Abstract
Objective:
Recent experience with surgery for enterocutaneous fistulae (ECF) at a specialist colorectal unit is reviewed to define factors relating to a successful surgical outcome.
Summary Background Data:
ECF cause significant morbidity and mortality and need experienced surgical management. Previous publications have concentrated on mortality resulting from fistulae, while factors affecting recurrence have not previously been a focus of analysis.
Methods:
Records were reviewed of patients who had ECF surgery (1994–2001). Management strategy involved early drainage of sepsis and nutritional support prior to elective ECF repair, with selective defunctioning proximal stoma formation.
Results:
A total of 205 patients were available (89 males, 43%; median age, 51 years; range, 16–86) years). ECF were related to Crohn's disease in 95, ulcerative colitis in 18, diverticular disease in 17, carcinoma in 25 (16 after radiotherapy), mesh ventral hernia repair in 21, and other causes in 29. Forty-one (20%) had undergone attempted fistula repair at other institutions. Initial management included CT-guided drainage of an intra-abdominal abscess in 23 patients, and total parenteral nutrition in 74 (36%). A total of 203 patients had definitive ECF repair. Forty-four had oversewing or wedge resection of the fistula, and 159 had resection and reanastomosis of the involved small bowel segment or ileocolic anastomosis. Ninety-day operative mortality was 3.5%. A total of 42 (20.5%) patients developed ECF recurrence within 3 months. Multivariate analysis demonstrated that recurrence was more likely after oversewing (36%) than resection (16%, P = 0.006).
Conclusions:
A strategy of drainage of acute sepsis, maintenance of nutritional support prior to surgery, and selective use of PS allows for primary closure in 80% of complicated ECF. Resection should be performed when feasible.
The management of 205 patients with enterocutaneous fistulas is reported. Drainage of acute sepsis, nutritional support, and surgery with selective proximal stoma use result in primary fistula closure in 80%. Multivariate analysis of factors predictive of closure is presented.
Enterocutaneous fistulae (ECF) involving the small bowel remain a challenging surgical problem.1 Patients are often nutritionally depleted and require skilled management to achieve fistula closure. Improved management strategies, such as antibiotic therapy and intravenous hyperalimentation, have reduced mortality; however, mortality up to 30% is reported in earlier series.2–5
Strategies for successful resolution of ECF include control of sepsis with imaging-guided drainage and antibiotic therapy, skin protection, maintenance of nutritional intake, and fluid and electrolyte balance. In some cases, ECF closure can also be promoted by the use of vacuum-assisted closure systems or fibrin glue, although these techniques have not been evaluated outside of small cases series and are not approved for this use yet.6,7 Although many fistulae will close with such conservative strategies, persistent fistulae require definitive surgical intervention. This generally involves a major laparotomy and should be delayed to allow resolution of intra-abdominal inflammatory adhesions for patients in the early postoperative phase.2,8–11
Most reports of surgery for ECF have been of case series, and factors predictive of fistula recurrence have not been clearly defined. We report a large series of ECF at a tertiary-referral colorectal unit and use multivariate analysis to examine patient and surgical-related factors that affect fistula recurrence rates after surgery.
METHODS
Patients with a surgical procedure for an external fistulous communication to skin originating from small bowel or an anastomosis or pouch constructed from small bowel were examined. They were excluded if no operation was performed or if a colocutaneous fistula was present without small bowel involvement. All patients were managed by a dedicated colorectal unit along unit guidelines; however, the choice and timing of the surgical procedure were at the discretion of the operating surgeon.
Cases were identified from all cases coded with a CPT code of 44640 (enterocutaneous fistula) between December 1994 and December 2001. All charts were reviewed and inclusion criteria confirmed. Data relating to initial and subsequent hospital stays were abstracted, including patient demographics, fistula etiology and duration, and nutritional and metabolic parameters such as body mass index), serum albumin, and duration of parenteral nutrition. Operative procedure details recorded included the number and site of fistulae, estimated blood loss, details of fistula resection or repair, and stoma formation. The data set was incomplete for some patients, and the number of patients is annotated for each statistic. American Society of Anesthesia grading was also extracted as it was the one independent assessment of the patients’ condition at the time of surgery that was available. Principles that were followed for the management of fistulae in these patients included preoperative antibiotics and thromboprophylaxis and drainage of abscesses by CT with later surgery. Anastomoses at risk were protected with defunctioning proximal stomas, particularly in patients with hypoalbuminemia, a septic focus intra-abdominally, or Crohn's disease (CD).
Nonparametric data are presented as median and range. Analysis was performed using the Mann-Whitney U test and Fisher exact test as appropriate with a P value less than 0.05 considered statistically significant. Multivariate analysis was undertaken to identify the primary factors predictive of ECF recurrence after surgery. Factors were considered in a multivariate logistic regression setting with recurrence as the outcome variable. Ethical approval was obtained from the Cleveland Clinic Institutional Review Board.
RESULTS
Between December 1994 and December 2001, 326 patients were identified with ECF. Following chart review, 205 patients fitted the review criteria of an external fistula involving small bowel for which surgery was performed.
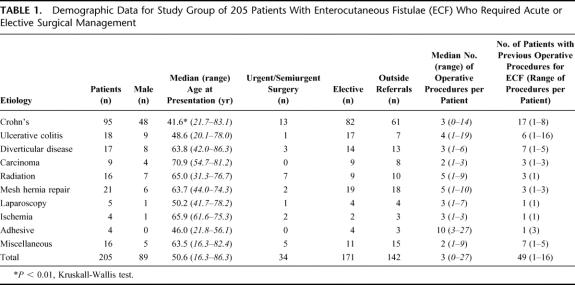
Demographic and etiologic data are presented in Table 1. Patients with CD made up the largest etiological group (46%) and were significantly younger than other groups. Four patients had ECF following procedures to deal with small bowel ischemia that was spontaneous (1 patient) or after a vascular procedure (3 patients). A total of 25 patients had fistulae resulting from treatment of cancer, including radiation in 16 (considered separately). Sixteen patients had other reasons for ECF: 3 postappendectomy, 2 postcolectomy for constipation, 2 for severe pancreatitis (1 after necrosectomy), 4 after open gynecological procedures, 1 after laparotomy for blunt trauma, 2 after open abdominal aortic aneurysm repair, 1 after open stenting of a benign biliary stricture, and 1 following right hemicolectomy for colonoscopic perforation. A total of 33 patients (32 CD, 1 following necrotizing pancreatitis) had spontaneous ECF, and the remainder occurred postoperatively.
TABLE 1. Demographic Data for Study Group of 205 Patients With Enterocutaneous Fistulae (ECF) Who Required Acute or Elective Surgical Management
A total of 142 patients (69%) were referred from outside hospitals or from other surgical services within this institution (Table 1). Forty-one of these patients had procedures to repair their ECF prior to their index operation at this department. Time from the last outside hospital procedure to referral was not available. A total of 63 patients were already patients of the colorectal service; 8 of these had had previous ECF procedures more than 6 months before the study period. The ECF had been present for a median of 5.8 months (range, 1 day to 21 years) prior to the index procedure. There was no difference between lengths of time for patients seen previously by this unit (median time, 4.5 months; range 1 day to 21 years) compared with outside referrals (median time, 6.4 months; range, 1 day to 12 years, P = 0.33).
Initial Management of ECF
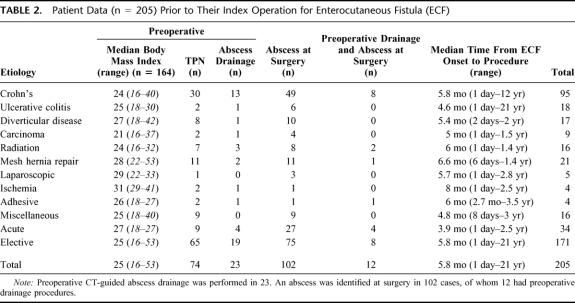
Patient data at the index operation are presented in Table 2. After admission, 23 patients had an initial drainage procedure for intra-abdominal abscess performed radiologically. A total of 74 patients were commenced on total parenteral nutrition (TPN) prior to their index procedure; however, the duration of TPN therapy was not available for all patients. There was insufficient evidence to indicate patients with preoperative TPN had total serum protein (P = 0.14) or serum albumin levels (P = 0.145) that differed from patients not receiving TPN.
TABLE 2. Patient Data (n = 205) Prior to Their Index Operation for Enterocutaneous Fistula (ECF)
Five patients (all acute) were taken to the operating room from the intensive care unit where they had been managed initially. On the day of operation, median white blood cell count was higher for acute compared with elective cases (median, 10.6; range, 3–23.4 vs. 7.8, range, 2.8–25.9, P < 0.05). ASA grading was a median of 3 (range, 1–4) for both acute and elective cases.
Initial Surgical Management of ECF
Initial operative procedures lasted a median of 4 hours (n = 163, range, 1–12 hours) and were longer for elective compared with acute cases (245 minutes, range, 60–720 vs. 210 minutes; range, 60–300 minutes, P = 0.006). Blood loss was a median of 350 mL (n = 179, range, 30–3600 mL), significantly greater for elective compared with acute cases (median, 400 mL; range, 50–3600 mL vs. 200 mL; range, 30–1200 mL, P = 0.001). Postoperative intensive care was needed for 23 patients (10 [30%] acute surgical cases and 13 [8%] elective cases). A total of 26 of the 74 patients stopped their TPN, and 13 others started TPN after surgery.
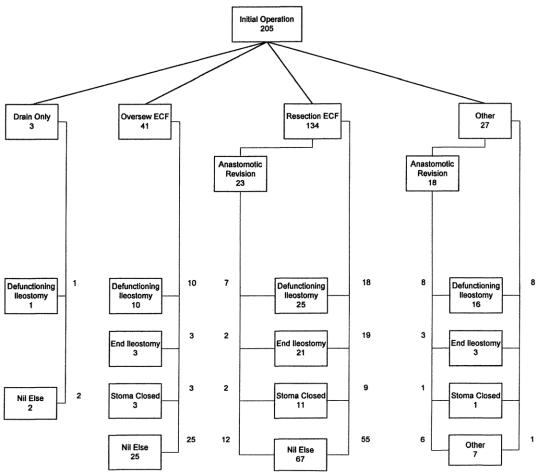
Figure 1 illustrates the procedures performed in these patients. The procedure was classified as a “resection” if the area of bowel containing the ECF was resected and a primary anastomosis formed. If the resection was less than the circumference of the bowel or the edges of the fistula were debrided and then closed, this was classified as “oversewing” or a wedge repair. If multiple ECF were present and resection and oversewing or multiple resections were performed, this was also classified as a “resection.” “Anastomotic revision” refers to the resection and revision of a previous small bowel or ileocolic anastomosis, rather than oversewing of an anastomotic leak. Such revisions were classified with the resection group for the purposes of multivariate analysis. All patients in the “oversewn” group had hand-sewn anastomoses, those in the resection group were either hand-sewn or stapled at the discretion of the operating surgeon. ECF were oversewn or resected and reanastomosed in an area of bowel thought appropriate and safe by the operating surgeon. Pathologic specimens from resection margins were not routinely taken. Fourteen patients had mesh removal and 10 had mesh placed to repair ventral hernias during this initial procedure.
FIGURE 1. Algorithm showing the initial surgical management of 205 patients requiring surgical treatment of ECF. The flowchart outlines procedures undertaken to deal with the fistula (drain only, oversewing, resection, or complex multiple resection with reanastomosis, nothing), requirement for anastomotic revision and reanastomosis, and stoma coverage (defunctioning proximal stoma, end ileostomy, stoma closure, or no stoma).
In 12 patients, this initial procedure was not definitive. It consisted of defunctioning stoma formation in 9 (3 of whom had abscess drainage), 2 with abscess drainage only, and 1 with removal of infected mesh from a ventral hernia repair. Two of this group did not proceed to definitive ECF repair. One subsequently died; the other had a defunctioning stoma only performed. The 2 with drainage only had later definitive management. The 8 who were defunctioned only had definitive procedures a median of 30 weeks later, and 7 of these had an ECF recurrence.
Findings at Operation
Undrained intra-abdominal collections or abscesses were found in 102 patients (50%). This was a more frequent finding in acute cases where 27 patients (79%) had intra-abdominal collections at operation compared with 75 elective cases (44%, P = 0.0001, Fisher exact test). Preoperative abscess drainage did not result in a significant reduction in the incidence of abscesses identified at surgery.
A total of 234 small bowel fistulae and 13 concomitant colonic fistulae were identified in the 205 patients. The small bowel fistulae were described as originating from duodenum in 2, jejunum in 80, “small bowel” in 15, ileum in 71, terminal ileum in 6, and 5 from an ileostomy (proximal to the fascia). The remaining 55 patients had ECF from anastomoses (1 small bowel anastomosis, 5 ileostomy closures, 30 ileocolic anastomoses, 5 ileorectal, 6 ileal pouch-anal, and 8 Koch pouch [proximal to the pouch]).
Definitive Surgical Management of ECF
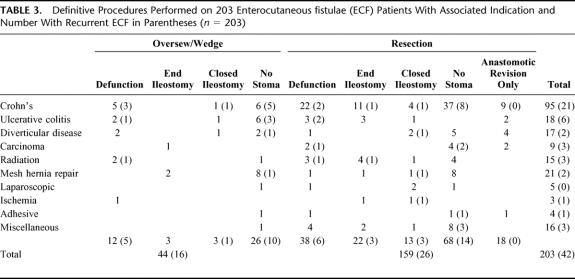
Definitive surgical management of ECF was performed in 203 patients. This was at the index ECF operation in 193 and at a second procedure in 10 of the 12 who had nondefinitive surgery initially. ECF recurrence occurred in 42 (21%) of these patients. A summary of the definitive procedure and associated ECF recurrence rates is presented in Table 3.
TABLE 3. Definitive Procedures Performed on 203 Enterocutaneous fistulae (ECF) Patients With Associated Indication and Number With Recurrent ECF in Parentheses (n = 203)
Timing of Surgery
Timing of definitive ECF surgery related to fistula onset is described in Table 2. Median time to definitive repair was 6 months (range, 1 day to 28 months). Time from fistula onset to definitive surgery was similar for those with ECF recurrence and for those with a successful procedure (median time, 6 months; range, 1 day to 22 months, P = 0.42, Mann-Whitney test). Those patients operated on between 2 and 12 weeks after their last surgery had an ECF recurrence rate of 28% (10 of 36). This was higher than those patients whose operation was delayed longer than 12 weeks (recurrence rate = 15% 17 of 114, P = 0.088, Fisher exact test) and those with early operations within 2 weeks of surgery (recurrence rate = 20%, 4 of 20, P = 0.37). Logistic regression analysis was used to generate a probability of fistula recurrence after definitive surgery relative to time since fistula onset. This is illustrated in Figure 2. It suggests that delaying surgery results in a higher chance of successful outcome (risk of recurrence at 3 months = 20.7%, at 12 months = 17.0%, P = 0.25).
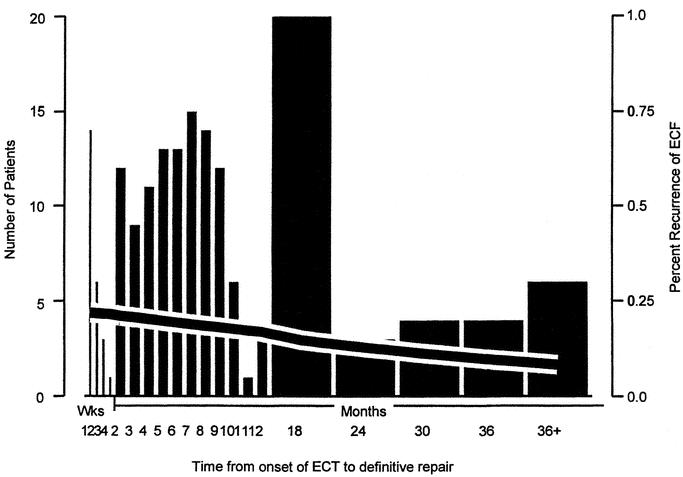
FIGURE 2. Relationship between number of definitive surgical procedures performed and probability of fistula recurrence relative to time since fistula onset (n = 203). Number of patients are represented by columns (left Y-axis) and recurrence by the black line (right Y-axis).
Outcome
A total of 203 patients had a definitive surgical procedure for ECF. Forty-two (21%) had recurrence within 3 months, including 3 after subsequent complications and 1 after stoma closure. These patients are identified with respect to their etiology and initial management in Table 3. There were 170 patients with postoperative ECF who had definitive surgery performed; 31 (18%) had fistula recurrence. This was not significantly different from those with spontaneous ECF (11 of 35 patients, 31%, P = 0.11, Fisher exact test).
A total of 119 (58%) of the 205 patients had one procedure only. Of these, 36 still have a stoma and 3 have an ongoing ECF (etiologic factors: necrotizing pancreatitis, radiation for intra-abdominal desmoid [drained only and subsequently died], and CD). The other 86 patients had subsequent surgical procedures. Thirty-one of the 89 patients (35%) with defunctioning or end stomas had these closed or rejoined. One of these 31 patients had a recurrence of ECF (carcinoid, initially oversewn, and defunctioned) without subsequent treatment.
Thirteen patients had surgery for complications resulting from definitive surgical procedures: 1 removal of infected mesh, 2 adhesiolysis for small bowel obstruction, 1 colonic resection for volvulus, 1 wound dehiscence, 1 wound infection requiring debridement, and 4 requiring drainage of intra-abdominal abscesses. Two had mesh repair of ventral hernias and one small bowel resection and stricturoplasty for recurrent CD without ECF. Three of these patients subsequently had ECF recurrence.
Six patients died within 3 months of definitive surgical management. Death was due to cardiac complications in 1, respiratory complications in 2, multiorgan failure in 2, and a variceal bleed in 1. One of these patients had a recurrence of ECF prior to death.
Predictors of Recurrence
Surgical repair technique was a significant predictor of ECF recurrence. Patients with a wedge repair or oversewing of an ECF had a recurrence rate of 32.7%, compared with 18.4% if the ECF was resected or the anastomosis revised (P = 0.004, Fisher exact test). No patient who had both a wedge repair and resection had ECF recurrence. If only a wedge repair/oversewing or resection was performed (n = 198), resection only still resulted in a better outcome with a recurrence rate of 17% (26 of 154) compared with 36% (16 of 44) for those oversewn only (P = 0.011 Fisher exact test).
Resection technique was also important in the CD subgroup. Analysis showed oversewing resulted in a recurrence rate of 75% (9 of 12) compared with a recurrence rate of 15% (12/83) for those resected (P < 0.0001, Fisher exact test). CD by itself did not influence recurrence (recurrence rate = 22%, P = 0.73, Fisher exact test). A multivariate logistic regression model was then constructed, which also identified oversewing or wedge repair as a primary predictor of recurrence.
Fistula closure was not adversely affected by previous attempts at closure at outside hospitals. A total of 28 of the 42 recurrent fistulae had attempts at ECF closure prior to referral (20% failure rate) compared with 14 recurrences of 62 cases (22% failure rate) for those whose only attempted fistula repair was at this department (P = 0.71, Fisher exact test).
Etiology, age, duration of ECF, number of previous surgeries, preoperative and postoperative use of TPN, defunctioning stoma use, presence of undrained abscess, or physiologic parameters (serum albumin, total protein, white blood cell count, body mass index, ASA) did not influence recurrence rate by univariate or multivariate analysis.
Long-term Follow-up
Median total follow-up for patients was 9.5 months (range, 2 months to 9 years from time of definitive treatment). Those with recurrent ECF had a longer follow-up of a median of 15 months compared with 8.5 months for those without recurrence, although this result was not statistically significant (P = 0.2).
Of those 42 patients with ECF recurrence following definitive surgery, 21 had further procedures for their ECF recurrence. This occurred a median of 8.5 months later (range, 1 day to 3.5 years). Four had subsequent recurrence.
Two patients had a further ECF remote from treatment, 7 months and 2 years later. Both had CD and had conservative management.
DISCUSSION
Most ECF requiring operative intervention arise postoperatively (84% in this series). These are usually caused by leakage from an anastomosis or from inadvertent enterotomy, following a procedure for inflammatory bowel disease, or one complicated by extensive adhesions or radiation injury.10,11
ECF may be catastrophic to the health and quality of life. Patients are often septic and malnourished; and if fistula output is high (>200 mL/day), severe metabolic disturbances can result.12 The availability of safe parenteral nutrition has reduced morbidity and mortality by permitting a period of conservative management during which a proportion of ECF can be expected to close spontaneously.13,14 This is less likely if the fistula is complicated by inflammatory bowel disease, previous radiation, bowel discontinuity, distal obstruction, short fistula tracts with mucocutaneous continuity, or persisting foreign bodies. Deciding on surgical repair is a major decision, as many centers report 30-day mortality rates of up to 10% to 20%.2–5
Initial fistula management should include controlling ECF effluent by pouching and skin protection. This is often difficult, especially with multiple fistulae or an open abdomen, and enterostomal nursing consultation should be sought early.15 Uncontrolled sepsis and sepsis-associated malnutrition have been shown to be important determinants of mortality.11,16,17 CT-guided drainage is the initial management of choice in patients presenting with spontaneous or postoperative intra-abdominal abscess.8 This can obviate the need for early operative intervention. If a fistula develops, a definitive procedure can be deferred with the drain left in to control further abscess formation.18,19
Radiologic drainage was necessary in 23 (11%) patients in this series. Twelve still had intra-abdominal collections noted at operation due to ECF persistence, with the drainage catheter acting to maintain a fistulous communication and provide partial drainage. TPN was initiated on patients with high output fistulae or proximal defunctioning jejunostomies, to decrease output and maintain nutritional status. This resulted in similar success rates following repair to those who were maintained on enteral nutrition.
Major abdominal surgery results in a dense peritoneal reaction that is maximal from the third to the tenth postoperative week.8,10,11 This is especially so when surgery is complicated by peritonitis or fistula formation. Surgery within this window may result in a more difficult dissection and risks compounding technical problems encountered.2,8,11 During this period, surgery is generally only undertaken if gangrene, peritonitis, or bleeding require it, and then should be limited to proximal defunctioning stoma formation and control of sepsis. In this series (Fig. 2), there were a small number of operative procedures in this window with 21 (10%) patients having definitive surgery between 2 weeks and 3 months after their last procedure, associated with a fistula recurrence rate of 28%.
Definitive surgery is generally delayed for several months, until physiologic deficits have been restored and intra-abdominal conditions are less hostile.20,21 This approach is supported by these results, where the chance of recurrence is lower if surgery is delayed, although this did not reach statistical significance (Fig. 2).
Successful surgery requires the resection of the ECF and associated diseased bowel back to an area free of edema and friability.9 The anastomosis should be clear of the fistula site and abscess cavity.1,8,10 This approach is associated with a greater rate of successful ECF closure, where fistula resection with or without defunctioning stoma resulted in successful ECF closure in 84% of patients, while fistula oversewing was successful in only 64% (P = 0.006), and surgical technique was an independent factor on multivariate analysis. These results are reminiscent of a previous series without multivariate analysis in which fistula closure was obtained in 57 of 66 patients (89%) having fistula resection, compared with only 19 of 32 (59%) after direct suture closure.4
It is important to note that, in some cases, a fistula may have been oversewn when it was not thought possible to perform a resection. This may have been because of an inability to adequately mobilize the bowel or concerns about the possibility of short bowel syndrome. If this is the situation, consideration should be given to protecting the repair with a proximal stoma or performing a bypass of that area.14
ECF remain a difficult management problem. The basis of management remains control of sepsis and fistula effluent, and nutritional maintenance. Early surgery should be limited to abscess drainage and proximal defunctioning stoma formation. Definitive procedures for persistent ECF should take place several months later with resection of the fistulous segment and reanastomosis of healthy bowel, when feasible. A low threshold is maintained for a defunctioning proximal stoma, particularly in the presence of residual sepsis, CD, and radiation enteritis. If such a stoma is proximal in the jejunum, postoperative TPN will be required. The application of this management strategy, in the setting of an institution with access to multispecialty care, resulted in an acceptable level of success with ECF closure with a very low mortality and morbidity. It is applicable to ECF due to a variety of etiologies with similar success, including CD.
Footnotes
Reprints: Conor P. Delaney, MCh, PhD, FRCSI(Gen), Department of Colorectal Surgery/A-30, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195. E-mail: delanec@ccf.org.
REFERENCES
- 1.Goligher JC. Resection with exteriorization in the management of faecal fistulas originating in the small intestine. Br J Surg. 1971;58:163–167. [DOI] [PubMed] [Google Scholar]
- 2.MacFadyen BV, Dudrick SJ, Ruberg RL. Management of gastrointestinal fistulas with parenteral hyperalimentation. Surgery. 1973;74:100–105. [PubMed] [Google Scholar]
- 3.Coutsoftides T, Fazio VW. Small intestine cutaneous fistulas. Surg Gynaecol Obstet. 1979;149:333–336. [PubMed] [Google Scholar]
- 4.Reber HA, Roberts C, Way LW, et al. Management of external gastrointestinal fistulas. Ann Surg. 1978;188:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre PB, Ritchie JK, Hawley PR, et al. Management of enterocutaneous fistulas: a review of 132 cases. Br J Surg. 1984;71:293–296. [DOI] [PubMed] [Google Scholar]
- 6.Cro C, George KJ, Donnelly J, et al. Vacuum assisted closure system in the management of enterocutaneous fistula. Postgrad Med J. 2002;78:364–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang TL, Chen MF. Randomized trial of fibrin tissue glue for low output enterocutaneous fistula. Br J Surg. 1996;83:112. [DOI] [PubMed] [Google Scholar]
- 8.Fazio VW. Intestinal fistulas. In: Keighley MRB, Pemberton JH, Fazio VW, et al. Atlas of Colorectal Surgery. New York: Churchill Livingstone, 1996:363–377. [Google Scholar]
- 9.Hill GL, Bambich CP. A technique for the operative closure of persistent external small-bowel fistulas. Aust NZ J Surg. 1981;51:477–485. [DOI] [PubMed] [Google Scholar]
- 10.Hill GL. Operative strategy in the treatment of enterocutaneous fistulas. World J Surg. 1983;7:495–501. [DOI] [PubMed] [Google Scholar]
- 11.Fazio VW, Coutsoftides T, Steiger E. Factors influencing the outcome of treatment of small bowel cutaneous fistula. World J Surg. 1983;7:481–488. [DOI] [PubMed] [Google Scholar]
- 12.Makhdoom ZA, Komar MJ, Still CD. Nutrition and enterocutaneous fistulas. J Clin Gastroenterol. 2000;31:195–204. [DOI] [PubMed] [Google Scholar]
- 13.Schirmer CC, Gurski RR, Gugel FL, et al. Alternative surgical treatment for complex enterocutaneous fistula. Int Surg. 1999;84:29–34. [PubMed] [Google Scholar]
- 14.Blackett RL, Hill GL. Postoperative external small bowel fistulas: a study of a consecutive series of patients treated with intravenous hyperalimentation. Br J Surg. 1978;65:775–778. [DOI] [PubMed] [Google Scholar]
- 15.Schaffner A, Hocevar BJ, Erwin-Toth P. Small-bowel fistulas complicating midline surgical wounds. J Wound Ostomy Continence Nurs. 1994;21:161–165. [DOI] [PubMed] [Google Scholar]
- 16.Soeters PB, Ebeid AM, Fischer JE. Review of 404 patients with gastrointestinal fistulas. Ann Surg. 1979;190:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zera RT, Bubrick MP, Sternquist JC, et al. Enterocutaneous fistulas: effects of total parenteral nutrition and surgery. Dis Colon Rectum. 1983;26:109–112. [DOI] [PubMed] [Google Scholar]
- 18.Sahai A, Bélair M, Gianfelice D, et al. Percutaneous drainage of intra-abdominal abscesses in Crohn's disease: short and long-term outcome. Am J Gastroenterol. 1997;92:275–278. [PubMed] [Google Scholar]
- 19.Ayuk P, Williams N, Scott NA, et al. Management of intra-abdominal abscesses in Crohn's disease. Ann R Col Surg Engl. 1996;78:5–10. [PMC free article] [PubMed] [Google Scholar]
- 20.Oakley JR, Steiger E, Lavery IC, et al. Catastrophic enterocutaneous fistulae: the role of home hyperalimentation. Cleve Clin Q. 1979;46:133–136. [DOI] [PubMed] [Google Scholar]
- 21.Mulholland MW, Delaney JP. Proximal diverting jejunostomy for compromised small bowel. Surgery. 1983;93:443–447. [PubMed] [Google Scholar]