Abstract
Objective:
To review recurrence patterns in completely resected gastric adenocarcinoma.
Summary Background Data:
Despite improvements in the surgical treatment of gastric adenocarcinoma, recurrence rates remain high in patients with advanced stage disease. Understanding the timing and patterns of recurrence is essential to develop effective adjuvant treatment strategies.
Methods:
A retrospective review of a prospectively maintained gastric cancer database was carried out. The timing and pattern of recurrence were reviewed. Univariate and multivariate analyses were performed to identify factors predictive of recurrence patterns.
Results:
From July 1985 through June 2000, 1172 patients underwent an R0 resection. Of these, 496 (42%) had recurrence and complete data on recurrence could be obtained in 367 patients (74%). Among the documented recurrences, 79% were detected within 2 years of operation. Locoregional sites were involved as any part of the recurrence pattern in 199 patients (54%). Distant sites were involved as any part of the recurrence in 188 patients (51%) and peritoneal recurrence was detected as any part of the recurrence in 108 patients (29%). On multivariate analysis, peritoneal recurrence was associated with female gender, advanced T-stage, and distal and diffuse type tumors; locoregional recurrence was associated with male gender and proximal location; distant recurrence was associated with proximal location, early T stage, and intestinal type tumors. The median time to death from the time of recurrence was 6 months.
Conclusions:
Recurrence after complete resection of gastric adenocarcinoma usually occurs within 2 years and is rapidly fatal. Patterns of recurrence are variable and may be associated with specific clinicopathologic factors.
Despite optimal surgical treatment, advanced gastric adenocarcinoma recurs at a high rate. This study reviews 367 patients who recurred after 1172 R0 resections over a 15-year period. Recurrence patterns are defined and clinicopathologic predictors of specific patterns are identified.
Long-term survival after potentially curative gastrectomy for advanced gastric cancer in the United States remains poor. In an American College of Surgeons survey, the overall 5-year survival for patients with completely resected gastric adenocarcinoma was 14%, but the majority of patients presented with advanced stage disease.1 More recent series in the United States have demonstrated that more patients present with early stage disease and that overall survival is improving. In a recent series of R0 resections from our institution, less than half the patients had stage III or IV disease and the overall 5-year survival was 49%.2 Nonetheless, patients with advanced stage disease continue to have recurrence at high rates, and overall recurrence rates are related to T and N staging. Improving operative technique and perioperative care have decreased operative mortality and morbidity, but have not improved stage-specific cancer survival. A number of prospective trials have been unable to prove a survival advantage for more extensive gastric resections3–5 or for more extensive lymphadenectomy.6,7 It is likely that, in addition to a standard R0 resection, improvements in adjuvant therapy will be necessary for improved cancer-specific outcomes in high-risk patients. A recent trial comparing gastrectomy with or without chemoradiation demonstrated a significant survival advantage for adjuvant therapy.8 Because adjuvant therapy focuses on specific areas of potential recurrence (locoregional, peritoneal, or distant/systemic), understanding and predicting the pattern of recurrence is critical to planning adjuvant strategies.
Data on recurrence patterns have been variable, in part because of differences in tumor biology, primary treatment, as well as the mode and timing of recurrence detection. Autopsy series typically describe endstage disease, often reporting untreated or undertreated patients, and are not representative of the early recurrence pattern. Although controversial, autopsy studies probably do not reflect the true biology of recurrence, but rather the end-stage of undertreated cancer.9–12 Planned “second-look” laparotomy was probably the best attempt to document early locoregional and peritoneal recurrence. However, it was performed in an era that lacked the modern radiologic capabilities that allowed diagnosis of early distant recurrence,13 and is currently unjustified. Clinical series may lack some accuracy in reliably detecting locoregional or peritoneal recurrences, but define the situation in which clinical decisions are made. Lastly, little is known about what clinicopathologic factors are associated with specific patterns of recurrence.
The goal of this study was to review recurrence patterns in a recent series of patients with documented recurrence after a complete resection of gastric adenocarcinoma at a single institution and to assess factors potentially predictive of the clinically detected pattern of recurrence.
METHODS
Utilizing a prospectively maintained gastric cancer database, all patients from July 1985 to June 2000 who underwent a curative gastrectomy at Memorial Sloan-Kettering Cancer Center were identified. Patients who had involved histologic margins (R1) or who had gross disease left behind during surgery (R2) were excluded. Demographic, pathologic, and treatment-related variables were prospectively recorded. A specific chart review of the timing and pattern of recurrence was performed. Patients in whom complete information on their recurrence could not be obtained were not included in the final analysis. Work-up required inclusion of complete radiologic imaging of the chest, abdomen, and pelvis as well as a complete history and physical examination. In patients whose recurrence was documented at an abdominal operation, some imaging of the chest was required. Serial imaging or biopsy was required to conclusively document recurrence. In some patients, no attempt was made to confirm recurrence, and these patients were excluded. Patients who developed what appeared to be anastomotic recurrences greater than 5 years after a gastrectomy for gastric adenocarcinoma were considered to have a new primary tumor.
Recurrences were categorized as locoregional, peritoneal, or distant. Detailed recurrence sites within each area were also recorded. Locoregional recurrence included dominant masses in the gastric bed, upper abdominal retroperitoneal lymph nodes, or anastomotic recurrence. Peritoneal recurrence was documented by positive cytology in ascitic fluid or by convincing peritoneal nodules on cross-sectional imaging. Distant metastases were further defined according to the specific organ involved. Periumbilical and cervical lymph nodes were considered distant metastases. Multiple recurrences in the same area (eg, anastomotic and gastric bed) were coded in a single category. The mode of recurrence detection was also recorded as histologic, radiologic, or clinical. Recurrence was considered histologically proven if it was documented by surgical biopsy, needle biopsy, or by cytology of appropriate fluid. Radiologic proof of recurrence was specifically reviewed in the context of the clinical situation and usually required sequential imaging demonstrating progression of metastatic lesions. In rare situations, specific recurrences were detected by clinical examination alone, such as in progressing subcutaneous nodules, or obvious diffuse peritoneal recurrence. Mediastinal lymph node recurrence was considered locoregional for gastroesophageal junction tumors and distant for the other gastric tumors. Tumors involving the ovaries (Krukenberg's tumor) were considered peritoneal. Multiple metastatic foci were considered 1 recurrence episode if they were diagnosed within a 3-month period. Although some patients had multiple recurrence episodes, this study analyzed the initial recurrence episode as defined above. Staging of the primary tumor was performed utilizing 1997 AJCC criteria.
Potential predictive factors for a given recurrence pattern were analyzed with χ2 and Fisher exact test for univariate comparisons. Disease-specific survival and recurrence-free survival was estimated by the methods of Kaplan and Meier, and log-rank test was used to determine univariate significance. Factors that were deemed of potential importance on univariate analysis (P < 0.10) were included in the multivariate analysis. Logistic regression was used for multivariate analysis of these factors. Predictive models were built using the significant risk factors, and their performance was evaluated using receiver operating characteristic curves. Statistical analysis was carried out with SPSS for Windows, version 10.0 (Statistical Package for the Social Sciences, SPSS, Inc., Chicago, IL) and SAS, version 8.0 (Statistical Analysis System, Cary, NC).
RESULTS
Demographics
From July 1985 through June 2000, 1172 patients underwent an R0 resection of gastric adenocarcinoma. Of these, 496 patients (42.3%) had recurrence at last follow-up. Complete data on recurrence could be obtained in 367 of these 496 patients (74%). These 367 patients make up the study group. The median age at the time of gastrectomy was 62 (range 21–92) and 258 (70%) were male.
Pathologic and Treatment Characteristics
Tables 1 and 2 summarize the characteristics of the 367 patients with documented recurrence. The 2 patients with T0 tumors presented with locally advanced gastroesophageal (GE) junction tumors that had a complete response to preoperative chemoradiotherapy. There were 14 T1 tumors, 7 of which were GE junction tumors. Two of these 7 GE junction tumors received neoadjuvant therapy. Of the 14 T1 tumors, 7 had lymph node metastases. Overall, these patients represent a group that presented with advanced tumors and underwent extensive surgical procedures. Thirty percent of the patients had another organ resected at the initial operation, and all but 70 patients (19%) had a D2 or D3 lymphadenectomy.
TABLE 1. Pathologic Characteristics of 367 Patients With Recurrence of Completely Resected Gastric Adenocarcinoma
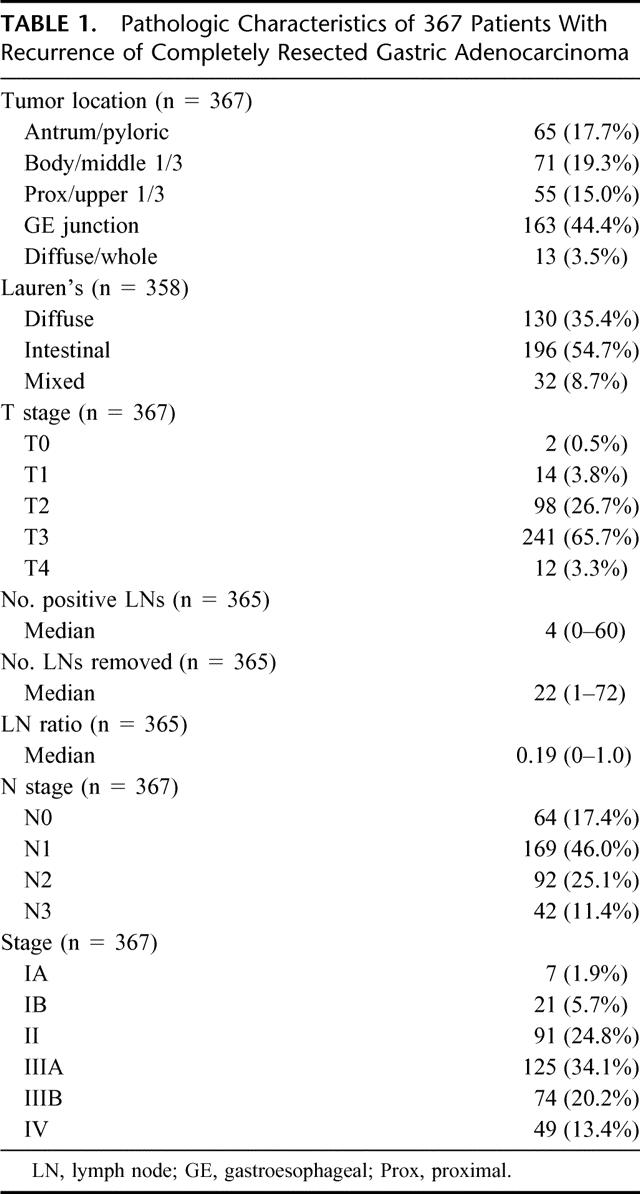
TABLE 2. Treatment-Related Characteristics of 367 Patients With Recurrence of Completely Resected Gastric Adenocarcinoma
Follow-up and Timing of Recurrence
The median follow-up from the time of operation for the 367 patients was 22 months (range 2–150). At last follow-up, 334 patients (91%) were dead of disease. The overall disease-free survival curve for all 1172 patients who had an R0 resection is illustrated in Figure 1 (median follow-up for survivors: 31 months). The curve demonstrates that the large majority of recurrences occurred in the first 2 years. Disease recurrence was rare after 4 years. Among the 367 patients with documented recurrence, 79% recurred within the first 2 years and 94% recurred within 4 years of resection. Documentation of what prompted the diagnosis of recurrence was obtained in 340 patients (93%). In 256 patients (75%), a symptom provoked a work-up and diagnosis of recurrence. The remaining 80 patients (25%) were asymptomatic and had recurrence discovered during routine follow-up, most commonly by routine cross-sectional imaging and occasionally by physical examination or serum tumor marker.
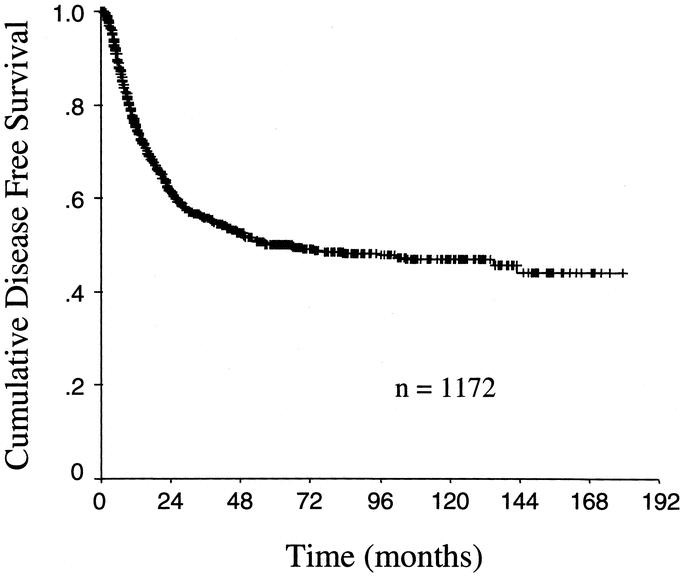
FIGURE 1. Kaplan-Meier disease-free survival plot for the whole cohort of 1172 patients who had complete margin negative resection of gastric adenocarcinoma.
Patterns of Recurrence
Of the 367 patients with documented recurrence, there were a total of 568 specific sites of initial recurrence that are detailed in Table 3. There was histologic proof of the recurrence in 291 of the 568 specific sites (51%) and in 269 sites, recurrence was documented radiologically. At 8 sites (1%) recurrence was documented on clinical grounds alone. Figure 2 illustrates the pattern of recurrence. Overall, 248 patients (68%) had recurrence involving a single area, 110 (30%) had recurrence involving 2 areas, and 9 (2.5%) had recurrence involving all 3 areas. Increasing nodal and overall stage was significantly associated with recurrence in multiple areas as compared with recurrence in a single area (data not shown). The locoregional area was involved as any part of the recurrence pattern in 199 patients (54%). Distant sites were involved as any part of the recurrence pattern in 188 patients (51%). Peritoneal recurrence was detected as any part of the recurrence pattern in 108 patients (29%).
TABLE 3. Specific Sites of Recurrence Within the 3 Areas
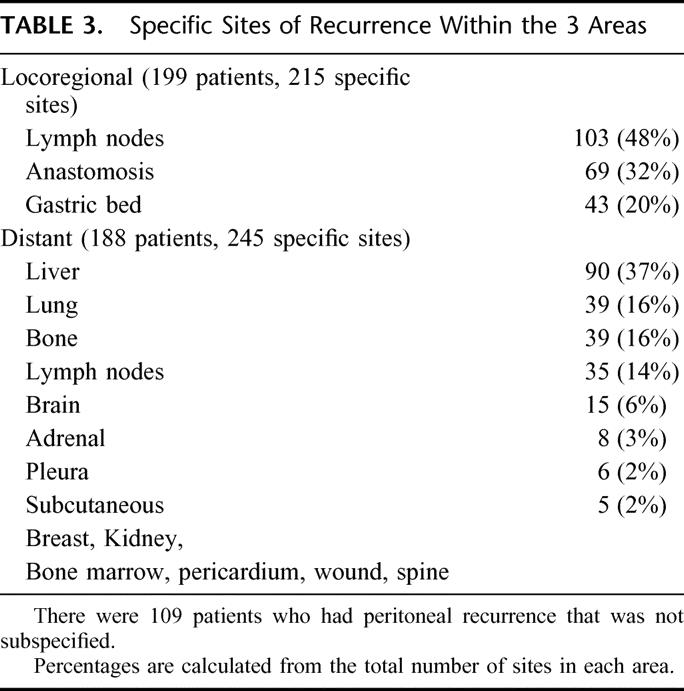
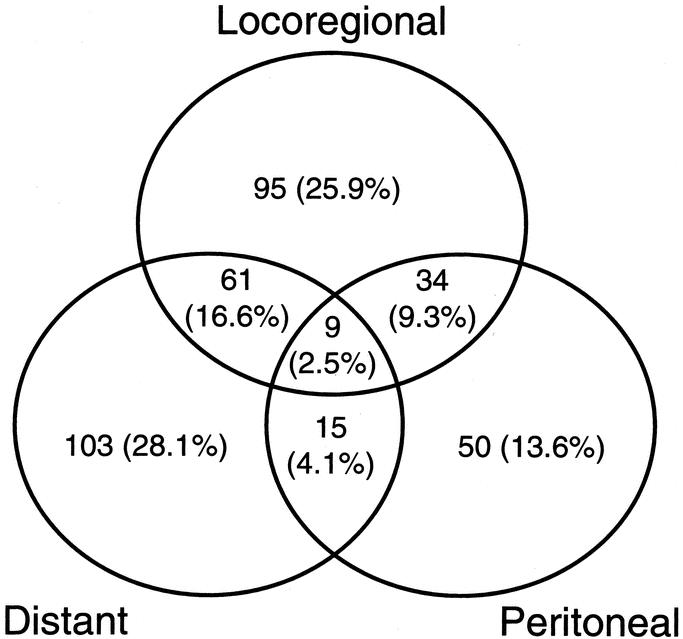
FIGURE 2. Plot of recurrence patterns in 367 patients with documented recurrence after complete resection of gastric adenocarcinoma.
Predicting the Pattern of Recurrence
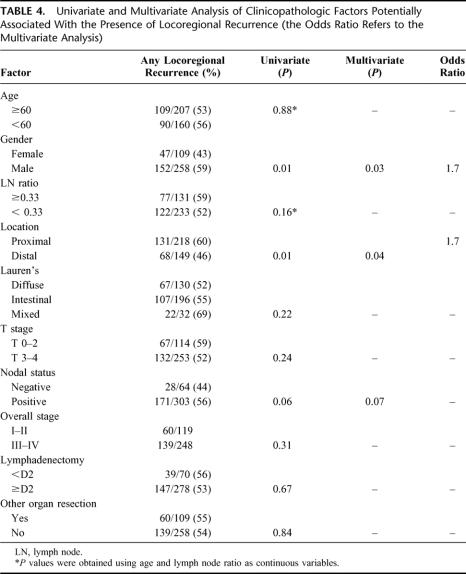
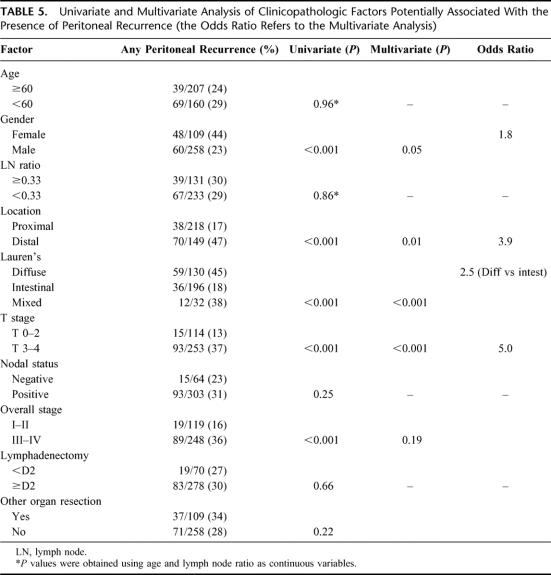
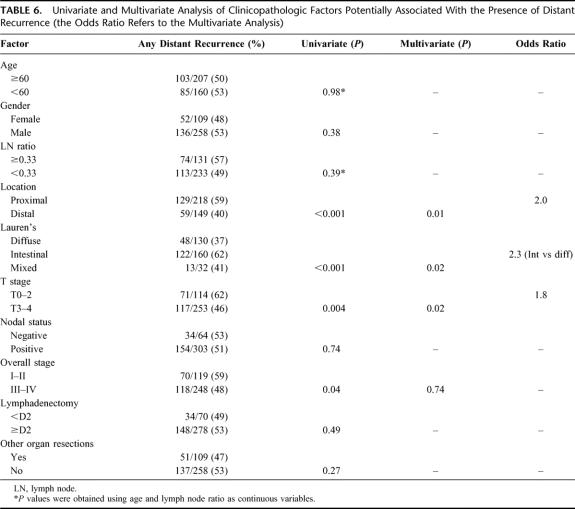
Tables 4, 5, and 6 detail the multivariate analysis of factors associated with the pattern of recurrence. Patients with locoregional and peritoneal recurrence were more likely to present with symptoms, whereas patients with distant metastases were more likely to be asymptomatic (data not shown). Nodal stage, extent of lymphadenectomy, and overall stage were notably not associated with any specific recurrence pattern. Of the 109 patients who had an additional organ resected, 77 (70%) involved a splenectomy and these factors were not associated with any specific recurrence pattern. Four factors were found to have statistically significant associations with specific patterns of initial recurrence: T stage, location, Lauren's subtype, and gender. Advanced T stage, distal location, diffuse subtype, and female gender were associated with higher rates of peritoneal recurrence as any part of the recurrence pattern. Proximal location and male gender were associated with locoregional recurrence as any part of the recurrence pattern. Proximal location, early T stage, and intestinal subtype were associated with distant recurrence as any part of the recurrence pattern. An isolated locoregional recurrence occurred in 95 (26%) patients. Female gender was the only factor significantly associated with this pattern of recurrence, whereas T-stage, N-stage, overall stage and extent of lymphadenectomy were notably not associated with an isolated locoregional recurrence.
TABLE 4. Univariate and Multivariate Analysis of Clinicopathologic Factors Potentially Associated With the Presence of Locoregional Recurrence (the Odds Ratio Refers to the Multivariate Analysis)
TABLE 5. Univariate and Multivariate Analysis of Clinicopathologic Factors Potentially Associated With the Presence of Peritoneal Recurrence (the Odds Ratio Refers to the Multivariate Analysis)
TABLE 6. Univariate and Multivariate Analysis of Clinicopathologic Factors Potentially Associated With the Presence of Distant Recurrence (the Odds Ratio Refers to the Multivariate Analysis)
Although statistically significant, the differences in recurrence patterns for each significant factor were not dramatic; however, 2 major patterns arose. With an increasing number of factors significantly associated with peritoneal recurrence, there was a high rate of peritoneal involvement (as high as 72%) and a low rate of locoregional and distant recurrence (as low as 31% and 22%, respectively). With an increasing number of factors significant for locoregional or distant recurrence, the opposite occurs: there is a low rate of peritoneal recurrence (as low as 13%) and a high rate of locoregional and distant recurrence (as high as 61% and 64%, respectively). We attempted to build a more comprehensive model predictive of recurrence patterns based on the significant associations found. The rationale for a more comprehensive approach was to account for the large number of possible combinations of factors any one patient might present with. When these factors were included in statistical predictive models, performance was not satisfactory and clinically relevant predictive models could not be developed.
Survival After Recurrence
Figure 3 demonstrates the survival time for patients once the diagnosis of recurrence has been made. Of the 367 patients with recurrence, 334 (91%) were dead of disease at last follow-up (91%). The median time from recurrence to death was 6 months. Seventy percent of the patients were dead within 1 year and 89% were dead within 2 years of the diagnosis of recurrence. On multivariate analysis, factors associated with a significantly shortened median time to death were higher T stage (4 versus 7 months, P = 0.007), involved lymph nodes (5 versus 9 months, P = 0.01), and presentation at recurrence with symptoms (4 versus 11 months, P < 0.0001). Older age, (analyzed as a continuous variable) was also a significant predictor of time to death (P = 0.01). Although initial recurrence in multiple areas was significantly associated with shortened survival (3 versus 8 months, P = 0.0001), the specific pattern of recurrence had no significant effect on the time to death.
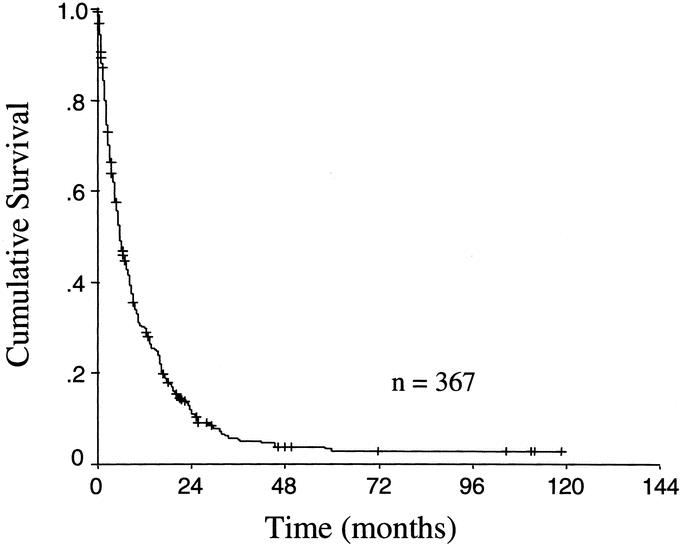
FIGURE 3. Kaplan-Meier survival plot of survival time after the diagnosis of recurrence in 367 patients with documented recurrent gastric carcinoma.
DISCUSSION
Results for the treatment of gastric adenocarcinoma in the United States have historically been poor. In general, patients have presented with advanced disease and had very high rates of recurrence.1 Increased detection of earlier stage disease, better preoperative staging, and better perioperative care have improved patient selection and operative survival, translating into improved long-term survival for patients with resected gastric adenocarcinoma. In an earlier publication recently published from our institution, 1038 R0 resections were reviewed with an actuarial 5-year survival of 49%.2 Nonetheless, patients with advanced disease still have very high rates of disease recurrence, which is essentially always lethal.14
There have been a number of attempts to improve cancer-specific outcomes in the surgical treatment of gastric adenocarcinoma. Three randomized trials have been unable to show a benefit for total gastrectomy over subtotal gastrectomy for antral or body tumors.3–5 Furthermore, 2 recent large multi-institutional randomized trials have not shown a benefit for extended lymph node dissection.6,7 Despite numerous previous negative trials evaluating adjuvant therapy for resected gastric adenocarcinoma,15 a recent multi-institutional trial evaluating gastrectomy with or without adjuvant chemoradiation demonstrated a 10% survival benefit for adjuvant therapy. The benefit was attributed to a decreased locoregional recurrence rate seen in the context of what many consider a substandard lymph node dissection.8 Complete surgical resection is the first step toward effective therapy, but is limited in its ability to cure advanced stage disease. More effective adjuvant therapy is needed if meaningful advances are to be made in the treatment of this disease.
Since adjuvant therapies focus on specific disease patterns (locoregional, peritoneal, or distant), and not all gastric cancers recur in the same manner, understanding the pattern, timing, and implications of recurrence is critical to the planning of adjuvant strategies. Clinicopathologic factors that predict failure patterns could potentially allow individualized and more effective adjuvant therapy strategies.
Many studies have attempted to analyze recurrence patterns in gastric cancer with variable results. The variability in the literature is multifactorial and relates to inconsistencies in treatment, as well as in the mode and timing of detection of recurrence.
Early attempts to describe recurrence pattern of gastric cancer relied on autopsy studies. Unfortunately, many of the series were in patients who were untreated or suboptimally treated.9,10 Although in some patients with early diffuse recurrence, autopsy studies may reflect the true biology of recurrence, the pattern of spread at the time of death likely reflects the end-stage of cancer with widespread disease and does not represent the initial, and potentially treatable or preventable recurrence pattern. Nonetheless, some value information was learned from these autopsy studies. Wisbeck et al analyzed 85 autopsies, 16 of which were in patients who had undergone a potentially curative gastrectomy. Locoregional recurrence was documented in 15 of 16 cases (94%), peritoneal recurrence in 8 of 16 (50%), and 7 of 16 (44%) had liver metastases. The authors correlated serosal involvement of the primary tumor with peritoneal recurrence (7 of 10 versus 1 of 6).11 In 1951, McNeer et al published an autopsy series of 92 patients who had undergone a potentially curative subtotal gastrectomy. The principal findings of the study were an 81% locoregional recurrence rate and that most of patients died with distant metastases.12
In 1948, Wangensteen began a practice of “second-look” laparotomy for patients with resected gastrointestinal cancer. Gunderson and Sosin reported these results for 107 patients with gastric cancer. The essential findings of the study were that 80% had a recurrence and 88% of these were locoregional, 54% involved the peritoneum, and 29% involved distant sites. These patients were from an era when radiologic capability of diagnosing distant disease was poor and almost certainly the rate of distant metastases was underdiagnosed.13 The morbidity of a second-look laparotomy was substantial and with few, if any patients benefiting from the procedure, this approach was abandoned.
Most current series that analyze recurrence patterns have relied on clinical follow-up. Landry et al followed 130 patients who underwent a curative gastrectomy, 88 of whom had recurrence. Of the 88 recurrences, 56% involved the locoregional area, 34% involved the peritoneum, and 76% involved distant sites including the peritoneum.16 In a recent series of 73 curative gastrectomies, Schwarz et al documented recurrence in 35 patients. Of these 35 recurrences, 40% involved locoregional sites, 54% involved the peritoneum, and 54% involved distant sites. This study found advanced T stage to be associated with peritoneal recurrence and advanced nodal stage to be associated with distant recurrence.17 A recent study from Korea analyzed recurrence patterns in 2038 patients who had undergone a potentially curative gastrectomy, 508 (25%) of whom had recurrence. Of these 508 recurrences, 33% involved locoregional sites, 44% involved the peritoneum, and 38% involved distant sites. Younger age, diffuse type, and undifferentiated tumors were associated with peritoneal recurrence. Older age and larger tumors were associated with distant recurrence, and older age, large tumors, diffuse type, and proximal tumors were associated with locoregional recurrence.18
We analyzed recurrence patterns in 1172 patients who underwent an R0 gastrectomy over a 15-year period at a single institution. These patients were largely treated with an extended lymph node dissection (81% D2 or greater). As is typical of many gastrointestinal cancers, most (80%) of the recurrences occurred within 2 years of surgical resection and death ensued rapidly thereafter. Nearly 90% of the patients with recurrence were dead of disease within 2 years of the diagnosis of recurrence. A number of factors were associated with a more rapid demise and included advanced T stage, lymph node metastases, symptomatic presentation, and recurrence in more than 1 area. Approximately two thirds of the patients had recurrence in a single area and recurrence patterns were compatible with previous clinical series, the major difference being the lower rate of documented peritoneal recurrence (29% of recurrences).
The large number of patients in this study allowed us to perform a multivariate analysis of factors predictive of specific recurrence patterns. Two major patterns arose. In the first pattern, patients had recurrence both locoregionally and distantly with low rates of peritoneal recurrence. This group included males with proximal, T1–2 and intestinal-type tumors. In this group of patients, clinically detected peritoneal recurrence was generally lower than 20%, and locoregional and distant recurrence accounted for the majority of the recurrence pattern. In the second pattern, patients had recurrence with higher rates of peritoneal disease and lower rates of locoregional and distant disease. This group included females with T3 or T4 tumors, distal location, and diffuse-type tumors. In this group of patients, peritoneal recurrence was as high as 72% whereas locoregional and distant recurrence accounted for less than 30% of the recurrence pattern.
Unlike the Korean study, we found that nodal metastases and age were not significantly associated with any specific recurrence pattern. Diffuse-type tumors were associated with peritoneal and locoregional recurrence in their study, but only peritoneal recurrence in ours. This may represent a difference in semantics because peritoneal disease in the tumor bed may have been called locoregional disease. Both studies found that proximal location was associated with a higher rate of locoregional recurrence and that advanced T stage and diffuse-type tumors are associated with higher rates of recurrence in the peritoneum. Differences in these 2 studies, such as the associations with gender in our study and the association with age in their study, are difficult to account for but may be related to follow-up practices and modes of detection of recurrence.
We also tried to build a predictive model based on the significant associations we discovered. The performance of these predictive models, however, was not satisfactory and a clinically applicable model could not be developed. This is consistent with the fact that the demonstration of statistically significant risk factors does not necessarily imply that predictive rules built on those factors will perform well. It appears that prediction of recurrence patterns of resected gastric adenocarcinoma based on clinicopathologic factors is elusive.
The findings in our study must be interpreted with some caution. First, 26% of the patients with recurrence did not have a recurrence pattern documented, and this limits the strength of our observations. Second, this retrospective study was based on clinical follow-up that varied from patient to patient and has changed during the past 15 years. It is well recognized that low-volume locoregional or peritoneal disease can be very difficult to diagnose clinically. However, it is in this background that decisions regarding treatment have to be made, making these findings relevant. Lastly, a retrospective review with imperfect follow-up makes analyzing factors such as adjuvant therapy impossible and potentially misleading. The majority of our patients were treated prior to any positive trials demonstrating a benefit to adjuvant therapy and therefore received heterogeneous treatments in an inconsistent manner, making a statistical analysis of this factor unreliable. We therefore did not include adjuvant therapy in this analysis.
A number of conclusions can be reached from this study. The majority of patients with gastric adenocarcinoma who have recurrence generally do so within the first 2 years after complete resection, and death from disease usually occurs rapidly. A number of factors are associated with a more rapid demise, and knowledge of these factors may be very useful to help practitioners, patients, and their families understand the implications of disease recurrence. Resected gastric cancer recurs in multiple patterns, and no single pattern dominates. Locoregional, peritoneal, and distant sites, all representing different mechanisms of spread, are common modes of recurrence. Lastly, we are beginning to understand that within the context of clinically detected recurrence, certain clinicopathologic factors are associated with specific patterns of recurrence, but clinically relevant predictive models were not possible to develop. Understanding these associations, however, may be useful in planning adjuvant strategies in certain cases.
ACKNOWLEDGMENTS
The authors would like to acknowledge the assistance of Marianne Benninati in maintaining our database.
Footnotes
Reprints: Michael D'Angelica, MD, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: dangelim@mskcc.org.
REFERENCES
- 1.Wanebo HJ, Kennedy BJ, Chmiel J, et al. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg. 1993;218:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karpeh MS, Leon L, Klimstra D, et al. Lymph node staging in gastric cancer: is location more important than number? An analysis of 1,038 patients. Ann Surg. 2000;232:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouzi JL, Huguier M, Fagniez PL, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg. 1989;209:162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson CS, Chung SC, Woods SD, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg. 1994;220:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozzetti F, Marubini E, Bonfanti G, et al. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. Dutch Gastric Cancer Group. N Engl J Med. 1999;340:908–914. [DOI] [PubMed] [Google Scholar]
- 7.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. [DOI] [PubMed] [Google Scholar]
- 9.Dupont B, Cohn I. Gastric adenocarcinoma. Curr Probl Cancer. 1980;4:4–46. [DOI] [PubMed] [Google Scholar]
- 10.Warwick M. Analysis of one hundred and seventy-six cases of carcinoma of the stomach submitted to autopsy. Ann Surg. 1928;88:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wisbeck WM, Becher EM, Russell AH. Adenocarcinoma of the stomach: autopsy observations with therapeutic implications for the radiation oncologist. Radiother Oncol. 1986;7:13–18. [DOI] [PubMed] [Google Scholar]
- 12.McNeer G, Van den Berg H, Donn F, et al. A critical evaluation of subtotal gastrectomy for the cure of cancer of the stomach. Ann Surg. 1951;134:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. [DOI] [PubMed] [Google Scholar]
- 14.Brennan MF, Karpeh MS Jr. Surgery for gastric cancer: the American view. Semin Oncol. 1996;23:352–359. [PubMed] [Google Scholar]
- 15.Weber SM, Karpeh MS. Randomized clinical trials in gastric cancer. Surg Oncol Clin N Am 2002;11:111–131, ix. [DOI] [PubMed] [Google Scholar]
- 16.Landry J, Tepper JE, Wood WC, et al. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:1357–1362. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol. 2002;9:394–400. [DOI] [PubMed] [Google Scholar]
- 18.Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. [DOI] [PubMed] [Google Scholar]