Abstract
Objective:
To evaluate the efficacy of a self-expanding plastic stent in the treatment of thoracic leaks after esophagectomy for cancer.
Summary Background Data:
Anastomotic leaks are a major cause of morbidity and mortality after esophageal resection. Treatment options range from aggressive surgery to conservative management, but there remains much controversy on the best treatment.
Methods:
Over a 6-year period (1998–2003), esophagogastric leaks were observed in 19 of 204 patients (9.3%) after esophagectomy. Between 1998 and 2000, anastomotic leaks were managed by reexploration (n = 7) or by conservative treatment (n = 3). Since 2001, insertion of self-expanding plastic stents was performed for all anastomotic leaks (n = 9). The short-term efficacy and long-term outcome of both treatments were analyzed.
Results:
Self-expanding plastic stents were successfully placed in all patients without procedure-related morbidity. Immediate leak occlusion was obtained in 8 of 9 patients. The mean healing time (time to stent removal) was 29 days. Compared with the conventional treatment group, patients who were treated with stents had earlier oral intake (11 days versus 23 days), a less extensive intensive care course (25 days versus 47 days), and shorter hospital stay (35 days versus 57 days). In-hospital mortality was 0% (0 of 9 patients) in the stent group and 20% (2 of 10 patients) in the other group. After a mean follow-up of 12 months, none of the patients developed a stricture after stenting, but a stricture occurred in 1 patient after conservative treatment.
Conclusions:
Self-expanding plastic stents can reduce leak-related morbidity and mortality after esophagectomy and may be considered a cost-effective treatment alternative.
A longitudinal study was performed to compare the treatment of gastroesophageal anastomotic leaks with self-expanding plastic stents to conventional treatment. Stenting appeared to be a promising treatment alternative and may be increasingly used in patients with isolated leaks.
Despite advances in the various treatment modalities, surgery continues to be the mainstay of treatment of patients with resectable esophageal cancer. Five-year survival rates as high as 40% to 50% have been achieved after curative resection in specialized centers.1,2 Esophagectomy remains a challenging operation that can be associated with considerable postoperative morbidity and mortality.3,4 The most frequently reported major complications include respiratory failure and anastomotic leaks. Cervical anastomoses have been associated with leakage rates of 10% to 20%, but leak-related mortality is low.5,6 Leaks from thoracic anastomoses occur in 5% to 10% of the cases and carry mortality rates as high as 30% to 60%.7–9 The most efficient treatment of such leaks remains controversial. Aggressive surgical treatment has been recommended by some authors, whereas others advocate a more conservative approach using perianastomotic drainage, total parenteral nutrition, nasogastric decompression, and broad-spectrum antibiotics.10,11
The aim of this study was to prospectively evaluate the efficacy of self-expanding plastic stents in the treatment of thoracic anastomotic leaks after esophagectomy and to compare the results with conventional treatment.
MATERIALS AND METHODS
Patients
From 1998 to 2003, esophagectomy with an esophagogastric anastomosis was performed in 204 patients with esophageal cancer. The standard technique was en bloc esophagectomy with a 2-field lymph-node dissection in the upper abdomen and mediastinum using the Lewis approach. Reconstruction was achieved by a gastric conduit (n = 179). In patients with poor cardiopulmonary function and lower third tumors, an esophageal resection carried out through an abdominal approach alone was considered to be appropriate. In these patients, an esophagogastrostomy was fashioned transhiatally in the lower mediastinum. (n = 25). Leaks of the esophagogastric anastomosis occurred in 19 of 204 patients (9.3%). The mean age of the patients was 62 years (range, 41–79) with a male to female ratio of 17 to 2. Sixteen patients had adenocarcinoma and 3 patients had squamous cell carcinoma of the esophagus. From June 1998 to December 2001, postoperative anastomotic leaks after esophagectomy were diagnosed in 10 patients at a mean of 7.5 days (range, 1–15 days) after surgery. In this time period, patients were treated by reoperation or by conservative management. Since January 2001, 9 thoracic leaks were diagnosed at a mean of 7.7 days (range, 2–10 days) after esophageal resection. These patients were managed by stent placement, i.v. antibiotics, and interventional drainage. Patient data were analyzed from a prospective database. Overall the clinicopathological features of both patient groups were comparable (Tables 1 and 2).
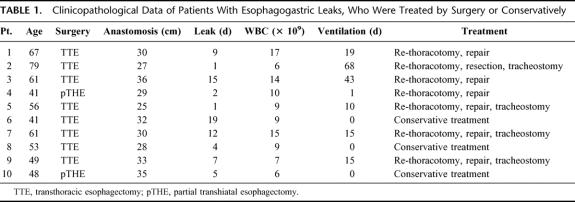
TABLE 1. Clinicopathological Data of Patients With Esophagogastric Leaks, Who Were Treated by Surgery or Conservatively
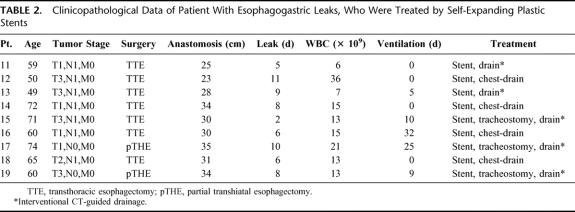
TABLE 2. Clinicopathological Data of Patient With Esophagogastric Leaks, Who Were Treated by Self-Expanding Plastic Stents
Surgical Technique
In 15 of the 19 patients, en bloc esophagectomy was performed using the Lewis approach.12 Transhiatal resection with an esophagogastrostomy in the lower mediastinum was performed in 4 high-risk patients. A pyloroplasty was not carried out. A stapled anastomotic technique (Proximate ILS, Ethicon GmbH, Norderstedt, Germany) using 21–25-mm cartridges was used in 18 patients. The stapled esophagogastric anastomosis was placed at the high point of the fundus away from the gastrotomy line. A nasogastric tube was introduced and the gastrotomy resection line was stapled and oversewn. Only 1 patient had a handsewn anastomosis.
Diagnosis and Treatment of Anastomotic Leaks
All patients underwent a routine contrast swallow examination between 5 and 10 days after the operation using water-soluble contrast. If there was an unclear contrast study or the clinical suspicion of a leak, the patients were examined by flexible upper gastrointestinal endoscopy. In critically ill patients, a methylene blue injection through the nasogastric tube was used as a diagnostic bedside test.
From June 1998 to December 2001, postoperative anastomotic leaks were diagnosed in 10 patients. Seven patients with extensive leaks and/or clinical signs of sepsis underwent reoperation. Reexploration with surgical repair was performed in 6 patients and resection of the esophagogastric anastomosis in 1 patient. Two patients required more than 1 reoperation, and a tracheostomy had to be fashioned in 4 patients, because of respiratory complications. Three asymptomatic patients with small volume leaks were managed conservatively. The nonoperative approach included absolute avoidance of oral intake, parenteral nutrition, nasogastric decompression, and i.v. application of broad-spectrum antibiotics.
Since January 2001, 9 consecutive patients with leaks of the esophagogastrostomy were managed by insertion of a self-expanding plastic stent (Polyflex-stent, Rüsch AG, Wiesbaden, Germany). The stent is commercially available worldwide and FDA approval was obtained in June 2003. The stent consists of an integral polyester braid that is completely covered with a silicone membrane (diameter: 2.5 cm, length: 9–15 cm). The proximal end of the stent is flared to prevent dislocation and warrant reliable leak occlusion (Fig. 1). Radioopaque markers at both ends and in the middle of the stent facilitate precise placement of the stent. The plastic stent was chosen instead of the more widely used metal stents because of its favorable characteristics. The soft material provides well-balanced radial force and adapts elastically to the esophageal wall, which warrants reliable leak occlusion (Fig. 2a and b). Furthermore, watertight stent-in-stent insertion is possible. The complete silicone coating prevents ingrowth and overgrowth of granulation tissue. It is therefore easy to reposition or remove the stent.
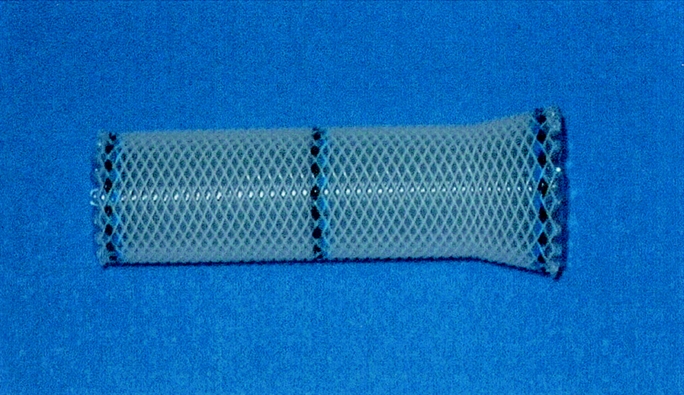
FIGURE 1. Self-expanding plastic stent consisting of a polyester mesh and a silicone membrane.

FIGURE 2. A: Endoscopic view of a large mediastinal leak. B: Occlusion of the leak by a self-expanding plastic stent. C: Stent retrieval and almost complete healing after 4 weeks
Stent placement was performed by a surgeon under endoscopic and fluoroscopic control. With the patient under conscious sedation with 3–5-mg midazolam, the stent applicator (diameter 14 mm) was introduced over a guide wire. The stent was deployed with at least 3–4-cm overlap proximal and distal to the leak. Correct placement of the stent and successful leak occlusion was confirmed by endoscopy and contrast-enhanced fluoroscopy with water-soluble contrast (Figs. 2 and 3). Perianastomotic and pleural drainage was obtained by existing chest drains or by insertion of additional drains under CT guidance. All patients received intravenous broad-spectrum antibiotics. After 2 weeks the stent was removed to assess healing of the leak (Fig. 4). Seven patients required a second stent, which was removed 7–14 days later. Retrieval of the stent was performed endoscopically using a special forceps. The stent was grasped at the proximal end and gently pulled out. Usually less than 10 minutes were required for this procedure. Complete healing of the leak was documented by endoscopy, contrast studies, and CT (Figs. 2–5).
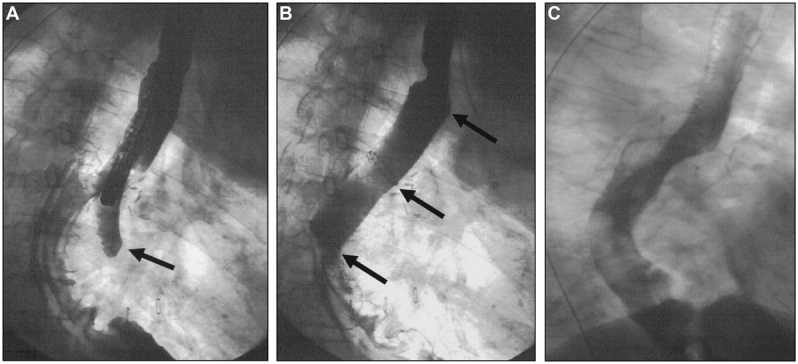
FIGURE 3. A: A contrast study demonstrates a leak of the esophagogastrostomy. B: Occlusion of the leak by a self-expanding metal stent (↑). C: Complete healing after 4 weeks.
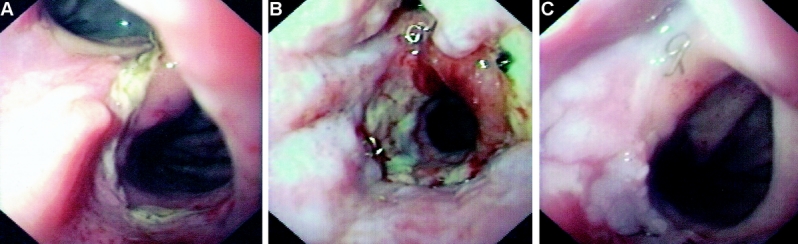
FIGURE 4. A: Endoscopic aspect of an anastomotic leak 6 days following esophagectomy. B: Change of the stent after 2 weeks reveals a residual leak. C: Retrieval of the stent and complete healing after 3 weeks.
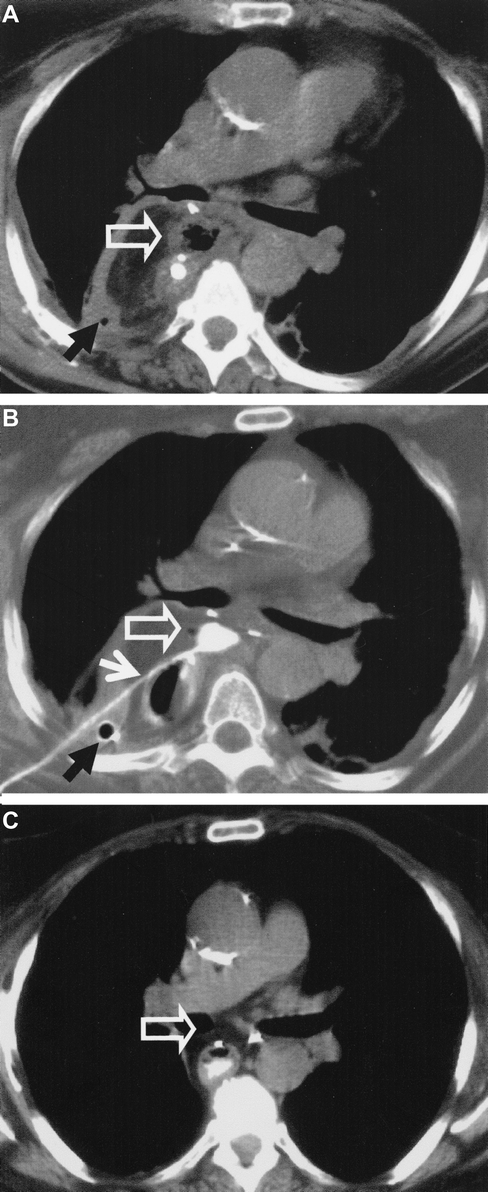
FIGURE 5. CT in a patient with anastomotic leakage after esophagectomy. A: The abscess (⇑) is not sufficiently drained by the chest tube (↑). B: CT-guided insertion of a drainage (↑) after endoscopic stent placement. C: Complete regression of the abscess (⇑) after 4 weeks.
RESULTS
Self-expanding plastic stents were successfully placed in all patients without procedure-related morbidity. Immediate leak occlusion was obtained in 8 of 9 patients (Figs. 2 and 3). The patients were allowed oral intake of fluid immediately after stent placement, and a solid diet was started 3 to 5 days later. Migration of the stent was observed in 2 patients after 3 and 12 days, respectively. Both stents were removed by flexible endoscopy and replaced by longer stents. Subsequently, only stents with a length of 12 cm or 15 cm were used. The mean healing time (time to stent retrieval) was 29 days. Perianastomotic and pleural drainage was obtained by existing chest drains in 4 patients and by insertion of additional drains under CT guidance in 5 patients (Fig. 5). None of the patients underwent rethoracotomy. Tracheostomy was performed in 3 patients to facilitate respiratory support. Compared with the conventional treatment group, patients who were treated with stents had earlier oral intake (mean: 11 days versus 18 days). Long-term respiratory support was required in 4 of 9 patients in the stent group and in 6 of 10 patients in the other group. The mean duration of ventilation was significantly shorter after stent insertion (9 days versus 17 days). Another advantage of stenting was the shortened intensive care course (mean: 25 days versus 47 days) and hospital stay (mean: 35 days versus 57 days). Mortality was not observed after stent insertion, whereas 2 patients died of septic complications and multiorgan failure after surgery (in-hospital mortality 0% versus 20%).
At the time of this report, the mean follow-up of the patients undergoing stent insertion is 12 months (range 3–26 months) Two of the patients died of metastatic disease and 7 patients are alive with no evidence of disease. None of the patients developed a stricture at the site of the anastomosis. In the conventional treatment group, 2 patients died of metastatic disease 3 months and 17 months after surgery, respectively. The remaining 6 patients are alive without evidence of tumor recurrence. A stenosis of the esophagogastrostomy was observed in 1 patient 4 months after nonoperative treatment of leakage. The stricture was successfully treated by endoscopic balloon dilatation.
DISCUSSION
Anastomotic leakage is the most important surgical complication after transthoracic esophagectomy. Many efforts have been made to identify etiological factors and to improve the technique for esophagogastric anastomosis.13 Nonetheless, thoracic anastomotic leakage remains a disastrous complication that is responsible for approximately 30% to 40% of postoperative deaths.14–16
There is no consensus on the most effective treatment of leaks of the esophagogastric anastomosis. Recently, Griffin et al have proposed an algorithm for the management of patients with mediastinal leaks.15 Patients with isolated leaks from the esophagogastric anastomosis were managed nonoperatively. Early reoperation was performed in cases with leakage from the gastrostomy line or necrosis of the gastric conduit. Despite rapid diagnosis and aggressive treatment, the mortality rates in both groups were 15% and 75% respectively, resulting in an in-hospital mortality of 32%.
From 1998 to 2000, we have used a similar approach to the treatment of anastomotic leaks after esophagectomy. The postoperative morbidity (28%) and overall mortality (20%) in this series were comparable to that of other studies.17 In our experience, conventional treatment of esophagogastric leaks was associated with high morbidity resulting in long-term parenteral nutrition and in extensive ICU and hospital stays.
Our hypothesis was that insertion of covered self-expanding stents could reduce septic complications of anastomotic leaks and would allow more rapid oral nutrition. In the last few years, self-expanding metal stents (SEMS) have been increasingly used for palliative treatment of patients with nonresectable esophageal cancer and esophago-respiratory fistulas. There are also sporadic reports on successful treatment of esophageal perforations with covered stents.18,19 However, the use SEMS in treatment of patients with postoperative complications has not been accepted, mainly because of the problems of stent retrieval and the well-recognized risk of late complications.20 Therefore, in the present study a new self-expanding plastic stent was investigated for the treatment of anastomotic leaks. One of the major advantages of this stent is that the complete silicone cover prevents ingrowth of granulation tissue, thus facilitating removal of the stent. Furthermore, the elasticity of the material and the high expansion force of the stent provide reliable occlusion of esophageal leaks. Since 2001, 9 consecutive patients with anastomotic leaks after esophagectomy were managed by insertion of a stent in combination with interventional drainage and intravenous antibiotics. The plastic stent was successfully inserted in all patients, and immediate leak occlusion was obtained in 89% of the cases. Usually the patients were allowed oral fluid intake at days 1–3 after stent insertion, and a normal diet was started afterwards. All leaks healed and the stents were removed after approximately 3–4 weeks. In addition to the chest drains, interventional drainage was required in 55% of the patients. The importance of adequate mediastinal and pleural drainage must be emphasized, because the stent prevents internal drainage into the gastric conduit. Patients who fail to improve clinically must be reinvestigated to identify and drain persisting fluid collections.
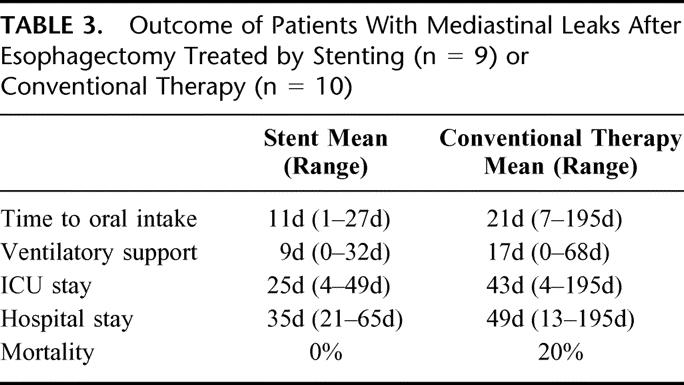
Compared with the conventional treatment group, patients who were managed with self-expanding plastic stents had earlier oral intake, a reduced rate and duration of long-term ventilation, and a shorter intensive care course and hospital stay (Table 3). In-hospital mortality was 0% after stent insertion and 20% after surgery or conservative treatment. These findings suggest that stenting of anastomotic leaks can reduce postoperative mortality, morbidity, and hospitalization. There are some limitations of longitudinal studies that compare consecutive time periods. However, the present study involves a rather short period of 6 years, and the surgical technique or the perioperative management was not changed substantially during this time period. The clinicopathological characteristics of both patient groups were comparable, but number of patients who manifested the leak more than 5 days after surgery was higher in the earlier treatment group. This may suggest that the patients in the conventional treatment group may have had more severe leaks than those in the stent group. On the other hand, the outcome of the patients in the conventional treatment group was even more favorable than in some other studies. Whooley et al observed a higher mortality (35%), a longer time to oral intake (50 days), and a longer hospital stay (63 days) in a group of 17 patients with thoracic leaks.16
TABLE 3. Outcome of Patients With Mediastinal Leaks After Esophagectomy Treated by Stenting (n = 9) or Conventional Therapy (n = 10)
Roy-Shoudry et al reported a series of 10 patients with gastroesophageal leaks that were successfully treated with self-expanding metal stents.21 Permanent stenting caused late complications in 4 of the 10 patients, ie, 3 cases of food blockage and 1 case of hemorrhage. The long-term fate of stents in benign disease has not yet been investigated. However, it has been shown in several studies that late complications, ie, obstruction, hemorrhage and perforation, occur in 20% to 30% of the patients after palliative stenting.22–24 Therefore, permanent stenting for anastomotic leaks is not justified after curative esophagectomy for esophageal cancer. Moreover, endoscopic removal of self-expanding metal stents is often very difficult, because granulation tissue grows into the uncovered flares of the stent. In one study, 1 or 2 sessions of argon plasma coagulation were required in 5 of 11 patients to remove the stent after successful treatment of esophagogastric leakage. Three of the patients subsequently developed a stenosis, which was probably due to extensive mucosal damage.25
We have not observed granulation tissue growing in or over the plastic stent. It was no problem to reposition or remove the stent endoscopically with a special forceps. Stent removal did not result in mucosal damage or any other complication. After a mean follow-up of 12 months, none of the patients has developed late complications related to temporary stenting with plastic stents. In conclusion, the results of this study suggest that short-time stenting with plastic stents is an emerging treatment alternative for thoracic leaks after esophageal resection. Management should be based on a multidisciplinary approach involving the surgeon, endoscopist, and interventional radiologist. Nonoperative treatment with stents must be rapidly instituted in patients with isolated esophagogastric anastomotic leakage to minimize mediastinal and pleural contamination. Adequate mediastinal and pleural drainage is crucial to reduce septic and respiratory complications. Surgical reexploration must be considered, if clinical improvement of the patient is not achieved with nonoperative treatment.
Footnotes
Reprints: Peter M. Schlag, Department of Surgery and Surgical Oncology, Charité Campus Buch and Helios Hospital Berlin, 13122 Berlin, Germany. E-mail: schlag@rrk-berlin.de.
REFERENCES
- 1.Hagen JA, DeMeester SR, Peters JH, et al. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg. 2001;234:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg. 2001;234:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562–572. [DOI] [PubMed] [Google Scholar]
- 4.Beitler AL, Urschel JD. Comparison of stapled and hand-sewn esophagogastric anastomoses. Am J Surg. 1998;175:337–340. [DOI] [PubMed] [Google Scholar]
- 5.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hankins JR, Attar S, Coughlin TR, et al. Carcinoma of the esophagus: a comparison of the results of transhiatal versus transthoracic resection. Ann Thorac Surg. 1989;47:700–705. [DOI] [PubMed] [Google Scholar]
- 7.Giuli R, Gignoux M. Treatment of carcinoma of the esophagus: retrospective study of 2400 patients. Ann Surg. 1980;192:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blewett CJ, Miller JD, Young JE, et al. Anastomotic leaks after esophagectomy for esophageal cancer: a comparison of thoracic and cervical anastomoses. Ann Thorac Cardiovasc Surg. 2001;7:75–78. [PubMed] [Google Scholar]
- 9.Hofstetter W, Swisher SG, Correa AM, et al. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg. 1995;169:634–640. [DOI] [PubMed] [Google Scholar]
- 11.Sauvanet A, Baltar J, Le Mee J, et al. Diagnosis and conservative management of intrathoracic leakage after oesophagectomy. Br J Surg. 1998;85:1446–1449. [DOI] [PubMed] [Google Scholar]
- 12.Lewis I. The surgical treatment of carcinoma of the esophagus, with special reference to a new operation for growths of the middle third. Br J Surg. 1946;34:18–31. [DOI] [PubMed] [Google Scholar]
- 13.Dewar L, Gelfand GG, Finley RJ, et al. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg. 1992;163:484–489. [DOI] [PubMed] [Google Scholar]
- 14.Putnam J-BJ, Suell DM, McMurtrey MJ, et al. Comparison of three techniques of esophagectomy within a residency training program. Ann Thorac Surg. 1994;57:319–325. [DOI] [PubMed] [Google Scholar]
- 15.Griffin SM, Lamb PJ, Dresner SM, et al. Diagnosis and management of a mediastinal leak following radical oesophagectomy. Br J Surg. 2001;88:1346–1351. [DOI] [PubMed] [Google Scholar]
- 16.Whooley BP, Law S, Alexandrou A, et al. Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer. Am J Surg. 2001;181:198–203. [DOI] [PubMed] [Google Scholar]
- 17.Whooley BP, Law S, Murthy SC, et al. Analysis of reduced death and complication rates after esophageal resection. Ann Surg. 2001;233:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mumtaz H, Barone GW, Ketel BL, et al. Successful management of a non-malignant esophageal perforation with a coated stent. Ann Thorac Surg. 2002;74:1233–1235. [DOI] [PubMed] [Google Scholar]
- 19.Serna DL, Vovan TT, Roum JH, et al. Successful nonoperative management of delayed spontaneous esophageal perforation in patients with human immunodeficiency virus. Crit Care Med. 2000;28:2634–2637. [DOI] [PubMed] [Google Scholar]
- 20.Kozarek RA, Raltz S, Marcon N, et al. Use of the 25mm flanged esophageal Z stent for malignant dysphagia: prospective multicenter trial. Gastrointest Endosc. 1997;46:156–160. [DOI] [PubMed] [Google Scholar]
- 21.Roy-Choudhury SH, Nicholson AA, Wedgwood KR, et al. Symptomatic malignant gastroesophageal anastomotic leak: management with covered metallic esophageal stents. AJR Am J Roentgenol. 2001;176:161–165. [DOI] [PubMed] [Google Scholar]
- 22.Christie NA, Buenaventura PO, Fernando HC, et al. Results of expandable metal stents for malignant esophageal obstruction in 100 patients: short-term and long-term follow-up. Ann Thorac Surg. 2001;71:1797–1801. [DOI] [PubMed] [Google Scholar]
- 23.Bartelsmann JF, Bruno MJ, Jensema AJ, et al. Palliation of patients with esophagogastric neoplasms by insertion of covered expandable modified Gianturco-Z endoprosthesis: experience in 153 patients. Gastrointest Endosc. 2000;51:134–138. [DOI] [PubMed] [Google Scholar]
- 24.Saxon RR, Barton RE, Katon RM, et al. Treatment of malignant esophageal obstructions with covered metal stents: long-term results in 52 patients. J Vasc Interv Radiol. 1995;6:747–754. [DOI] [PubMed] [Google Scholar]
- 25.Doniec JM, Schniewind B, Kahlke V, et al. Therapy of anastomotic leaks by means of covered self expanding metallic stents after esophagectomy. Endoscopy. 2003;35:652–658. [DOI] [PubMed] [Google Scholar]