Abstract
Objective:
This study aimed at: (1) documenting the evolution of surgical results of esophagectomy in a high-volume center, (2) identifying predictive factors of pulmonary complications and mortality, and (3) examining whether preoperative chemoradiation therapy would complicate postoperative recovery.
Summary Background Data:
Pulmonary complications and mortality rate after esophagectomy remain substantial, and factors responsible have not been adequately studied. Neoadjuvant chemoradiation is widely used; it is hypothesized that this may lead to adverse postoperative outcome.
Methods:
Prospectively collected data were used to analyze outcome in 421 patients with intrathoracic squamous cell esophageal cancer who underwent resection. Logistic regression analyses determined independent predictors of pulmonary complications and death. Two time periods were compared: period I (January 1990 to June 1995) and period II (July 1995 to December 2001). In the later period, neoadjuvant chemoradiation therapy was introduced.
Results:
Transthoracic resections were carried out in 83% of patients. Neoadjuvant chemoradiation was given to 42% of patients in period II. Major pulmonary complications occurred in 15.9%, and were primarily responsible for 55% of hospital deaths. Thirty-day and hospital mortality rates were 1.4% and 4.8%, respectively. Logistic regression analysis identified age, operation duration, and proximal tumor location as risk factors for pulmonary complications, whereas advanced age and higher blood loss were predictive of mortality. Chemoradiation did not lead to worse outcome. When period I and II were compared, hospital mortality rate reduced from 7.8% to 1.1%, P = 0.001, with correspondingly less blood loss (median blood loss was 700 ml (range: 200–2700 (period I) and 450 ml (range: 100–7000) (period II), P < 0.01).
Conclusion:
A 1.1% mortality rate was achieved in the last 6 years of the study period. Preoperative chemoradiation did not result in worse outcome. Reduction in mortality rate correlated with decreased blood loss.
After esophagectomy, advanced age, long operation duration, and proximal tumor location were associated with postoperative pulmonary complications; whereas advanced age and blood loss were independent predictors for hospital death. Neoadjuvant chemoradiation did not lead to more adverse outcome. Reduction in mortality rate during the study period correlated with decreased blood loss.
In 1940, Oschner and DeBakey reviewed the world literature and collected reports of 191 esophageal resections with a 72% mortality rate.1 Much progress has since been made, but even in recent reports, a hospital mortality rate of around 10% is still encountered.2,3 In a multicenter trial of preoperative chemotherapy involving 802 patients published recently, the 30-day mortality rate was 10%.4
Patients suffering from squamous esophageal cancer are frequently heavy smokers, consume much alcohol, and have chronic diseases. Many are also malnourished as a result of reduced food intake from malignant stenosis. The surgical trauma that esophagectomy imposes is perhaps the greatest among general surgical operations, often involving the abdomen, chest, and neck. The operation itself is technically complex, and the margin of error is small. It is not surprising that esophagectomy is associated with high complication and death rates.
The most frequently seen surgical complication after esophagectomy is anastomotic leakage, and contributes to substantial morbidity.5,6 As surgical technique and perioperative care improves, the incidence of leakage and its related morbidity and mortality is reduced in many specialized centers. The current anastomotic leakage rate in the authors’ institution is 3%, with an overall leak-related mortality rate of 1%.7 The most common medical complication is arrhythmia, but in most cases it is benign. Pulmonary complications are the most common serious morbidity after esophagectomy.8,9 Major respiratory complications can reach above 30%, even in experienced centers. Most report a complication rate of around 20%.10
Neoadjuvant therapies such as chemotherapy or chemoradiation have been widely applied in recent years.11 These treatment regimens have been variably linked to increased or similar postoperative complication rates,12–14 and their roles remain controversial.
The objectives of the present study were to (1) document the evolution of surgical care and results of esophagectomy in a high-volume center in the 1990s, (2) identify factors responsible for pulmonary complications and deaths after esophagectomy, and (3) examine whether introducing neoadjuvant chemoradiation therapy would complicate postoperative recovery.
METHODS
Between January 1990 and December 2001, 894 patients with esophageal cancer who had no prior treatment were managed at the Department of Surgery, University of Hong Kong Medical Center at Queen Mary Hospital. In this study, patients who had cervical esophageal cancers were excluded because of their different oncological characteristics and treatment protocols. Only patients with squamous cell cancers were included. Out of a total of 687 such patients, 421 underwent resection. These patients were the subjects of the present study.
Patient Management Protocols
Preoperative Preparation and Staging
Surgical treatment was the preferred treatment option. Patients were selected for nonsurgical treatment if they had locally advanced unresectable disease, or nonlocal–regional metastases, when medical–surgical risks were prohibitive, or in those who declined surgery.
Patients had basic hematological and biochemical tests, pulmonary function tests, electrocardiograph, and chest radiograph taken. Further cardiological assessments were selectively applied when indicated. All patients had a barium contrast study, an endoscopy, bronchoscopy, and since May 1996, endoscopic ultrasound examination. Other imaging studies included an ultrasound scan of the neck, and CT scan of the thorax and abdomen. Positron emission tomography scans were not available during the study period.
Patients were advised to stop smoking and to quit alcohol. Chest physiotherapy and incentive spirometry were instituted. High-caloric and high-protein diet supplements were given. An endoscopically placed fine-bore nasogastric feeding tube was used for feeding in those with high-grade stenosis, while awaiting completion of preoperative investigations.
Surgical Techniques
The surgical techniques have previously been described.15,16 A Lewis-Tanner esophagectomy via an abdominal right thoracotomy approach was most commonly used. For patients who had a tumor of the superior mediastinal segment, a 3-phase esophagectomy was carried out; an alternative was a split-sternum esophagectomy.17 Transhiatal esophagectomy was selectively applied for tumors of the lower third of the esophagus. It was performed mainly in a randomized controlled trial from 1990 to 1994 comparing transhiatal and transthoracic approach to lower third cancers, the results of which were published.18
Thoracoscopic esophageal mobilization was introduced in the latter part of 1994.19,20 This procedure was selected only for poor-risk patients, and it has largely replaced the need for transhiatal esophagectomy.
Lymphadenectomy usually involved a 2-field lymphadenectomy with dissection of lymph nodes around the celiac trifurcation and also an infracarinal mediastinal lymph node dissection. Lymph nodes of the superior mediastinum were sampled or resected when found. In patients who underwent transhiatal resection, no formal mediastinal lymphadenectomy was performed, and only accessible lymph nodes were sampled. Cervical lymphadenectomy was not carried out routinely for our study of recurrence patterns suggested limited value of neck dissection,21 and that survival advantage of cervical lymphadenectomy was not proven.22
Reconstruction of intestinal continuity was usually restored with a gastric conduit placed in the right thoracic cavity (after Lewis-Tanner esophagectomy), or via the orthotopic route when the anastomosis was carried out in the neck. In the obviously palliative cases where residual mediastinal disease was evident, the retrosternal route was chosen in a 3-phase esophagectomy. The colon was used in patients with a prior gastrectomy, the right ileo-colon being the preferred conduit.23 A hand-sewn anastomosis was constructed by a 1-layer continuous technique with absorbable monofilament suture. The circular stapler was also used during the early part of the study period.24
When a thoracotomy was performed, before 1995 a conventional chest tube was placed before closure and was connected to underwater seal (ARGYLE chest tube 28 Fr, Division of Sherwood Medical, USA). In 1995, pleural drainage was effected selectively by means of a small 18-Fr vacuum system (Astratech, Molndal, Sweden), and since 1996, it was used routinely.25
Patients were not given neoadjuvant or adjuvant treatment except in the context of clinical trials. A randomized controlled trial comparing preoperative chemotherapy and resection alone was carried out during 1989 to January 1995, the results of which have been published.26 Since mid-1995, chemoradiation was introduced and with this, a substantial change in treatment policy occurred. Patients with potentially resectable tumor on preoperative investigations entered into a trial that compared neoadjuvant chemoradiation and surgical resection alone. For patients with locally advanced tumors (T4) or nonregional metastatic spread (eg, cervical lymph nodes), they were palliated with upfront chemoradiation therapy. In those patients who showed significant downstaging, surgical resection was offered. The later 2 studies commenced in mid-1995.
Postoperative Care
Patients were routinely extubated in the recovery room unless they were judged to have poor cardiopulmonary reserve, or if surgery had been complicated by intraoperative events and prolonged. Patients were cared for in a high-dependency unit initially. They were given a limited amount of intravenous fluid for the first few days, usually 1 to 2 L over a 24-hour period. Intravenous albumin supplement was given for those whose albumin concentration was less than 35 g/L. Bronchoscopic suction of sputum was used liberally to aid expectoration of retained secretion and sputum. Routine feeding jejunostomy was not used. Epidural analgesia was the preferred postoperative analgesic method, which has been routine since 1989.
Patients were started on oral fluid usually by the fourth day after surgery and gradually advanced in volume. A contrast study was performed on the seventh day, and if normal, diet was advanced as tolerated.
Data Analysis
Complications were categorized as medical or surgical. Medical complications included cardiac (myocardial infarction, heart failure, arrhythmia), major pulmonary (bronchopneumonia and aspiration pneumonia [diagnosed with radiologic, clinical and microbiological features, with or without evidence of aspiration], and respiratory failure [diagnosed with blood gas criteria with or without ventilatory support]), renal failure, hepatic failure, and stroke. Surgical complications were recorded as anastomotic leakage, nonanastomotic leakage, gangrene of the conduit, chylothorax, hemorrhage, intraabdominal sepsis, recurrent laryngeal nerve palsy, and gastric outlet obstruction. A 30-day mortality was defined as death within 30 days of esophageal resection, whereas any death in the hospital after surgery was recorded as a hospital death. After recovery from surgery at Queen Mary Hospital, it was not uncommon for patients to convalesce at an offsite medical facility for socioeconomic reasons. Any death occurring during convalescence was also recorded as a hospital death, including that caused by progression or recurrence of malignant disease.
Two endpoints were specifically analyzed with logistic regression analyses for independent risk factors: major pulmonary complications and hospital mortality. Perioperative variables that were examined included patients’ age, gender, history of smoking, duration of presenting symptom, preexistent pulmonary or cardiac disease, diabetes, cirrhosis, abnormal electrocardiogram (ischemic changes or arrhythmia), abnormal chest radiograph, serum hemoglobin level, albumin level, arterial oxygen tension, arterial carbon dioxide tension, percent of predicted forced expiratory volume in 1 second (FEV1), percent of predicted forced vital capacity (FVC), and use of neoadjuvant therapy (chemotherapy or chemoradiation). Operative factors that were evaluated included the type of resection (transthoracic versus nontransthoracic), site of anastomosis, route of reconstruction, organ used for esophageal substitution, amount of blood loss, duration of operation, and the use of epidural analgesia for postoperative pain relief. Tumor-specific variables included the level of tumor (an upper third tumor defined as tumor located between the thoracic inlet and the tracheal bifurcation), pTNM stage, and the completeness of resection; R0 versus R1/R2 resections.
The study period was divided into 2 halves, from January 1990 to June 1995 (period I), and from July 1995 to December 2001 (period II). The reason for this is that neoadjuvant chemoradiation therapy was introduced in the second period. The aim was to examine its impact on surgical results. It was hypothesized that chemoradiation therapy could complicate postoperative recovery.
Continuous variables were expressed as mean (SD) when the variable is parametric and median (range) when nonparametric. Multivariate analyses were carried out with logistic regression using forward selection algorithm. Statistical significance was taken at P < 0.05. All statistical analyses were performed using the SPSS statistical package (version 8.0, SPSS Inc., Chicago, IL).
RESULTS
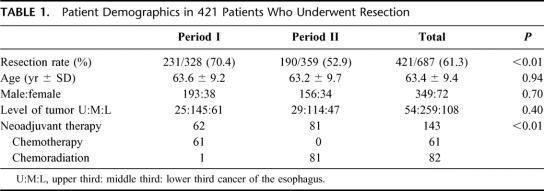
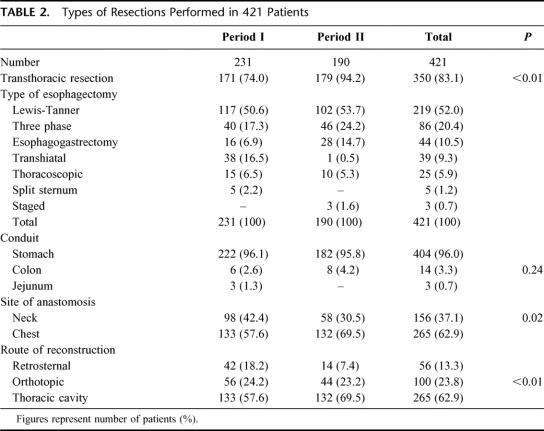
The demographics of the patients divided into 2 periods are shown in Table 1. The overall resection rate was 61.3% but was significantly less in period II (52.9%) compared with period I (70.4%), P < 0.01. This corresponded to the introduction of upfront chemoradiation for patients with advanced tumors (T4 or stage IV disease), with resections performed in those with significant responses, so the number of palliative resections was reduced. A total of 143 patients had preoperative therapy, 82 with chemoradiation and 61 had chemotherapy. Except in 1, all patients who had chemoradiation were managed after July 1995, whereas all patients with preoperative chemotherapy were resected before that time. The types of resections are shown in Table 2. Transthoracic resections were preferred for both periods, but were significantly more frequent in period II, when transhiatal resections were mostly abandoned after our randomized trial.18 The site of the esophageal anastomosis, and the route of reconstruction being more in the thoracic cavity in period II was also a reflection of this change in technique. The overall median blood loss for the study period was 600 mL (range, 100–7000 mL), and the median operation duration was 235 minutes (range, 120–520 minutes). The amount of blood loss was less for period II, the respective median blood loss for period I and II were 700 mL (range, 200–2700 mL) and 450 mL (100–7000 mL), P < 0.01. The operating times were 215 minutes (range, 120–520 minutes) and 255 minutes (range, 165–480 minutes) for periods I and II, respectively, P < 0.01.
TABLE 1. Patient Demographics in 421 Patients Who Underwent Resection
TABLE 2. Types of Resections Performed in 421 Patients
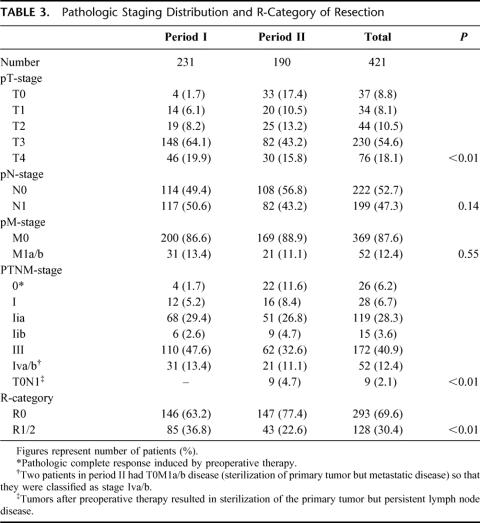
Pathologic stage distributions and R-category of resections are shown in Table 3. Tumor stage distributions differed when patients were compared before and after July 1995, stage III/IV diseases were found in 61% of patients in period I compared with 43.7% in period II, P < 0.01, and R0 resections were also more prevalent in period II, at 77.4% compared with 63.2%, P < 0.01.
TABLE 3. Pathologic Staging Distribution and R-Category of Resection
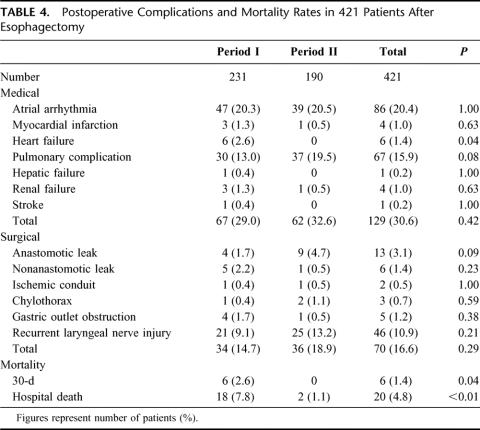
Postoperative complications and mortality rates are listed in Table 4. Atrial arrhythmias were most common, but most were benign and were reflective of underlying pulmonary problems or surgical sepsis.27 Major pulmonary complications occurred in 67 patients (15.9%), including pneumonias and respiratory failure. Temporary tracheostomy was performed in 97 patients (23%), mainly to aid sputum suction. The incidences of pulmonary complications were slightly higher in period II, though not reaching statistical significance, at 13% and 19.5%, P = 0.08. Other medical and surgical complications as listed did not differ significantly between the 2 time periods.
TABLE 4. Postoperative Complications and Mortality Rates in 421 Patients After Esophagectomy
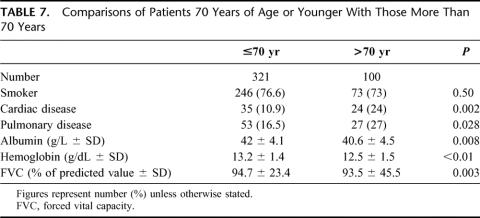
Both 30-day and hospital mortality rates were lower in period II. The primary causes of death were listed in Table 5. Pulmonary complications were responsible for 11 (55%) of in-hospital deaths. Since 1995, there were only 2 deaths, 1 from anastomotic leakage, and another patient died of a sudden myocardial infarction after apparent recovery from her esophagectomy.
TABLE 5. Primary Causes of Death
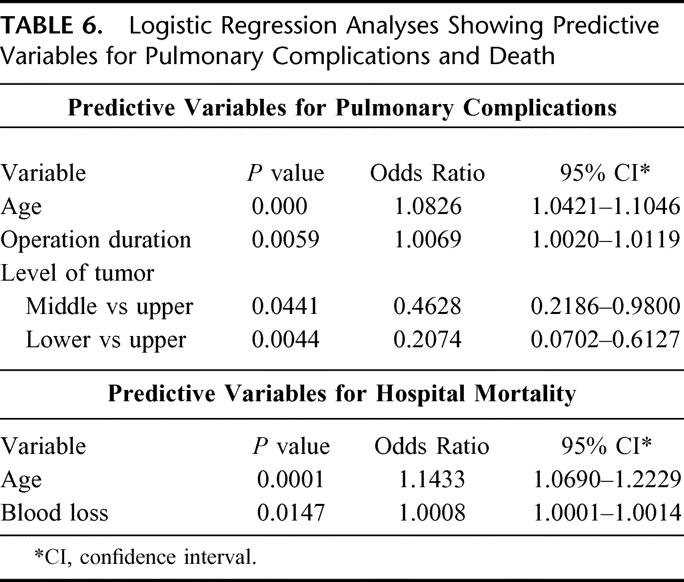
Logistic regression analyses were used to identify factors predictive of pulmonary complications and death. They are shown in Table 6. Advanced age, long operation duration, and proximal tumor location were factors predictive of pulmonary complications, whereas advanced age and more blood loss were predictive of hospital mortality. In particular, preoperative chemotherapy and chemoradiation were not identified as significant predictive factors.
TABLE 6. Logistic Regression Analyses Showing Predictive Variables for Pulmonary Complications and Death
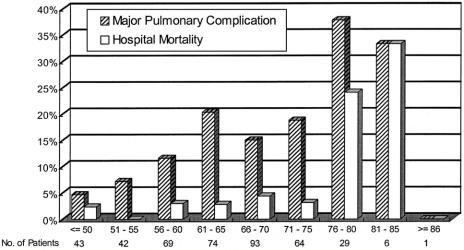
Patients with or without pulmonary complications were compared with regard to the factors identified on logistic regression. The mean age was 67.6 ± 8.2 years for patients with complications compared with 62.6 ± 9.36 for patients without, P < 0.01. The incidence of pulmonary complications was 25% in those older than 70 years of age, and was 13% in the younger patients, P = 0.006. Figure 1 shows the incidence of pulmonary complications with advancing age. In patients 70 years or younger, pulmonary complications developed in 9 of the 62 (14.5%) patients who had preoperative chemoradiation compared with 16 of 84 (19%) who had resection alone, P = 0.51. For patients older than 70 years, the corresponding figures were 4 of 19 (21.1%) and 8 of 25 (32%), P = 0.51.
FIGURE 1. The incidence of pulmonary complications and hospital mortality rates with respect to age.
The median operation durations were 255 minutes (range: 150–450) and 230 minutes (range: 120–520), P = 0.001, for those with and without pulmonary complications. Patients with pulmonary complications also had more proximal tumors. Distribution of tumor locations were 16 patients (24%) for upper third tumors; 42 (63%) for middle third tumors; and 9 (13%) for lower third tumors; compared with 38 (11%) for upper third tumors; 217 (61%) for middle third tumors; and 99 (28%) for lower third tumors for patients without pulmonary morbidity, P = 0.002. The chances of having pulmonary complications for patients with upper, middle, and lower third tumors were 29.6%, 16.2%, and 8.3%, respectively.
Patients who were and were not hospital deaths were compared with regard to the factors identified on logistic regression. The mean age was 71 ± 9.4 years for patients who died, compared with 63 ± 9.3 years for the survivors, P < 0.01. Hospital mortality rate was 11% in those older than 70 years, and was 2.8% in the younger patients, P = 0.002. Figure 1 shows the increase in mortality rate with advancing age. The median blood losses were 725 mL (range, 250–2700 mL) and 600 mL (range, 100–7000 mL) for patients who died and survived, respectively, P = 0.02.
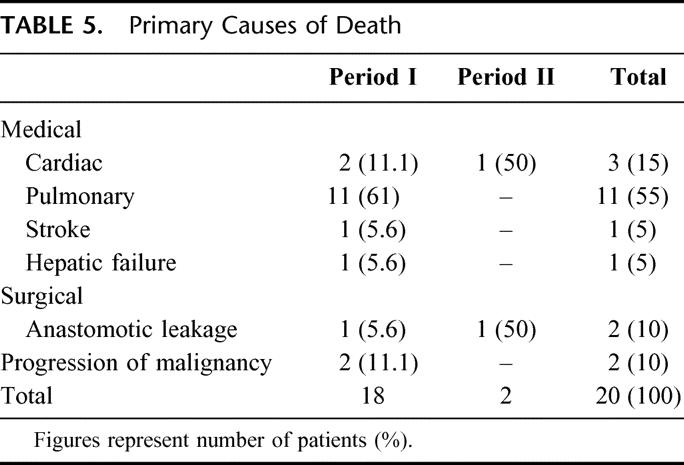
Because advanced age was an adverse factor for both pulmonary complications and mortality, patients younger than 70 years were compared with those who were older to identify differences in demographics, and operative and tumor factors (Table 7). Elderly patients had a higher prevalence of preexisting cardiopulmonary diseases, lower preoperative albumin and hemoglobin levels, and lower percent predicted FVC values. Other factors also tested included the prevalence of diabetes, cirrhosis, alcohol intake, pO2 and pCO2 levels, percent of predicted FEV1 value, location of primary tumor, the use of thoracotomy for resection, route of placement of the esophageal substitute organ, site of anastomosis, use of epidural analgesia, blood loss, duration of operation, stage of tumor, and the intent of resection (R0 versus R1/2). None was significantly different between the 2 groups (data not shown). To test for potential bias in the use of chemoradiation therapy in patients of different age groups, patients in period II were separately analyzed (because such treatment was only given in period II). The use of chemoradiation was not age related; it was similarly given to 62 of 146 (42.5%) patients 70 years or younger and to 19 of 44 patients (43.2%) older than 70 years, P = 1.0.
TABLE 7. Comparisons of Patients 70 Years of Age or Younger With Those More Than 70 Years
The incidence of pulmonary complications did not differ significantly between the 2 time periods. Of the 3 factors identified, only operation duration was longer in period II. For hospital deaths, mean age did not differ significantly between the 2 periods, but blood loss was less in period II, as described previously.
DISCUSSION
In this study, we have identified advanced age, tumor location above the tracheal bifurcation, and operation duration as independent risk factors for pulmonary complications, Similarly, advanced age and blood loss were predictive factors for hospital mortality. A hospital mortality rate of 1.1% was achieved in the most recent 6 years.
Previous studies from the authors’ institution that analyzed risk factors for esophagectomy looked at patients recruited over a long time period, and many were recruited in the 1980s.8,9 Much has changed in the management of esophageal cancer in the last decade. In this study, we examined patients treated in the 1990s, so that it reflected present-day management.
Pulmonary complications remain most common and serious after esophagectomy. Major pulmonary morbidities occurred in 15.9% of our patients, and were responsible for 55% of hospital deaths. This rate of respiratory morbidity is similar to that reported in the literature, although in some series it could be as high as 33%.3,12,28,29 Various factors have been implicated that could predispose to pulmonary morbidity: these include advanced age, history of smoking, cirrhosis and diabetes, abnormal chest radiograph or lung disease, spirometric or nutritional parameters, blood loss and volume transfused, low serum albumin, preoperative chemoradiation therapy, general performance status, inadequate postoperative analgesia, and stage of disease.8,12,29–35
In our patient cohort, advanced age was identified as a risk factor for both pulmonary complications and death after esophagectomy. The chance of developing a major respiratory complication was twice in those older than 70 years, and the death rate was 4-fold. The adverse factors responsible for worse outcome in elderly patients seemed to be the higher prevalence of preexisting cardiopulmonary diseases. Other tumor and operative factors were not significantly different. One should further strive to refine patient selection, and optimize patients’ cardiorespiratory status preoperatively in this group of at-risk patients. Whether correcting the lower preoperative albumin and hemoglobin deficits in elderly patients would improve outcome remains to be seen, although doing so would be intuitive.
Patients with proximally located tumor were also more at risk. Tumors behind the trachea are anatomically unfavorable, especially in the context of radical resections aiming at cure. One study demonstrated a higher proportion of T4 tumors, lower resectability rate, less R0 resections, more blood loss, and more recurrent laryngeal nerve injury for tumors above the tracheal bifurcation.36 In the present study, patients with upper third tumor had a 3.5 times risk of developing pulmonary complications compared with those with their lower third counterparts. The more extensive upper mediastinal dissection behind the trachea, and possibly more recurrent laryngeal nerve injury may have contributed to worse outcome.
Longer operation duration and more intraoperative blood loss were associated with more pulmonary complications and hospital deaths, respectively. Both factors may be associated with more advanced tumors, when tumor resection was more problematic, although the stage of tumor per se was not found to be significant on multivariate analysis. A longer operating duration, especially of one-lung anesthesia, may produce more atelectasis and predisposes patients to respiratory problems. Decreased blood loss and blood transfusion has been shown in other studies to correlate with decreased hospital death rate as well as long-term survival after major cancer surgery, such as hepatectomy.37 The reduction in blood loss in the second half of this study coincided with the lower mortality rate. The surgeon can play an active role in refining technique of resection, meticulous hemostasis can be achieved, and if this can also be accomplished expeditiously, better outcome is expected.
The mortality rate was reduced in period II and correlated with less blood loss. Other factors deserved more discussion, and may also have contributed to better results. These are related to 3 areas: patient selection, change in operative technique, and modifications of perioperative care.
Certainly patient selection was more stringent for period II. The resection rate was lower, and a lower pathologic stage distribution was evident. This was brought about by tumor downstaging by preoperative chemoradiation. In addition, the number of palliative resections was reduced because patients with locally advanced or metastatic tumors were treated by upfront chemoradiation, and only those with good response were selected for surgery. The relative contributions of selection and tumor downstaging were not easily segregated. However, the lower stage distribution was primarily a result of lower pT-stage; the p-N and p-M stages were not significantly different between the 2 periods. pT0 disease was found in 17.4% of patients in period II compared with 1.7% in period I, and this could only be a result of preoperative therapy. Indirectly, the amount of blood loss may be related to T-stage, because the difficulty of resection (hence blood loss) is more dictated by the locally advanced tumor, rather than distant metastases.
In studies that report on improvement of surgical results over time, more stringent patient selection often comes into play, whether by excluding patients with high medical risk34,38 or by treating those with advanced tumors by alternative means.39 One has to be cautious that overselection of patients for resection may deny many the benefits of tumor extirpation. Our resection rate of 52.9% even in period II was still high by comparison when compared with series with an unbiased referral pattern.40 In addition, we have evidence to show that our change in treatment strategy also resulted in better survival for the whole cohort of patients treated, whether by surgical resection or not.41
The main obvious change in surgical technique was the diminishing use of transhiatal resections in period II, and the advent of thoracoscopic esophagectomy. Transhiatal esophagectomy was mainly performed for patients recruited in our randomized trial, and no significant differences in blood loss and postoperative morbidity and mortality was found when compared with transthoracic resection.18 The surgical approach (transthoracic versus transhiatal) also was not identified to be of relevance by multivariate analysis. Thoracoscopic esophagectomy was introduced in 1994; however, it was only selectively applied and its frequency of use was similar in about 5% of patients for the 2 periods. In addition, it was not shown to result in lower complication rates when compared with transthoracic resections.19 Other differences in techniques between the 2 periods were either minor—for example, the use of a smaller chest drain,25—or are difficult to quantify, for instance, experience and various steps taken to reduce blood loss.
Perhaps the most important advance in perioperative care in esophagectomy in the 1990s was the use of epidural analgesia, which was shown to reduce complications and death rate.35 However, this technique was already routinely used during the whole study period, given to 82% of patients in the series. Again, other changes in postoperative management, for instance, being more vigilant in diagnosing complications, and more liberal in bronchoscopic sputum aspiration, though important, are difficult to measure.
There is increasing evidence that surgical volume and outcome are related in major cancer surgery, both in terms of mortality and cost.42–45 Our institution had experience in managing more than 2000 patients with esophageal and cardia cancer since 1982. The death rate has been reduced to 1.1% in the second half of the series. It is hoped that with further refinement in patient selection, surgical technique, and perioperative care, the mortality rate after esophagectomy can be eliminated.
Footnotes
Reprints: Professor John Wong, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, Hong Kong. E-mail: jwong@hku.hk.
REFERENCES
- 1.Oschner A, DeBakey M. Surgical aspects of carcinoma of the esophagus. J Thoracic Surg. 1941;10:401–445. [Google Scholar]
- 2.Jamieson GG, Mathew G. Surgical management of esophageal cancer: the Western experience. In: Daly JM, Hennessy TPJ, Reynolds JV, eds. Management of Upper Gastrointestinal Cancer. London: WB Saunders; 1999:183–199. [Google Scholar]
- 3.Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg. 2001;72:306–313. [DOI] [PubMed] [Google Scholar]
- 4.Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727–1733. [DOI] [PubMed] [Google Scholar]
- 5.Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: A review. Am J Surg. 1995;169:634–640. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi SK, Naunheim KS. Complications of transhiatal esophagectomy. Chest Surg Clin N Am. 1997;7:601–610. [PubMed] [Google Scholar]
- 7.Whooley BP, Law S, Alexandrou A, et al. Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer. Am J Surg. 2001;181:198–203. [DOI] [PubMed] [Google Scholar]
- 8.Law SY, Fok M, Wong J. Risk analysis in resection of squamous cell carcinoma of the esophagus. World J Surg. 1994;18:339–346. [DOI] [PubMed] [Google Scholar]
- 9.Whooley BP, Law S, Murthy SC, et al. Analysis of reduced death and complication rates after esophageal resection. Ann Surg. 2001;233:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75:217–222. [DOI] [PubMed] [Google Scholar]
- 11.Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562–572. [DOI] [PubMed] [Google Scholar]
- 12.Avendano CE, Flume PA, Silvestri GA, et al. Pulmonary complications after esophagectomy. Ann Thorac Surg. 2002;73:922–926. [DOI] [PubMed] [Google Scholar]
- 13.Doty JR, Salazar JD, Forastiere AA, et al. Postesophagectomy morbidity, mortality, and length of hospital stay after preoperative chemoradiation therapy. Ann Thorac Surg. 2002;74:227–231. [DOI] [PubMed] [Google Scholar]
- 14.Eguchi R, Ide H, Nakamura T, et al. Analysis of postoperative complications after esophagectomy for esophageal cancer in patients receiving neoadjuvant therapy. Jpn J Thorac Cardiovasc Surg. 1999;47:552–558. [DOI] [PubMed] [Google Scholar]
- 15.Law S, Wong J. Esophagogastrectomy for carcinoma of the esophagus and cardia, and the esophageal anastomosis. In: Baker RJ, Fischer JE, eds. Mastery of Surgery. Philadelphia: Lippincott Williams & Wilkins; 2001:813–827. [Google Scholar]
- 16.Law S, Wong J. Esophagectomy without thoracotomy. In: Baker RJ, Fischer JE, eds. Mastery of Surgery. Philadelphia: Lippincott Williams & Wilkins; 2001:828–836. [Google Scholar]
- 17.Moorehead RJ, Paterson I, Wong J. The split-sternum approach to carcinoma of the superior mediastinal esophagus. Dig Surg. 1989;6:114–117. [Google Scholar]
- 18.Chu KM, Law SY, Fok M, et al. A prospective randomized comparison of transhiatal and transthoracic resection for lower-third esophageal carcinoma. Am J Surg. 1997;174:320–324. [DOI] [PubMed] [Google Scholar]
- 19.Law S, Fok M, Chu KM, et al. Thoracoscopic esophagectomy for esophageal cancer. Surgery. 1997;122:8–14. [DOI] [PubMed] [Google Scholar]
- 20.Law SY, Fok M, Wei WI, et al. Thoracoscopic esophageal mobilization for pharyngolaryngoesophagectomy. Ann Thorac Surg. 2000;70:418–422. [DOI] [PubMed] [Google Scholar]
- 21.Law SY, Fok M, Wong J. Pattern of recurrence after oesophageal resection for cancer: clinical implications. Br J Surg. 1996;83:107–111. [DOI] [PubMed] [Google Scholar]
- 22.Law S, Wong J. Two-field dissection is enough for esophageal cancer. Dis Esophagus. 2001;14:98–103. [DOI] [PubMed] [Google Scholar]
- 23.Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch Surg. 2003;138:303–308. [DOI] [PubMed] [Google Scholar]
- 24.Law S, Fok M, Chu KM, et al. Comparison of hand-sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg. 1997;226:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law S, Boey JP, Kwok KF, et al. Pleural drainage after transthoracic esophagectomy: experience with a vacuum system. Dis Esophagus. 2004;17:81–86. [DOI] [PubMed] [Google Scholar]
- 26.Law S, Fok M, Chow S, et al. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114:210–217. [DOI] [PubMed] [Google Scholar]
- 27.Murthy S, Law S, Whooley BP, et al. Atrial fibrillation after esophagectomy is a marker for postoperative morbidity and mortality. J Thorac Cardiovasc Surg. 2003;126:1162–1167. [DOI] [PubMed] [Google Scholar]
- 28.Griffin SM, Shaw IH, Dresner SM. Early complications after Ivor Lewis subtotal esophagectomy with two-field lymphadenectomy: risk factors and management. J Am Coll Surg. 2002;194:285–297. [DOI] [PubMed] [Google Scholar]
- 29.Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg. 2000;231:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagawa H, Kobori O, Muto T. Prediction of pulmonary complications after transthoracic oesophagectomy. Can J Surg. 1994;81:860–862. [DOI] [PubMed] [Google Scholar]
- 31.Griffin S, Desai J, Charlton M, et al. Factors influencing mortality and morbidity following oesophageal resection. Eur J Cardiothorac Surg. 1989;3:419–423. [DOI] [PubMed] [Google Scholar]
- 32.Tsutsui S, Moriguchi S, Morita M, et al. Multivariate analysis of postoperative complications after esophageal resection. Ann Thorac Surg. 1992;53:1052–1056. [DOI] [PubMed] [Google Scholar]
- 33.Nishi M, Hiramatsu Y, Hioki K, et al. Pulmonary complications after subtotal oesophagectomy. Br J Surg. 1988;75:527–530. [DOI] [PubMed] [Google Scholar]
- 34.Bartels H, Stein HJ, Siewert JR. Preoperative risk analysis and postoperative mortality of oesophagectomy for resectable oesophageal cancer. Br J Surg. 1998;85:840–844. [DOI] [PubMed] [Google Scholar]
- 35.Tsui SL, Law S, Fok M, et al. Postoperative analgesia reduces mortality and morbidity after esophagectomy. Am J Surg. 1997;173:472–478. [DOI] [PubMed] [Google Scholar]
- 36.Kato H, Tachimori Y, Watanabe H, et al. Thoracic esophageal carcinoma above the carina: a more formidable adversary? J Surg Oncol. 1997;65:28–33. [DOI] [PubMed] [Google Scholar]
- 37.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siewert JR, Stein HJ, Feith M, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2000;232:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pye JK, Crumplin MK, Charles J, et al. One-year survey of carcinoma of the oesophagus and stomach in Wales. Br J Surg. 2001;88:278–285. [DOI] [PubMed] [Google Scholar]
- 41.Law S, Kwong DL, Kwok KF, et al. Improvement in treatment results and long-term survival of patients with esophageal cancer: impact of chemoradiation and change in treatment strategy. Ann Surg. 2003;238:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 43.Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119:1126–1132. [DOI] [PubMed] [Google Scholar]
- 44.Dimick JB, Cattaneo SM, Lipsett PA, et al. Hospital volume is related to clinical and economic outcomes of esophageal resection in Maryland. Ann Thorac Surg. 2001;72:334–339. [DOI] [PubMed] [Google Scholar]
- 45.Patti MG, Costantini M, Godwin DH, et al. A hospital's annual rate of esophagectomy influences the operative mortality rates. J Gastrointest Surg. 1998;2:186–192. [DOI] [PubMed] [Google Scholar]