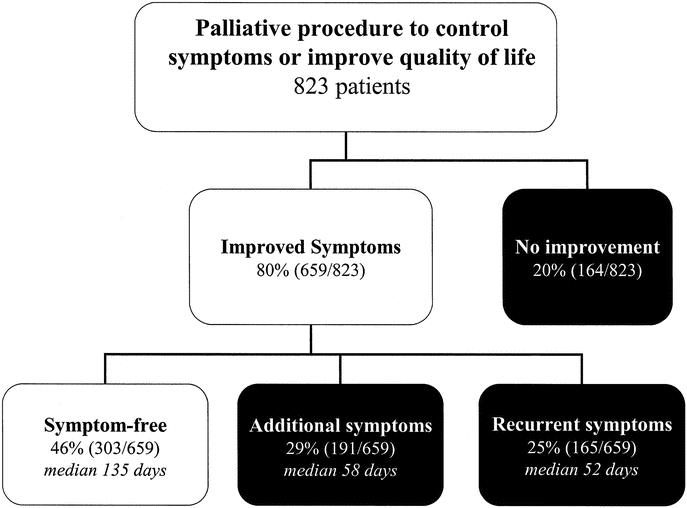
FIGURE 2. Although symptoms usually improved following the initial palliative procedure, recurrent or additional symptoms often develop during follow-up.
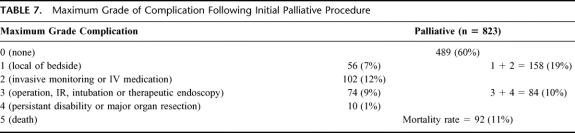
Table 7 lists the maximum grade complication following the initial palliative intervention. In the postoperative period, a complication was identified in 40% (334/823) of all patients. The mean length of hospitalization was longer in those patients who had a postoperative complication (16 ± 1.3 days versus 9 ± 0.8 days, P < 001). Patients who experienced a complication were less likely to have documented improvement in their chief complaint (67% [225/334] versus 89% [434/489], P < 0.001). A high-grade postoperative complication was associated with a reduction in observed symptom improvement to 17% (14/84), P < 0.001. The 30-day postoperative mortality rate was 11% (92/823). Palliative procedures contributed to 36% (92/253) of the total 30-day postoperative mortality (P < 0.001) encountered at our institution during the period of study. These often reflect the terminal nature of this care and are reflective of progression of disease in many instances. Although patients with an endoscopically based procedure had fewer nonfatal perioperative complications (endoscopic [18% (37/209)] versus operative [39% (205/522)]), P < 0.001), they experienced a higher 30-day mortality (endoscopic [15% (38/247)]) versus operative (9% [54/576], P = 0.017).
TABLE 7. Maximum Grade of Complication Following Initial Palliative Procedure
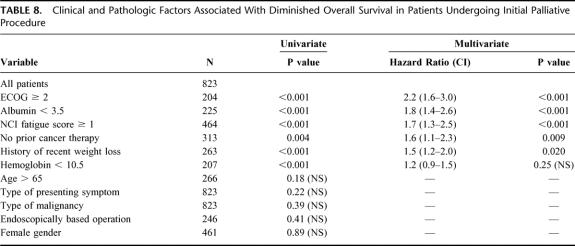
To identify the opportunity for durable symptom relief, the overall survival following a palliative procedure (median 194 days) was documented in Figure 3. Univariate and multivariate analysis was performed to identify factors associated with overall survival in this unique patient population (Table 8) and demonstrated that ECOG ≥2, albumin >3.5, NCI fatigue scale ≥1, no previous history of cancer therapy, a history of recent weight loss, and hemoglobin <10.5 were associated with a diminished overall survival. On multivariate analysis, independent factors adversely associated with overall survival were poor performance status (ECOG ≥2 or NCI fatigue score of ≥1), poor nutrition (albumin <3.5 or significant weight loss) and no previous cancer therapy.
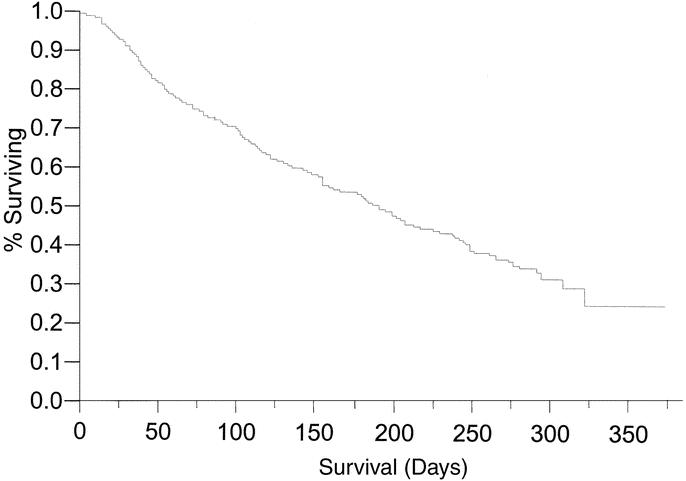
FIGURE 3. Overall survival in patients following initial procedure performed with palliative intent.
TABLE 8. Clinical and Pathologic Factors Associated With Diminished Overall Survival in Patients Undergoing Initial Palliative Procedure
DISCUSSION
Over 600,000 Americans will lose their battle against cancer each year and be faced with end of life issues. As they transition from curative to comfort care, the patient, family and physician will seek reliable information to properly frame difficult treatment choices at a highly vulnerable time. The patient is forced to consider a therapy that may eliminate a concern but cannot deliver the most desired outcome. No longer able to support the patient through the toxicity of difficult curative treatments, the family may feel compelled to protect the patient from any harm. Well-schooled in risk assessment of patients prior to initiating multidisciplinary strategies supported by randomized clinical trials, the physician must propose therapy for gravely ill patients solely seeking resolution of a symptom. At this time of great need, palliative decisions are often based on individual expectations and anecdotal experience rather than well-characterized information regarding the risks and benefits for specific groups of patients. A patient-centric understanding of the value assigned to resolution of a symptom is essential in allowing this important concordance between patient, family and physician to occur. Considerations relating to the medical condition and performance status of the patient, the extent and prognosis of the cancer, knowledge of the natural history of the primary and secondary symptoms, potential durability of the procedures and quality of life expectations of the individual patient will aid this discussion.
An established and reproducible definition of palliative surgery predicated on providing symptom control and optimizing quality of life was used in this study to evaluate all operations performed at our institution.1,3,4,9,10 Palliative interventions accounted for 6% of all procedures performed in this comprehensive cancer center and exceeded the number of esophagectomies, gastrectomies, pancreatectomies and hepatectomies combined. This finding is consistent with a report from the City of Hope National Medical Center, which demonstrated, using a broader definition of surgical palliation, that 12.5% of the surgical procedures performed were palliative in nature.11 Palliative procedures are performed by all surgical specialties. Though perhaps not experts in breast cancer, neurosurgeons will treat the brain or spinal metastases, orthopedic surgeons the pathologic fractures and thoracic surgeons the malignant pleural effusions at an incurable stage of this disease. Comparable symptoms may demand different responses based upon the biology of each primary disease. These, often subtle, distinctions must be appreciated to provide the finest care. Factors such as symptom severity, the degree of symptom resolution, the timing and choice of procedure, the durability of the intervention, associated complications, and patient preferences all play major roles in determining the overall benefit of the palliative operation.
In this study, experienced clinicians selected patients for palliative procedures that resulted in improvement or resolution of specific symptoms 80% of the time. There are no validated quality of life instruments solely focused on palliative surgical outcomes. It is far easier to identify patients who fail to improve than it is to distinguish those who experience the greatest benefits. The impact of a specific palliative intervention also will differ for each individual patient.3,4 Complete relief of dyspnea for a patient with a malignant pleural effusion for even a few days may be of significant value while lifelong partial improvement of gastrointestinal obstruction with a gastrostomy may be of modest importance. Experience with a prospective pilot study using standardized quality of life instruments exposes the challenge in interpreting this representation of outcome following palliative interventions.4 As patients progress through end of life phases, global quality of life status may so overwhelm the picture that an accurate depiction of the overall benefit of an intervention to solve a specific symptom may be lost. It is difficult to measure degrees of success in the actively dying patient.
The opportunity for durable improvement in quality of life is evidenced by the median symptom-free survival of 135 days in patients having a median survival of 194 days. Nearly one half of the palliative patients developed new or recurrent symptoms that required additional palliative interventions within 2 months of the initial palliative procedure. The observation that new symptoms may arise or that the initial symptom can recur is an important finding in this study and confirms earlier retrospective studies in advanced gastric cancer and locally recurrent rectal cancer that similarly demonstrated this phenomenon.8,10 The limited median overall survival of 194 days following a palliative intervention was demonstrated in this study and was independently associated with patient specific factors such as poor nutrition, poor functional status, and previous treatment history. Consideration of anticipated survival helps to define a period when the requirements of symptom control must be met and adds a perspective that is useful when considering the risk-benefit ratio for an individual patient.10 Accurate estimations of survival also are valuable in helping patients define their own treatment preferences. In a large cohort of terminally ill cancer patients, substantially different treatment preferences were expressed by patients based on their understanding of anticipated survival.12 To achieve the goal of making care at the end of life consistent with their values, patients and families need to be openly informed of such estimates.1,4
Palliative care ideally selects treatment that will maximize quality of life and minimize complications. Although consideration of risk in terms of treatment related toxicity or morbidity and mortality is an important part of the surgical decision making process, attention to this element should not be the sole factor in making decisions about palliative therapy. A balanced view is essential as risk assessment techniques honed in the practice of patient selection for curative procedures may overly influence the clinician's decision to deny an opportunity for quality of life improvement during the obvious terminal phase of a patient's life. Yet the overly zealous intervention in the actively dying patient seeking a futile procedure must be recognized and avoided. In this study, perioperative complications had a considerable influence of the patients’ already limited anticipated survival. Significant 30-day perioperative morbidity (29%) was seen following the palliative procedures evaluated in this study and was associated with increased time in hospital (mean 16 days) and a decreased chance of symptom resolution (17%). Death occurred within 30 days of operation in 11% of the palliative patients representing both progression of disease and acceleration of time to death due to complications. This is in marked contrast to the 30-day operative mortality of 0.4% associated with elective curative intent surgical oncology procedures at this institution. Palliative interventions were associated with a disproportionate 30-day operative mortality accounting for approximately one third of all deaths while representing only 6% of the procedures. The significant influence of palliative procedures on the overall operative mortality data from an institution also suggests that palliative intent should be considered as a specific data element when comparing surgical outcomes across institutions.
Observations from this study now provide some of the critical data required to frame these palliative decisions. Symptom resolution can be anticipated in 80% of patients though further intervention may be required for either new (25%) or recurrent (25%) symptoms. These procedures are associated with significant operative morbidity (40%), mortality (10%) and limited anticipated overall survival (approximately 6 months). Further expansion of this database will permit more precise symptom, disease and patient specific descriptions of these global observations and aid in the determination of therapeutic superiority, optimal timing and overall effectiveness of surgical palliation of advanced cancer.
Discussions
Dr. William C. Wood (Atlanta, Georgia): Dr. Miner, I congratulate you first on reporting this experience, which is 10 times the size of the majority of the publications on surgery for palliation in the literature to this point. It is another in a series of contributions by you and your co-authors to this field.
We now have some outcome data from extensive experience in an area with little prior critical examination, and it is humbling that in only 80% are we able to achieve symptom control, and that in less than half of these does this persist. I have an observation and 2 questions for you, Dr. Miner.
First, I have found that the toughest decisions are those made with the patient not to perform palliative surgery; for example, to allow a uremic death from a recurrent pelvic tumor rather than relieve that obstructive uropathy and allow progressive pelvic nerve pain and a more miserable death.
My first question: Can you now identify for us the 20% of operations that are not likely to result in symptom improvement, those that we should avoid? For example, attempts at GI fistula closure, if I understand your table.
The second question: Persold's group from Ulm, Germany, have suggested that much improvement could be achieved in the care of terminally ill patients if we move from palliative symptom control to anticipatory surgery for impending events and operated on the patients who are more fit rather than waiting until they are more frail and actually suffering from these symptoms? Your comments, please.
Dr. Thomas J. Miner (Providence, Rhode Island): The patients in this series were clearly highly selected for operation. They represent the patients we decided to perform a procedure on. Mostly, one would assume, people we thought would do best. Because the selection bias in our patients is so high, it is difficult to determine, however tempting, which patients are best qualified for each particular palliative intervention. The much harder question is: What happens to those patients we say “no” to? This is something we are unsure of and currently investigating. These data will be required to answer that question in the future.
During the study we also asked the surgeon the surgical intent of the procedure. We specifically asked whether the procedure was done explicitly for active symptoms or in anticipation of future symptoms. Initial results from this data set have yet to be presented but confirm a principle demonstrated in this report. We are fairly good at fixing anticipated symptoms. However, as patients progress through their disease, other symptoms often develop. Furthermore, procedure related morbidity is still significant and anticipated survival does not appear to be substantially longer. Additional care and involvement from the surgeon is often required beyond the management of those anticipated symptoms.
Footnotes
Reprints: David P. Jaques MD, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021. E-mail:
REFERENCES
- 1.Foley KM, Gelbrand H. Improving palliative care for cancer, Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 2.The American College of Surgeons Committee on Ethics. Statement on principles guiding care at the end of life. Bull Amer Col Surg. 1998;83:4. [PubMed] [Google Scholar]
- 3.Miner TJ, Jaques DP, Tavaf-Motamen H, et al. Decision making on surgical palliation based on patient outcome data. Amer J Surg. 1999;177:150–154. [DOI] [PubMed] [Google Scholar]
- 4.Miner TJ, Jaques DP, Shriver CD. A prospective evaluation of patients undergoing surgery for the palliation of an advanced malignancy. Ann Surg Oncol. 2002;9:696–703. [DOI] [PubMed] [Google Scholar]
- 5.McCahill LE, Krouse RS, Chu DZ, et al. Decision making in palliative surgery. JACS. 2002;195:411–422. [DOI] [PubMed] [Google Scholar]
- 6.Easson AM, Crosby JA, Librach SL. Discussion of death and dying in surgical textbooks. Am J Surg. 2001;182:34–39. [DOI] [PubMed] [Google Scholar]
- 7.Martin RC, Jaques DP, Brennan MF, et al. Achieving R0 resection for locally advanced gastric cancer: is it worth the risk of multiorgan resection? JACS. 2002;194:568–577. [DOI] [PubMed] [Google Scholar]
- 8.Miner TJ, Jaques DP, Karpeh MS, et al. Defining palliative surgery in patients receiving non-curative resections for gastric cancer. JACS. 2004; in press. [DOI] [PubMed]
- 9.Porzsolt F, Tannock I. Goals of palliative cancer therapy. J Clin Oncol. 1993;11:378–381. [DOI] [PubMed] [Google Scholar]
- 10.Miner TJ, Jaques DP, Paty P, et al. Symptom control of locally recurrent rectal cancer. Ann Surg Oncol. 2002;10:72–79. [DOI] [PubMed] [Google Scholar]
- 11.Krouse RS, Nelson RA, Farell BR, et al. Surgical palliation at a cancer center: incidence and outcomes. Ach Surg. 2001;136:773–778. [DOI] [PubMed] [Google Scholar]
- 12.Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–1714. [DOI] [PubMed] [Google Scholar]