Abstract
Objective:
To clarify the relationship between intratumoral dihydropyrimidine dehydrogenase (DPD) expression and response to 5-fluorouracil (5-FU) liver perfusion chemotherapy (LPC) in pancreatic cancer patients, we evaluated DPD expression immunohistochemically in resected pancreatic cancer tissues.
Summary Background Data:
Pancreatic cancer is considered a disease with a poor prognosis even if aggressive resection is performed. One of the main causes of death is hepatic metastasis soon after surgery. As a treatment, we have assessed adjuvant LPC via the portal vein using 5-FU just after pancreatectomy for advanced pancreatic cancer since 1994. However, the results remain unsatisfying.
Methods:
Sixty-eight resected specimens were obtained from patients with pancreatic cancer from 1988 to 2000. Formalin-fixed paraffin-embedded tissues were immunostained with polyclonal anti-DPD antibody. The relation between intratumoral DPD expression and the prognoses of pancreatic cancer patients was investigated statistically.
Results:
Of the 68 tumors studied, 27 carcinomas (39.7%) were DPD(+), and 41 (60.3%) were DPD(−). In the DPD(+) group, there was no significant difference between the LPC(+) and LPC(−) subgroups, whereas in the DPD(−) group the LPC(+) subgroup showed a significantly higher survival rate than the LPC(−) subgroup. Moreover, in the LPC(+) group, overall survival in the DPD(−) subgroup was significantly better than in the DPD(+) subgroup.
Conclusions:
An immunohistochemical evaluation of intratumoral DPD expression might be useful in predicting responsiveness to 5-FU-based chemotherapy in pancreatic cancer patients. In the DPD(−) group, liver perfusion chemotherapy using 5-FU via the portal vein is effective adjuvant therapy for pancreatic cancer once pancreatectomy has been performed.
Of 68 pancreatic cancer tissues immunostained, 27 carcinomas (39.7%) have expressed dihydropyrimidine dehydrogenase (DPD) antigen. In the DPD(−) group, patients who received adjuvant 5-fluorouracil liver perfusion chemotherapy (LPC) had a better prognosis than those who did not. In the LPC(+) group, patients with DPD(−) tumors had a better prognosis than those with DPD(+) tumors.
Pancreatic cancer is considered a disease with a poor prognosis even if aggressive resection is performed. In our department, we have aggressively performed extended radical resections with wide lymphoidectomy and portal vein resection for advanced pancreatic cancers.1,2 However, the overall survival rate is extremely low. One of the main causes of death is hepatic metastasis soon after surgery.3–7 Several studies have demonstrated that adjuvant liver perfusion chemotherapy (LPC) via the portal vein and/or hepatic artery is effective in pancreatic cancer.8–11 Ishikawa et al8 reported efficacy of adjuvant LPC using 250 mg/d/body of 5-fluorouracil (5-FU) via both portal vein and hepatic artery (125 mg /d/body from each route). Takahashi et al9 reported adjuvant LPC using 250 mg/d/body via the portal vein decreased the liver metastases. Since 1994, we have assessed adjuvant LPC via the portal vein using 5-FU (250 mg/d/body) continuously for 3 weeks just after radical pancreatectomy for advanced pancreatic cancer. This 5FU-based chemotherapy reportedly improved the long-term survival rate of pancreatic cancer patients who had undergone extended pancreatectomy.12 However, the results remain unsatisfying, and 5-FU LPC is not recognized as a standard treatment.
5-FU is catabolized to 2-fluoro-β-alanine via 3 enzymes, among which dihydropyrimidine dehydrogenase (DPD) is the first and rate-limiting enzyme in the pathway.13 It is widely expressed in most normal tissues, and its content is highest in the liver and peripheral mononuclear cells.14 DPD expression in tumor cells has been reported to vary considerably compared with its expression in normal tissues.15 In addition, its intratumoral expression could cause 5-FU degradation prior to 5-FU engagement in the anabolic pathway, resulting in a correlation with responsiveness to 5-FU.13 Several clinical studies have shown that DPD-positive tumors are associated with 5-FU resistance and a poor outcome.16–22 Thus, we speculated that a better prognosis in pancreatic cancer patients might be obtained by extracting an indication of 5-FU LPC by intratumoral DPD expression. Moreover, there have been no reports concerning the correlation between DPD expression and 5-FU LPC in pancreatic cancer.
Immunohistochemistry has the advantage of permitting the evaluation of protein expression in situ using paraffin-embedded blocks of specimens. Takenoue et al23 investigated DPD expression using immunohistochemistry for colon carcinoma, and demonstrated that the immunohistochemical score was correlated with the protein levels of DPD.
We performed an immunohistochemical study of intratumoral DPD expression in pancreatic cancer and investigated the relationship between intratumoral DPD expression and the response to 5-FU LPC in resected pancreatic cancer patients. Our investigation may provide grounds for a reevaluation of LPC in the treatment of postoperative pancreatic cancer patients.
METHODS
Clinical Samples
Sixty-eight resected specimens were obtained from patients with pancreatic cancer from 1988 to 2000. None of them had macroscopic liver metastases or peritoneal dissemination at the time of their surgery. Fifteen patients had further lymph nodes metastases beyond regional lymph nodes. Uniform surgical techniques were used in every operation.2 Portal vein resections were performed in 40 of the 68 cases. The diagnosis was confirmed by histopathological examination of the resected tissues. The histologic examinations were carried out routinely using hematoxylin and eosin stain according to the rules of the tumor-node-metastasis (TNM) classification (UICC TNM classification of malignant tumors. 6th edition. 2001). None of these patients received chemotherapy prior to surgery or after discharge. Since 1994, we have added adjuvant LPC via the portal vein after surgery. Following radical pancreatectomy with wide lymphadenectomy for advanced pancreatic cancer, a 16-gauge catheter was inserted into one of the branching vessels of the superior mesenteric vein, and the catheter tip was placed in the portal vein. For 3 weeks just after the operation, 250 mg/d/body of 5-FU was infused via the portal vein continuously. In this study, 36 of 60 patients with pancreatic cancer except stage I received 5-FU LPC.
Immunohistochemistry for DPD
The expression of DPD was studied immunohistochemically using a polyclonal antibody for recombinant human DPD provided by the Second Cancer Research Laboratory, Honno Research Center, Taiho Pharmaceutical Co., Ltd., Saitama, Japan. The immunohistochemical staining procedure was as follows: resected specimens of pancreatic cancer were fixed in 4% buffered formalin and embedded in paraffin. Then, 4-μm tissue sections were cut from the paraffin-embedded cancer lesions. Deparaffinized sections were treated with 100% methanol containing 0.3% hydrogen peroxide to inhibit endogenous peroxidase. After deparaffinization and dehydration, sections were incubated with 0.2% trypsin at room temperature for 30 minutes and then treated with 100% methanol containing 0.3% hydrogen peroxidase to inhibit endogenous peroxidase. After being washed 3 times in PBS, nonspecific bindings were blocked by preincubation with Vectastain (SAB kit; Vector Laboratory, Inc.) at room temperature for 30 minutes according to the INC recommendation. The sections were then incubated with antihuman rDPD antibody at 4°C overnight. After being washed 3 times in PBS, slides were reacted with biotinylated antirabbit goat IgG (SAB kit) at room temperature for 10 minutes as a secondary antibody. After being washed 3 times in PBS, the sections were then incubated with streptavidin-conjugated to horseradish peroxidase for 5 minutes. Sections were immersed in 0.25% 3,3-diaminobenzidine tetrahydrochloride (DAB) solution in 50-mm Tris-HCl buffer (pH 7.6) containing 10-mm hydrogen peroxide and 10-mm sodium azide until staining was complete. Finally, the sections were counterstained with Mayer hematoxylin solution.
Assessment of Immunostaining
The grade of staining was evaluated without previous knowledge of the clinicopathological details. Since DPD antigen was strongly stained in the cytoplasm of islet cells, we regarded them as a positive control of immunostaining. The intensity of staining was scored as follows: grade 0, not stained; grade 1, stained only faintly or unevenly; grade 2, stained weakly compared with islet cells; grade 3, stained as strongly as islet cells. If the product was grade 0 or 1, the sample was considered DPD(−), and if grade 2 or 3, the sample was considered DPD(+). Three specimens in which cells including the islet cells were not stained might have been inadequately fixed, although the possibility that these represent DPD-deficient cases cannot be completely denied. These specimens were excluded from the current study.
Statistical Analysis
Survival curves were analyzed by the Kaplan-Meier method, and differences between individual curves were evaluated by log-rank test. For the clinicopathological features of the patients, P values were calculated by χ2 test and Student t test, with P < 0.05 considered significant.
RESULTS
Intratumoral DPD Expression of Pancreatic Carcinoma
Intratumoral DPD staining appeared in the form of a granular cytoplasmic staining pattern (Fig. 1). The numbers of the tumors classified into grade 0, 1, 2, and 3 were 38, 3, 21, and 6, respectively. Of the 68 tumors studied, 27 carcinomas (39.7%) were DPD(+), and 41 carcinomas (60.3%) were DPD(−).
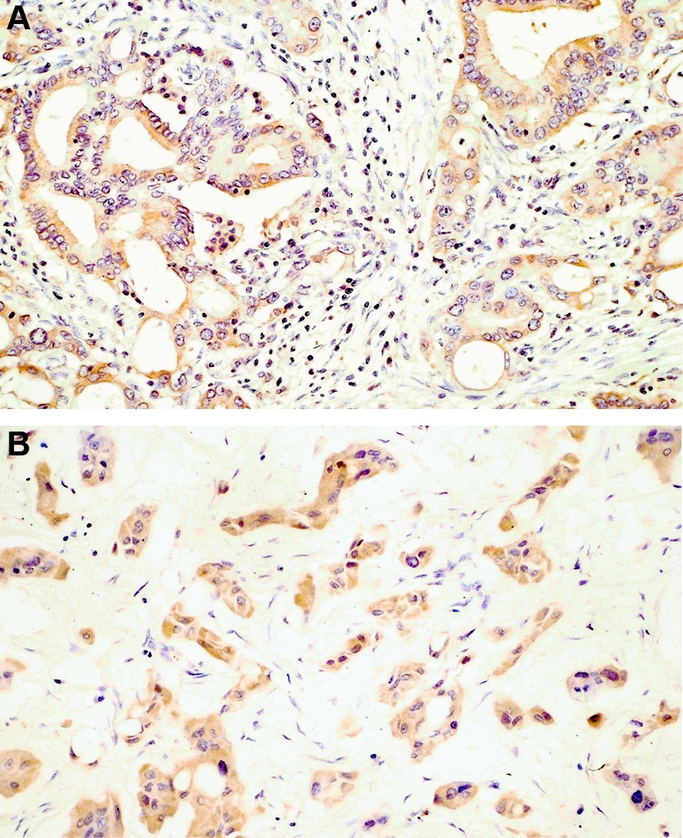
FIGURE 1. Immunohistochemical staining for DPD. A, DPD(+) moderately-differentiated adenocarcinoma. B, DPD(+) poorly-differentiated adenocarcinoma.
Clinicopathological Factors and DPD Expression
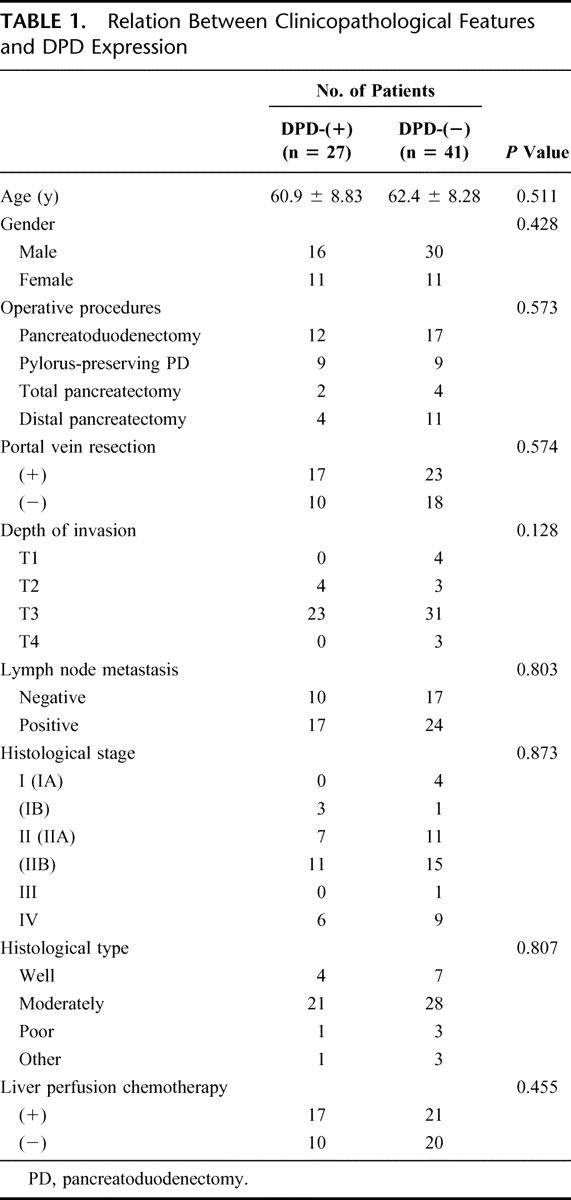
The relation between clinicopathological factors and DPD expression is summarized in Table 1. No significant differences were shown between the DPD(−) and DPD(+) groups. There was no correlation between intratumoral DPD staining and other patient prognostic factors such as tumor status, nodal status, or tumor differentiation.
TABLE 1. Relation Between Clinicopathological Features and DPD Expression
Association Between Intratumoral DPD Expression and Survival of Pancreatic Cancer Patients
The relation between intratumoral DPD expression and the prognosis of patients with pancreatic cancer in stages II (IIA, IIB), III, and IV was investigated statistically. In the DPD(−) group, the LPC(+) subgroup showed a significantly higher survival rate compared with the LPC(−) subgroup (P < 0.0001) (Fig. 2A), whereas in the DPD(+) group, there was no significant difference between the LPC(+) and LPC(−) subgroups (Fig. 2B). In the LPC(+) group, overall survival in the DPD(−) subgroup was significantly better than in the DPD(+) subgroup (P < 0.04) (Fig. 3A), whereas in the LPC(−) group, overall survival was no different between the DPD(−) and DPD(+) subgroups (Fig. 3B).
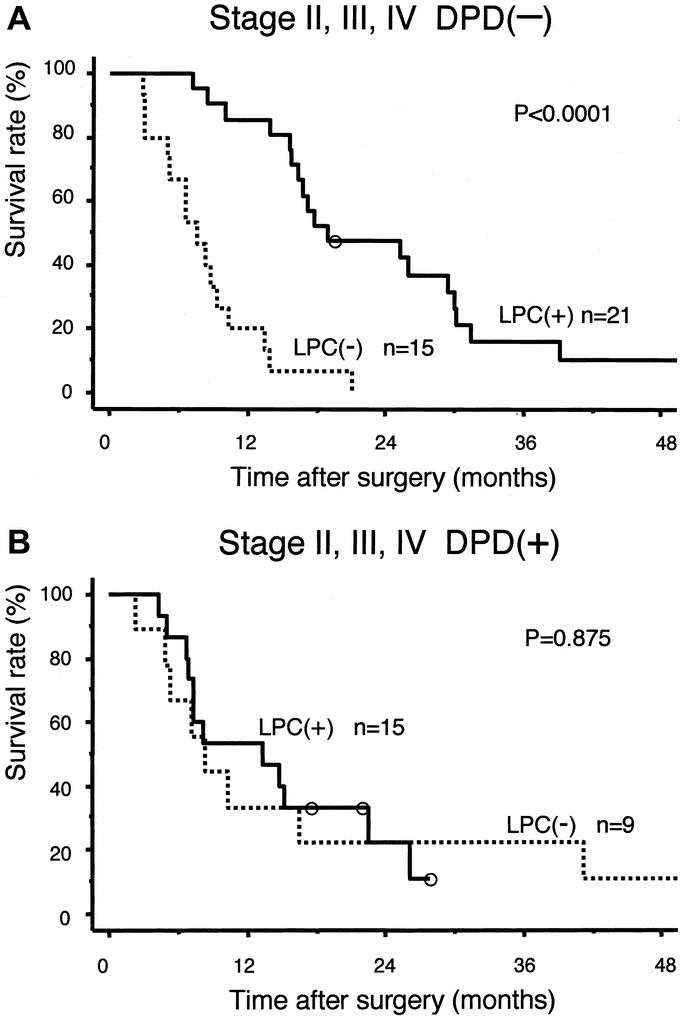
FIGURE 2. Overall survival by LPC in stages II, III, and IV. A, In DPD(−) group, LPC(+) subgroup showed significantly better survival than LPC(−) subgroup. B, In DPD(+) group, there was no significant difference between LPC(+) and LPC(−) subgroups.
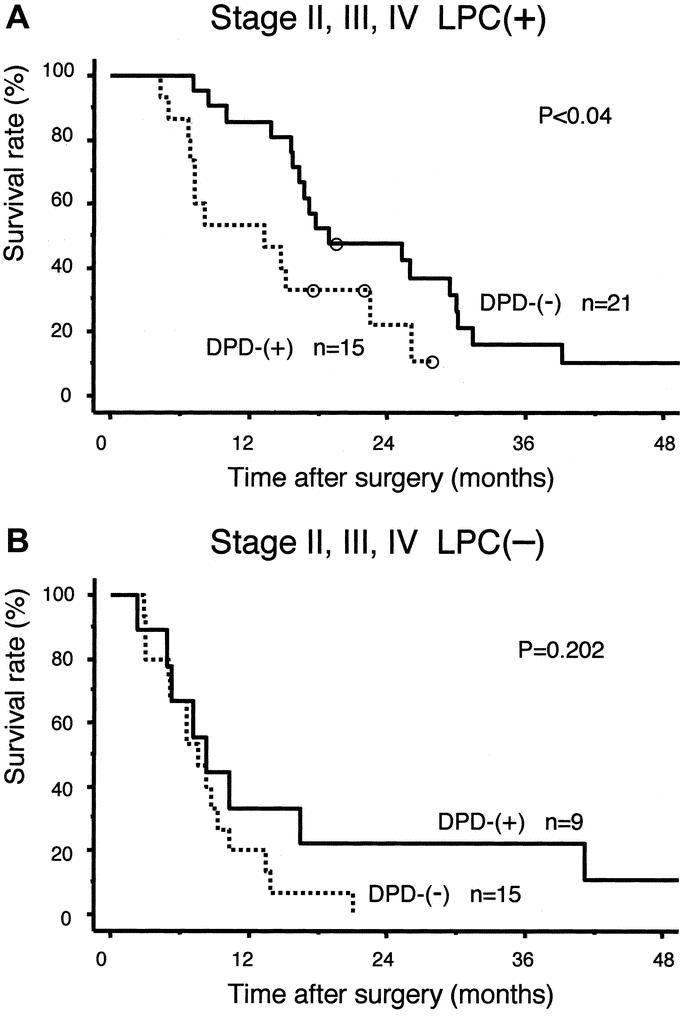
FIGURE 3. Overall survival by DPD expression in stages II, III, and IV. A, In LPC(+) group, DPD(−) subgroup showed significantly better survival than DPD(+) subgroup. B, In LPC(−) group, there was no significant difference between DPD(−) and DPD(+) subgroups.
DISCUSSION
5-FU is recognized as an effective chemotherapeutic agent against gastrointestinal cancer, and its metabolic pathways are becoming clear. Among the several molecules involved in those pathways, previous studies have demonstrated the biologic importance of the intratumoral activity of TS, the target enzyme of 5-FU, and DPD, the first enzyme to metabolize 5-FU.13,24–27 Several clinical studies on gastrointestinal and other cancers which have been widely treated by 5-FU-based chemotherapy have demonstrated that intratumoral DPD expression is associated with 5-FU resistance and poor outcomes.16–22 To our knowledge, this is the first clinical report demonstrating a correlation between intratumoral DPD expression and the survival of patients with pancreatic carcinoma. It clearly revealed that intratumoral DPD expression was associated with the survival of pancreatic cancer patients treated with 5-FU LPC. This finding supports a close correlation between intratumoral DPD activity and responsiveness to 5-FU. An immunohistochemical evaluation of intratumoral DPD expression might be useful in predicting responsiveness to 5-FU-based chemotherapy in pancreatic cancer patients, and DPD expression may be a beneficial indicator in LPC applications using 5FU.
Furthermore, earlier research in our department revealed that intratumoral TS expression was also associated with the survival of pancreatic cancer patients treated with 5-FU LPC.12 Salonga et al and Huang et al reported that both DPD and TS-negative patients showed a good survival prognosis in colorectal and lung cancer, respectively.15,16 In previous study in our department, TS-positive tumors had a good response to 5-FU in pancreatic cancer.12 Although the small number of patients involved seems insufficient to draw conclusions, the DPD(−)TS(+) subgroup in LPC(+) group have a markedly improved survival rate (data not shown). However, even if DPD(−)TS(+) patients received 5-FU LPC, most of them had hepatic metastases or local recurrences within 3 years after radical resection. To obtain further prognostic improvement, it will be necessary to consider additional adjuvant treatments following LPC. For example, 5-FU oral agents following LPC treatment may improve the survival rate. On the other hand, for DPD(+) patients, DPD inhibitors such as S1 or another agent such as gemcitabine may be needed.28–30 Furthermore, it is expected that new therapeutic breakthroughs such as gene therapy will be developed in the near future.
In conclusion, in the DPD(−) group, LPC using 5FU is a promising therapy even for stage IV pancreatic cancer when pancreatectomy is aggressively performed with wide lymphadenectomy and portal vein resection. The immunohistochemical evaluation of DPD is not only technically simple and easy to perform but accurately determines the expression level in cancer cells. We believe that a clinical application based on these new biologic advances may well prolong and save lives, not to mention save time and money spent on ineffective treatments.
Footnotes
Reprints: Shin Takeda, MD, Department of Surgery II, Nagoya University School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan. E-mail: shinta@med.nagoya-u.ac.jp.
REFERENCES
- 1.Nakao A, Nonami T, Harada A, et al. Portal vein resection with a new antithrombogenic catheter. Surgery. 1990;108:913–918. [PubMed] [Google Scholar]
- 2.Nakao A, Takagi H. Isolated pancreatectomy for pancreatic head carcinoma using catheter bypass of the portal vein. Hepatogastroenterology. 1993;40:426–429. [PubMed] [Google Scholar]
- 3.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. [DOI] [PubMed] [Google Scholar]
- 4.Kayahara M, Naganawa T, Ueno K, et al. An evaluation of radical resection for pancreatic cancer based on the recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118–2123. [DOI] [PubMed] [Google Scholar]
- 5.Sperti C, Pasquali C, Piccoli A, et al. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. [DOI] [PubMed] [Google Scholar]
- 6.Nakao A, Inoue S, Nomoto S, et al. Extended radical surgery for pancreatic adenocarcinoma: indications and oncological problems. Asian J Surg. 1997;20:192–197. [Google Scholar]
- 7.Inoue S, Nakao A, Kasai Y, et al. Detection of hepatic metastasis in pancreatic adenocarcinoma patients by two-stage polymerase chain reaction/restriction fragment length polymorphism (PCR/RFLP) analysis. Jpn J Cancer Res. 1995;86:626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa O, Ohigashi H, Sasaki Y, et al. Liver perfusion chemotherapy via both the hepatic artery and portal vein to prevent hepatic metastasis after extended pancreatectomy for adenocarcinoma of the pancreas. Am J Surg. 1994;168:361–364. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi S, Ogata Y, Miyazaki H, et al. Aggressive surgery for pancreatic duct cell cancer: feasibility, validity, limitations. World J Surg. 1995;19:653–660. [DOI] [PubMed] [Google Scholar]
- 10.Link KH, Gansauge F, Rilinger N, et al. Celiac artery adjuvant chemotherapy: result of a prospective trial. Int J Pancreatol. 1997;21:65–69. [DOI] [PubMed] [Google Scholar]
- 11.Beger HG, Gansauge F, Buchler MW, et al. Intraarterial adjuvant chemotherapy after pancreaticoduodenectomy for pancreatic cancer: significant reduction in occurrence of liver metastasis. World J Surg. 1999;23:946–949. [DOI] [PubMed] [Google Scholar]
- 12.Takeda S, Inoue S, Kaneko T, et al. The role of adjuvant therapy for pancreatic cancer. Hepatogastroenterology. 2001;48:953–956. [PubMed] [Google Scholar]
- 13.Fischel JL, Etienne MC, Spector T, et al. Dihydropyrimidine dehydrogenase: a tumoral target for fluorouracil modulation. Clin Cancer Res. 1995;1:991–996. [PubMed] [Google Scholar]
- 14.Ho DH, Townsend L, Luna MA, et al. Distribution and inhibition of dihydrouracil dehydrogenase activities in human tissues using 5-fluorouracil as a substrate. Anticancer Res. 1986;6:781–784. [PubMed] [Google Scholar]
- 15.Naguib FNH, Kouni MH, Cha S. Enzymes of uracil catabolism in normal and neoplastic human tissues. Cancer Res. 1985;45:5405–5412. [PubMed] [Google Scholar]
- 16.Inada T, Ogata Y, Kubota T, et al. 5-Fluorouracil sensitivity and dihydropyrimidine dehydrogenase activity in advanced gastric cancer. Anticancer Res. 2000;20:2457–2462. [PubMed] [Google Scholar]
- 17.Ishikawa Y, Kubota T, Otani Y, et al. Dihydropyrimidine dehydrogenase and messenger RNA levels in gastric cancer: possible predictor for sensitivity to 5-fluorouracil. Jpn J Cancer Res. 2000;91:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salonga D, Danenberg KD, Johnson M, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–1327. [PubMed] [Google Scholar]
- 19.Huang CL, Yokomise H, Kobayashi S, et al. Intratumoral expression of thymidylate synthase and dihydropyrimidine dehydrogenase in non-small cell lung cancer patients treated with 5-FU-based chemotherapy. Int J Oncol. 2000;17:47–54. [PubMed] [Google Scholar]
- 20.Fujiwaki R, Hata K, Nakamoto K, et al. Gene expression for dihydropyrimidine dehydrogenase and thymidine phosphorylase influences outcome in epithelial ovarian cancer. J Clin Oncol. 2000;18:3946–3951. [DOI] [PubMed] [Google Scholar]
- 21.Mizutani Y, Wada H, Fukushima M, et al. The significance of dihydropyrimidine dehydrogenase (DPD) activity in bladder cancer. Eur J Cancer. 2001;37:569–575. [DOI] [PubMed] [Google Scholar]
- 22.Horiguchi J, Takei H, Koibuchi Y, et al. Prognostic significance of dihydropyrimidine dehydrogenase expression in breast cancer. Br J Cancer. 2002;86:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takenoue T, Kitayama J, Takei Y, et al. Characterization of dihydropyrimidine dehydrogenase on immunohistochemistry in colon carcinoma, and correlation between immunohistochemical score and protein level or messenger RNA expression. Ann Oncol. 2000;11:273–279. [DOI] [PubMed] [Google Scholar]
- 24.Beck A, Etienne MC, Cheradame S, et al. A role for dihydropyrimidine dehydrogenase and thymidylate synthase in tumor sensitivity to fluorouracil. Eur J Cancer. 1994;30A:1517–1522. [DOI] [PubMed] [Google Scholar]
- 25.Langenbach RJ, Danenberg PV, Heidelberger C. Thymidylate synthase: mechanism of inhibition by 5-fluoro-2′-deoxy-uridylate. Biochem Biophys Res Commun. 1972;48:1565–1571. [DOI] [PubMed] [Google Scholar]
- 26.Spears CP, Gustavsson BG, Berne M, et al. Mechanisms of innate resistance to thymidylate synthase inhibition after 5-fluorouracil. Cancer Res. 1998;48:5894–5900. [PubMed] [Google Scholar]
- 27.Rustum YM, Harstrick A, Cao S, et al. Thymidylate synthase inhibitors in cancer therapy: direct and indirect inhibitors. J Clin Oncol. 1997;15:389–400. [DOI] [PubMed] [Google Scholar]
- 28.Shirasaka T, Shimamoto Y, Ohshima H, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–557. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto S, Boku N, Ohta A, et al. Study Group of S-1 for Gastric Cancer: clinical implications of immunoreactivity of thymidylate synthase and dihydropyrimidine dehydrogenase in gastric cancer treated with oral fluoropyrimidines (S-1). Int J Oncol. 2000;17:653–658. [DOI] [PubMed] [Google Scholar]
- 30.Burris HA 3rd, Moore MJ, Anderson J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. [DOI] [PubMed] [Google Scholar]


