Abstract
Objective:
To describe a standardized technique for ileal graft procurement in the setting of living related bowel transplantation.
Summary Background Data:
Living donor transplantation has been successfully developed for kidney, liver, pancreas, and lung transplantation. More recently, living related small bowel transplantation (LR-SBTx) has been developed with the aim of expanding the pool of intestinal graft donors and reducing the mortality in patients on the waiting list. To date, a total of 25 LR-SBTx worldwide have been reported to the international registry. We herein report the largest single center experience.
Methods:
A segment of ileum, 150 to 200 cm, is resected 20 cm proximal to the ileocecal valve (ICV), which is always preserved. The arterial inflow is given by the terminal branch of the superior mesenteric artery and venous outflow by a proximal segment of the superior mesenteric vein. The entire bowel is measured intraoperatively and at least 60% of intestine length is left in the donor.
Results:
Since 1998, we have performed 9 terminal ileum resections for small bowel donation. None of the donors has experienced persistent alteration of bowel habits or malabsorption; only 1 minor wound complication has occurred.
Conclusions:
Terminal ileal resection with preservation of the ICV seems to assure fast functional recovery of the donor and has minimal postoperative complications.
Living donor small bowel transplant can represent a valid treatment of patients with intestinal failure. The aim of this paper is to describe the evaluation protocol used for the small bowel donor, the surgical technique for the procurement of the graft, and to report on the clinical follow-up of our donors.
In the last decade, small bowel transplant (SBTx) has become a valuable surgical option to treat selected patients affected by irreversible intestinal failure.1–4 Total parenteral nutrition (TPN) still represents the first therapeutic line in the management of this disease. However, those patients who develop life-threatening complications are considered for SBTx. Currently, cadaveric small bowel allografts are associated with satisfactory outcomes.2,5 Although there is a relatively small number of candidates for SBTx, the waiting time is relatively long, averaging 220 days, and mortality on the waiting list is very high.6 In pediatric patients, age 0 to 5 years, reported mortality on the waiting list is up to 60%.6
Living donor small bowel transplant (LR-SBTx) is not routinely performed yet, but it can represent a valid option for patients with intestinal failure. It can reduce waiting time, thus decreasing mortality and preventing progression of the complications caused by TPN. To date, a total of 25 cases of LR-SBTx have been reported to the international Intestinal Transplant Registry (ITR) and the United Network for Organ Sharing (UNOS).6,7
The operation first proposed by Gruessner consists of the use of a long segment of donor terminal ileum as the graft for the recipient. The inflow is given by the ileocolic branch of the superior mesenteric artery (SMA) and the outflow by the ileocolic effluent of the superior mesenteric vein (SMV).8 Excellent results in terms of function have been obtained by transplanting grafts of about 200 cm in the adult patients, whereas in the pediatric patients the length of the graft can be reduced to 150 cm. Since 1998, we have performed 9 terminal ileum resections for small bowel donation, which represents the largest single center series. In all cases the ICV, in continuity with a segment of 20 cm of terminal ileum and at least 60% of small bowel, have been preserved in the donor. In this paper, we describe the evaluation protocol used for the small bowel donor, the surgical technique for the procurement of the graft, and report on the clinical follow-up of our donors.
MATERIALS AND METHODS
Patients
From 1998 to 2003, 9 adult patients (mean age 31 years, range: 25–53 years), 5 male and 4 female, 7 white and 2 African American, underwent terminal ileal resection for small bowel donation. The recipients (6 adults and 3 children) were affected by irreversible intestinal failure due to: 3 gastroschisis (pediatric recipients), 3 short bowel syndrome (SBS) posttrauma, 1 SBS secondary to SMA thrombosis, 1 SBS secondary to Crohn's disease, and 1 SBS secondary to Churg-Strauss syndrome. Mean follow-up for the donors is 25 months (range: 1–54 months). Demographic donor information has been obtained by review of their hospital records. Information regarding bowel habits, weight, and general conditions has been obtained by phone interviews or personal visits.
Donor Selection
Potential living related small bowel donor selection starts with the preliminary determination of ABO blood type and human leukocyte antigen (HLA).
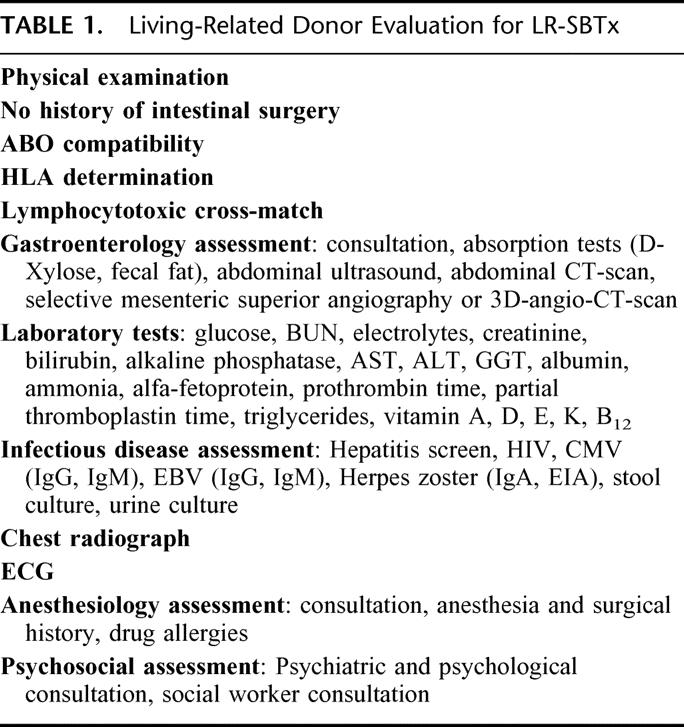
The donor and recipient ABO blood types must be compatible; in the presence of multiple potential donors, the candidate with best HLA match is selected. A careful evaluation of possible cardiopulmonary risk factors must follow; Table 1 summarizes the stepwise evaluation of potential donors for LR-SBTx.
TABLE 1. Living-Related Donor Evaluation for LR-SBTx
The size match between the donor and recipient, critical in cadaver cases, is less critical in the setting of LR-SBTx because only a segment of small bowel is used.
To evaluate anatomy and patency of the donor's superior mesenteric vessels, conventional selective angiography is performed. Abdominal ultrasound and CT scan complete the study of the donor's abdomen to rule out unknown associated pathologies. In the last 4 donors, the evaluation of the splanchnic vasculature was performed with angio-CT scan and 3-dimensional imaging reconstruction. This test provides images that are comparable to those of the angiography, but is less invasive and shortens the list of tests that the donor must undergo (Fig. 1).9
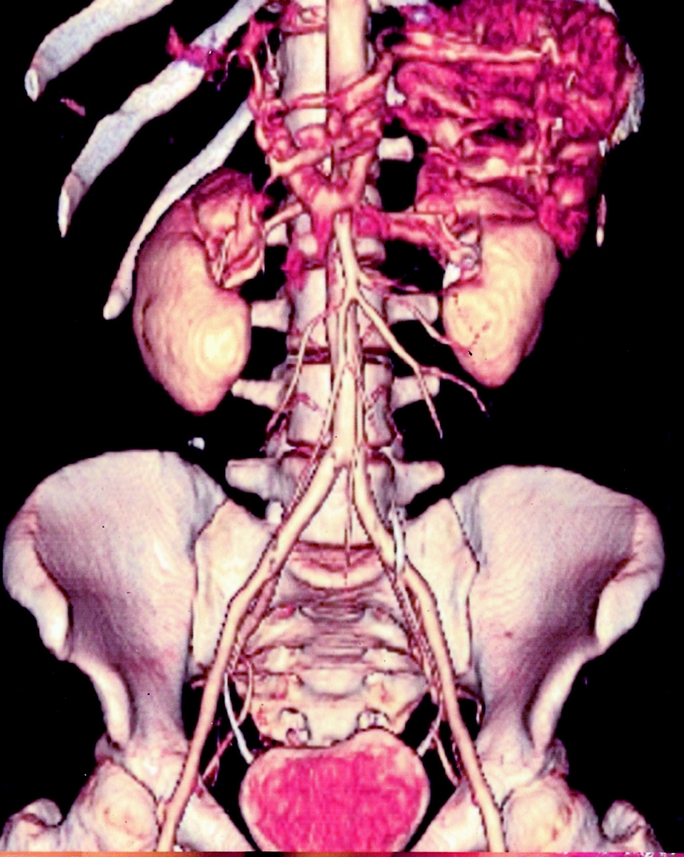
FIGURE 1. 3D CT scan reconstruction of superior mesenteric artery (SMA) and its major branches.
Intestinal decontamination of the graft is performed with a mechanical bowel preparation 1 day prior to surgery. Two doses of 45 mL of phosphosoda are given 4 hours apart on the afternoon before the surgery. Three doses of Neomycin 1 g per os and metronidazole 500 mg are given 18, 17, and 10 hours prior to surgery. A single perioperative dose of antibiotic prophylaxis with intravenous cefoxitin 2 g IV is also administered.
Anatomy
The ileum is supplied by the SMA, the intestinal branches of which reach the attached border of the bowel, and run between the serous and muscular coats with frequent inosculations to the free border, where they also anastomose with other branches running around the opposite surface of the gut. From these vessels, numerous branches are given off, which form an intricate plexus in the submucous tissue. The veins have a similar course and arrangement to the arteries.
The SMA is a large vessel that supplies the entire length of the small intestine; it also supplies the cecum and the ascending part of the colon and about one half of the transverse portion of the colon (Fig. 2).10,11
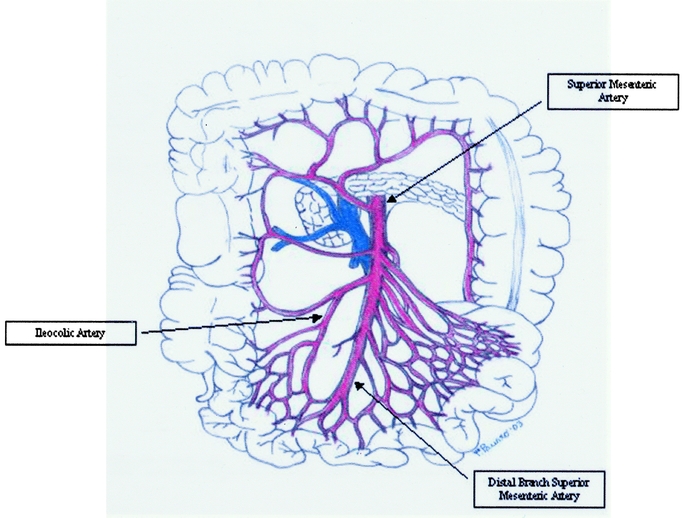
FIGURE 2. Small bowel vascular anatomy: SMA, ileocolic artery (IA) and its major branches.
The intestinal arteries arise from the convex side of the SMA. They are usually between 12 and 15 in number, and are distributed to the jejunum and ileum. The IA is the lowest branch arising from the concavity of the SMA. It passes downward and to the right within the mesentery toward the right iliac fossa, where it divides into a superior and an inferior branch; the inferior anastomoses with the end of the SMA, the superior with the right colic artery.
The inferior branch of the IA runs toward the upper border of the ileocolic junction and supplies the colic, anterior and posterior cecal, the appendicular artery, and the ileal branches. The colic branch passes upward on the ascending colon, the anterior and posterior cecal are distributed to the front and back of the cecum, and the appendicular artery descends behind the termination of the ileum and enters the mesenteriole of the vermiform process; it runs near the free margin of this mesenteriole and ends in branches that supply the appendix. The ileal branch runs upward and to the left on the lower part of the ileum, and anastomoses with the termination of the SMA.
To maintain optimal blood flow to the remaining small bowel of the donor and with the aim of preserving the branches of the ileocolic artery feeding the ileocecal valve (ICV), the vessel chosen for the inflow of the graft is the terminal branch of the superior mesenteric artery. By transillumination of the mesentery and palpation, this branch is recognized just distal to the take-off of the ileocolic branch. The jejunal and ileal branches of the SMA take-off proximal to the take-off of the ileocolic artery and the distal branch of the SMA and assures normal blood flow to the jejunum and proximal ileum. The segment of the SMV that drains the graft is identified just next to the arterial branch.
Surgical Technique
After administration of preoperative prophylactic antibiotics, the patient is brought to the operating room. An epidural catheter is placed for analgesia. A midline laparotomy from just above the umbilicus to the pubis is performed. After a general exploration of the abdominal cavity, the length of the small bowel is measured using an 18-inch umbilical tape. The umbilical tape is placed over the antimesenteric border of the small bowel. First, the bowel is measured in its entire length from Treitz to ICV. Then, after controlling the position of the vascular arcades of the last portion of the terminal ileum, the 200 or 150 cm of bowel destined to become the graft is measured, starting from 20 cm from the ICV. Lastly, the remainder of the bowel is measured again to assure that at least 60% is left for the donor. The segment of ileum to become the graft is marked with simple stitches of different material to recognize proximal and distal ends on the back table. Manipulation of the bowel induces peristalsis; it is not unusual that the same bowel segment measured more than once yields different lengths. It has become our practice to accept the first measurement as the final one.
The location of the distal branch of the SMA is identified with palpation and transillumination. The mesentery is scored and the terminal branch of the SMA is dissected free from the take-off of the ileocolic branch distally for about 2 cm. All the major branches of the SMA supplying the jejunum and proximal ileum are left intact. Small side branches (less than 1 mm in diameter) that rarely are present in the dissected segment of the SMA branch can be tied off. The segment of the SMV draining the graft is visualized next to the artery and dissected free for 2 to 3 cm. A segment of about 2 cm of free vessel is needed for the anastomosis in the recipient. Mobilization of the vessels is facilitated with the use of vessel loops (Fig. 3). Next, the mesentery is divided in a “V”-shaped fashion with the tip of the V at the vessel take-off and extending toward the stitches marking the segment of ileum to become the graft. Lastly, the small bowel is divided using a gastrointestinal anastomosis stapler.
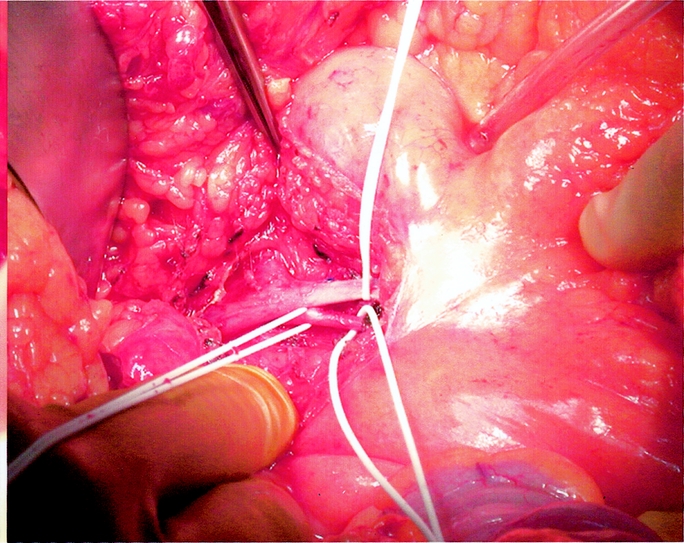
FIGURE 3. Distal portion of the SMA and SMV are dissected.
The removal of the graft is performed applying spoon vascular clamps to both vessels 1–2 mm distal to the take-off of the ileocolic vessels. The vascular tissue left on the clamp will allow safe closure of the stumps without jeopardizing the in- and outflow in the remaining bowel. Once the vessels are cut and the graft is removed (Fig. 4), the stumps are oversewn with 5–0 polypropylene sutures in running fashion. On the back table, the graft is perfused through the artery with University of Wisconsin solution until the perfusate is clear.
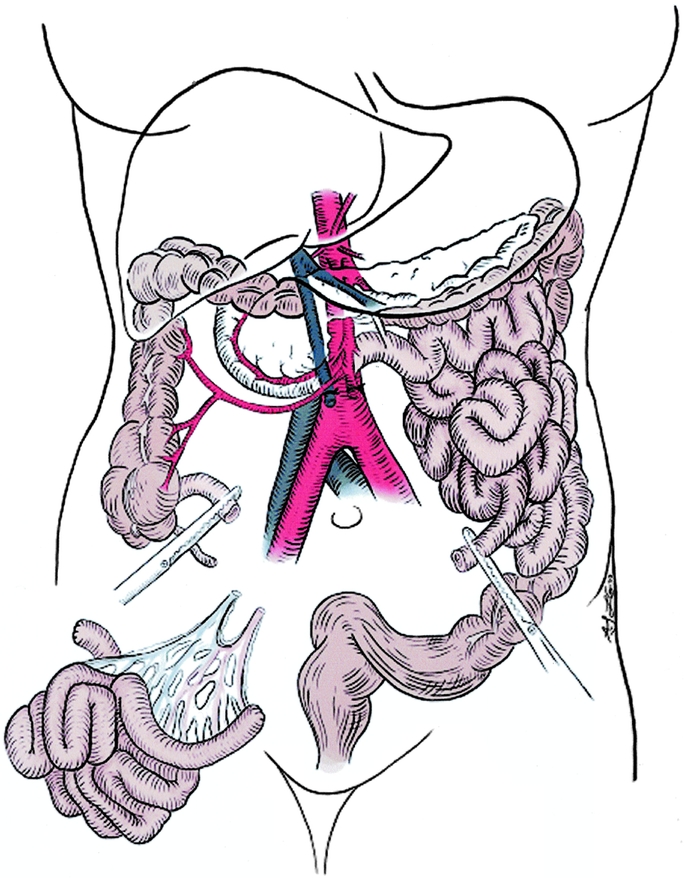
FIGURE 4. Operative sketch at the completion of bowel resection.
The 2 ends of the small bowel are approximated in a side-to-side fashion, and a functional end-to-end stapled anastomosis is performed. The abdomen is irrigated with saline solution, and the incision is closed in standard fashion.
Statistical Analysis
For univariate data analysis, categorical variables were analyzed using the χ2 test and, when applicable, Fisher exact test. Continuous variables were analyzed parametrically using the t test and nonparametrically using the Mann-Whitney U test. For all univariate statistical tests, P values less than 0.05 were considered significant.
RESULTS
All the procedures were completed as planned. The median operative time was 118 minutes (range: 95–175 minutes). None of the donors required blood transfusion during or after surgery. There was no operative mortality or morbidity. The length of postoperative hospital stay ranged between 3 and 4 days. One donor suffered a minor wound infection that healed promptly with conservative treatment. No donor had a significant change in intestinal function in the early postoperative period, and all patients were discharged home on regular diet. During the period of close follow-up, ranging from 1 to 57 months, no donor has had complaints of significant modification of preoperative bowel habits, weight loss, or change in diet. The total number of bowel movements (BM) was reported to range between 1 and 3 per day, with a mean of 1.5/d. This change was not statistically different from before the operation. By retaining the most distal 20 cm of terminal ileum, no donor has developed macrocytic anemia due to vitamin B12 deficit. Vitamin B12 assays were available in 6 of the donors at an interval ranging from 3 months to 4 years postdonation. The mean vitamin B12 serum level was 371 pg/mL (range: 283–700), with a normal value at our institution ranging from 250 to 1,100 pg/mL. All of the levels were found to be within normal limits. Body weight decreased in only 3 patients in the postoperative period. The average weight loss was 2.5 kg (range: 0–5 kg), which was not statistically significant. No donor reported changes in lifestyle, work habits, or psychosocial conditions after the small bowel donation.
In all 9 cases, the segmental ileal graft has provided enough function to enable the recipients to wean off TPN at least initially. The 1- and 3-year actuarial patient and graft survival for our 9 recipients has been 78% and 67%, respectively. One recipient lost his graft to posttransplant lymphoproliferative disease. At the present time, all recipients are off TPN.
COMMENTS
Living donor small bowel transplantation is a relatively new procedure.8,12,13 In selected cases, it is a very good treatment for irreversible intestinal failure, greatly shortening the time that the patients remain on TPN.6 Intestinal failure requiring long-term TPN is plagued by line infection, sepsis, central veins thrombosis, repeated procedures for central venous access, cholestatic liver disease, and liver failure.1,2 In pediatric patients, the situation is worsened by growth failure.14 The quality of life for patients supported by long-term TPN is generally poor and the cost to society is very high.13 Most importantly, the mortality on the waiting list for a cadaveric graft has been reported to be as high as 25% for adult recipients and 60% for pediatric recipients.6,7 Because LR-SBTx virtually eliminates the waiting time, it is an especially attractive option in pediatric recipients. A total of 25 cases of LR-SBTx have been reported to the United Network for Organ Sharing (UNOS) and the Intestinal Transplant Registry (ITR). The patient and graft survival has been similar to the rates achieved with bowel transplant from cadaveric donors.7 Since we started our program of LR-SBTx in 1998, we have performed 9 cases to date. Our patient and graft survival compare favorably with the outcomes of cadaver bowel transplant reported by ITR and UNOS.
Temporary, partial organ insufficiency is expected in both the donor and the recipient in all living donor operations. It may manifest itself as early increased creatinine in kidney transplantation,15 bilirubin in liver transplantation,16 and diarrhea in small bowel transplantation.8 The clinical concept of living donor transplantation is that the organ insufficiency is partial and temporary, and that the donor will regain his function entirely, while providing enough to support the recipient. In living donor small bowel transplantation, when total recovery of organ function is not obtained, the donor may suffer from persistent diarrhea. Conversely, if not enough intestine is provided to the recipient, independence from TPN may not be gained. A small bowel graft consisting of only 60 cm of distal jejunum and proximal ileum proved insufficient to achieve TPN independence in the recipient,17 whereas the donor of a graft consisting of distal ileum, ICV, and a portion of the cecum experienced a long period of diarrhea and dysvitaminosis.12 Two other reports of attempts at jejunum transplantation resulted in vascular complications due to complex vascular reconstructions.18 The goal of donor safety and recipient TPN independence has been achieved by transplanting a sufficient length of the distal ileum with the preservation of the ICV in the donor. The preservation of the last 20 cm of the terminal ileum and ICV prevents lipid and/or vitamin B12 malabsorption, and does not accelerate intestinal transit time in the donor. Furthermore, the distal SMA and SMV constitute a single vascular pedicle of adequate size. To date, we did not experience any vascular thrombosis. The first living donor small bowel transplantation using a standardized technique was performed by Gruessner8 and has constituted the conceptual and technical basis for the 9 living donor small bowel transplants performed at our center. The length of the graft is a balance between patient and donor needs. It has been well demonstrated that intestinal grafts develop early adsorption adaptation, characterized by increased length and size of the villi.19 A similar adaptation, although of less magnitude, is also expected in the donor. On the basis of all these considerations, we decided to use 150–200 cm of small bowel consisting of the terminal ileum, preserving the last 20 cm. We found that a graft of around 200 cm in adult recipients and 150 cm in pediatric recipients is sufficient to obtain TPN independence. The donor's vascular anatomy is important in guiding the resection of the distal ileum. By choosing as the graft inflow the distal branch of the SMA direct vascularization to the distal 20 cm of the ileum, the ICV and the proximal cecum is preserved. The other option for the vascular inflow of the graft could be the ileocolic artery, but choosing this vessel would mean to leave only the right colic and the terminal branch of the SMA to provide inflow for the proximal cecum and the ICV. Theoretically, this could constitute an area of low-flow/ischemia with potential problems for the healing of the ileoileostomy and the motility of this portion of the intestine.
The length of bowel left in the donor is the other important technical point. There is no precise method to obtain bowel length in the donor prior to the operation. Measuring the bowel from IV to ligament of Treitz intraoperatively remains the only option. Nonetheless, even intraoperatively, repeated measurements yield different lengths. It seems that the use of an umbilical tape with minimal manipulation of the bowel to avoid stretching and stimulating peristalsis remains the method that yields the most accurate measurement. Because the presence of the ICV is a protective factor against fast intestinal transit, we have been very careful to leave at least 60% of the initial length to the donor. Although the number of our donors is still relatively small, this policy has proved successful, because none of the donors has experienced significant changes in bowel habits or weight loss. The period of partial intestinal insufficiency that can be characterized by increased number of BM/d, weight loss, and vitamin B12 deficiency has been limited in time and is of minimal discomfort for the donor.
At the present time, no donor has complained of shortened intestinal transit time, new onset of food intolerance, or had signs of anemia due to vitamin B12 malabsorption. Longer follow-up is necessary to determine the incidence of postsurgical intestinal adhesions, which could be the only real long-term complication in small bowel donors.
These early results from the largest cohort of small bowel donors reported seem to indicate that in terms of donor safety, living donor small bowel transplantation can be proposed as a realistic solution for patients affected by irreversible intestinal failure. In comparison to liver, kidney, and pancreas living donor operations, the resection of the terminal ileum is technically less challenging and presents little immediate or long-term risks for the donor.
ACKNOWLEDGMENTS
The authors thank Peter S. Knight for editorial review of the manuscript and the surgical photograph (Fig. 3). We also wish to thank Laura Gibson and Allison Long for the sketch of the bowel resection (Fig. 4).
Footnotes
Reprints: Enrico Benedetti, MD, FACS, Associate Professor of Surgery, Chief, Division of Transplantation, University of Illinois at Chicago, 840 South Wood Street, Clinical Sciences Building, Room 402, Chicago, IL 60612. E-mail: enrico@uic.edu.
REFERENCES
- 1.Kaufman SS, Atkinson JB, Bianchi A, et al. Indications for pediatric intestinal transplantation: a position paper of the American Society of Transplantation. Pediatr Transplant. 2001;5:80–87. [DOI] [PubMed] [Google Scholar]
- 2.Fishbein TM. The current state of intestinal transplantation. Transplantation. 2004;78:175–178. [DOI] [PubMed] [Google Scholar]
- 3.Kato T, Thompson JF, Eskind LB, et al. Intestinal and multivisceral transplantation. World J Surg. 2002;26:226–237. [DOI] [PubMed] [Google Scholar]
- 4.Goulet O, Michel JL, Brousse N, et al. Can intestinal transplantation constitute treatment for intestinal failure ? Ann Chir. 1999;53:412–421. [PubMed] [Google Scholar]
- 5.Fishbein TM, Kaufman SS, Florman SS, et al.Isolated intestinal transplantation: proof of clinical efficacy. Transplantation. 2003;76:636. [DOI] [PubMed] [Google Scholar]
- 6.The United Network for Organ Sharing (UNOS) registry. Available at: http://www.unos.org.
- 7.The Intestinal Transplant Registry (ITR). Available at: http://www.intestinaltransplant.org/. Accessed January 7, 2002.
- 8.Gruessner RW, Sharp HL. Living-related intestinal transplantation: first report of a standardized surgical technique. Transplantation. 1997;11:271–274. [DOI] [PubMed] [Google Scholar]
- 9.Panaro F, Testa G, Balakrishnan N, et al. Living related small bowel transplantation in children: 3-dimensional computed tomography donor evaluation. Pediatr Transplant. 2004;8:65–70. [DOI] [PubMed] [Google Scholar]
- 10.Gray H, The Bartleby.com edition of Gray's Anatomy of the Human Body. http://www.bartleby.com.
- 11.Rohen JW, Yokochi C, Lutjen-Drecoll. Color Atlas of Anatomy: A Photographic Study of the Human Body, 5th edition. Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 12.Pollard SG. Intestinal transplantation: living related. Br Med Bull. 1997;53:868–878. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe B. Current indications for and prospects of living related intestinal transplantation. Curr Opinion Organ Transplant. 2000;5:290–294. [Google Scholar]
- 14.Ghobrial RM, Farmer DG, Amersi F, et al. Advances in pediatric liver and intestinal transplantation. Am J Surg. 2000;180:328–334. [DOI] [PubMed] [Google Scholar]
- 15.Ellison MD, McBride MA, Taranto SE, et al. Living kidney donors in need of kidney transplants: a report from the organ procurement and transplantation network. Transplantation. 2002;74:1349–1351. [DOI] [PubMed] [Google Scholar]
- 16.Beavers KL, Cassara JE, Shrestha R. Practice patterns for long-term follow-up of adult-to-adult right lobectomy donors at US transplantation centers. Liver Transpl. 2003;9:645–648. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi T, Suita S. Segmental small-intestinal transplantation: a comparison of jejunal and ileal grafts. Surgery. 2002;131(suppl):S294–S300. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto Y, Uemoto S, Inomata Y, et al. Small bowel transplantation using grafts from living-related donors. Two case reports. Transpl Int. 2000;13(suppl 1):S179–S184. [DOI] [PubMed] [Google Scholar]
- 19.Benedetti E, Baum C, Cicalese L, et al. Progressive functional adaptation of segmental bowel graft from living related donor. Transplantation. 2001;27:569–571. [DOI] [PubMed] [Google Scholar]


